Imatinib as preoperative therapy in Chinese patients with recurrent or metastatic GISTs
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal system and are thought to originate from intestinal cells of Caja (1). Before the specific tyrosine kinase inhibitor imatinib was investigated for GISTs treatment in 2000, GISTs proved refractory to any treatment other than surgery, including conventional chemotherapy and radiation therapy, making observation alone the standard of care after surgical resection (2,3). Imatinib is a success story of molecular biology that has dramatically altered the management of patients with gastrointestinal stromal tumours. However, as patient follow up has continued, secondary resistance has appeared because responses to imatinib are of limited duration. Indeed, the median time to progression during imatinib mesylate treatment is 2 years (4). The current clinical practice at The University of Texas M.D. Anderson Cancer Center for patients with either locally advanced, recurrent, or metastatic GISTs is to use imatinib as first-line treatment and then to consider surgical resection when the tumor has demonstrated adequate response to allow for complete resection of any residual disease (5). However, the indications, timing, and role of surgery for these patients are not well established. The effect of preoperative imatinib on surgical resection rates and postoperative outcome in patients with advanced GISTs is unknown. In the present study, we aimed to define the role of surgery and its long-term outcome in patients with advanced GISTs treated with imatinib preoperatively.
Patients and methods
Patient characteristics
We reviewed the medical records of 113 patients with unresectable recurrent or metastatic GISTs who had been treated with imatinib at Fudan University Cancer Center, Shanghai, China, between June 2003 and June 2011. Twenty-two patients who underwent surgery after imatinib treatment were enrolled into the study.
Imatinib treatment
Front-line imatinib treatment consisted of 400 mg once daily. Patients who experienced disease progression before surgery were treated with 600 or 800 mg imatinib daily. Tumor response to imatinib was evaluated in all patients with serial CT imaging. According to the Response Evaluation Criteria in Solid Tumors criteria (6), an experienced radiologist retrospectively re-reviewed all radiographic data for the 22 patients in our study and categorized the response to imatinib based on changes in tumor size, degree and extent of enhancement, and the presence or absence of solid nodules within the tumor. Consistent with Southwest Oncology Group (SWOG) criteria, an increase in the sum of the longest diameters of the target lesions alone was not regarded as disease progression if accompanied by definite cystic change in the tumor suggesting necrosis (7). A resistant lesion was confirmed by radiographic imaging, such as computed tomography (CT), magnetic resonance imaging (MRI). Radiographic evaluations were carried out every 3 months during imatinib treatment.
Disease status at the time of surgery was classified into two clinical categories. The first category included patients who achieved partial response (PR), stable disease (SD) or complete response (CR) to imatinib. These patients were classified as having responsive disease (RD). The second category included patients who experienced secondary resistance and primary resistance to imatinib during the treatment. Primary resistance was defined as progression occurring in patients who had never shown any response to imatinib or progression encountered within 3 months after initiation of imatinib treatment. Secondary resistance was defined as progression occurring in patients who initially had a response to imatinib treatment or a progression free survival interval exceeding 3 months (8). These patients were classified as having progression disease (PD).
Surgical intervention
The standard approach is to plan resection at the point of maximal response when the disease has stabilized but not begun to progress. In patients with RD, a reasonable time to consider surgery is at 12 months after starting imatinib treatment (4,9). All surgical procedures in this study were elective. In RD patients with no evidence of change in tumor size or enhancement throughout treatment for at least two months, the resection was considered by the surgeon based on a review of computed tomography (CT) imaging by experienced radiologists. However, in PD patients, surgery was done as soon as progression occurred.
Mutation analysis
Eight pretreatment formalin-fixed and paraffin-embedded surgical specimens from primary tumors were available in our institution, but most specimens were from other medical centers. Tumor specimens from the second resections done after imatinib therapy were all from our center. The fresh samples were flash frozen in liquid nitrogen at the time of surgical resection and maintained at -80 °C. Genomic DNA was extracted using a standard phenol-chloroform organic extraction protocol. Adequate DNA for mutation analysis was obtained, which was then analysed for mutation of KIT exon 9, 11, 13, 17, and PDGFRA exon 12 and 18 as described previously in the literature (10).
Statistical analysis
Progression-free survival (PFS) was measured from the date of surgery to the date of documented disease progression or death from any cause. Overall survival (OS) was measured from the date of surgery to death from any cause. Survival curves were calculated according to the Kaplan-Meier method. We used the Statistical Package for the Social Sciences, version 13.0 (SPSS, Chicago, IL). P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 22 patients (13 men and 9 women) underwent surgery for GIST after preoperative treatment with imatinib. The median age of the patients was 49.3 years (range from 33 to 72 years) at surgery after imatinib treatment. All patients received operations in our institution, including 4 patients who experienced more than 2 excisions. Primary sites of GIST were the stomach in 7 patients (31.8%), small intestine in 7 patients (31.8%), colon and rectum in 4 patients (18.2%), extra-gastrointestinal in 4 patients (18.2%). At the time of surgery, 15 patients had locally recurrent GISTs, and 7 patients had metastatic GISTs, including 3 tumors which metastasized to the liver, 2 tumors which metastasized to the peritoneum, and 2 tumors which metastasized to extragastrointestinal sites. At the first surgery in 2 patients, the surgeon found that the whole abdominal cavity and pelvic cavity were covered with hundreds of the GISTs (Table 1).
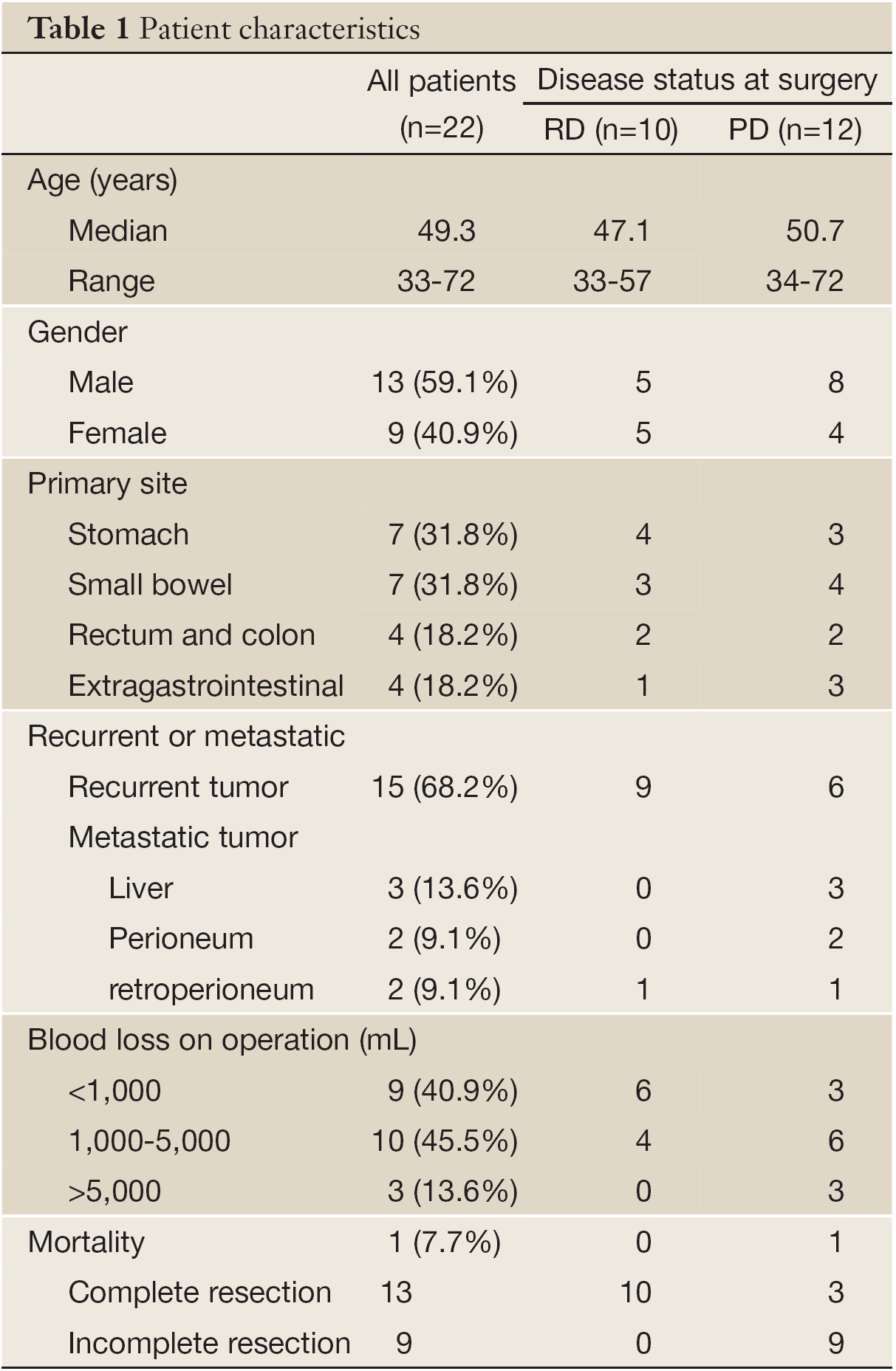
Full table
Preoperative imatinib treatment
Prior to surgery, the median duration of imatinib treatment for all patients was 14 months (range, 2-51 months), 18.5 months (range, 5-51 months) for patients with RD, and 8 months (range, 2-30 months) for patients with PD. Except for one patient who showed primary resistance during treatment, 21 of 22 patients had an initial response to imatinib. Of the 22 patients treated, 1 (4.5%) patient had a complete response, 4 (18.2%) patients had a partial response, 5 (22.7%) had stable disease, and 12 (54.6%) had progressive disease (Table 2). Of the 12 patients with progressive disease, one exhibited primary resistance to imatinib, while 11 developed secondary resistance to imatinib. In RD group, treatment was interrupted in 4 patients after a range of 2 to 12 months, mostly for financial reasons or drug adverse effects. After imatinib treatment was resumed in these 4 patients, all patients were still sensitive to imatinib and disease control continued. Prior to surgical resection, 8 (36.4%) patients were administered 400 mg daily, 7 (31.8%) patients, including 3 RD cases and 4 with secondary resistance, were escalated to 600 mg a day, and 7 (31.8%) patients were escalated to 800 mg per day due to different tolerance. In the 14 patients who were administered higher doses, 10 (71.4%) patients who had shown secondary resistance continued to experience disease progression and 4 (28.6%) patients had partial remission, which classified them as RD patients. Of the 11 patients with PD, except one hospital death and three who continued imatinib treatment due to experiencing local disease progression, imatinib therapy was discontinued and postsurgical treatment was with Chinese medicine or sunitinib.
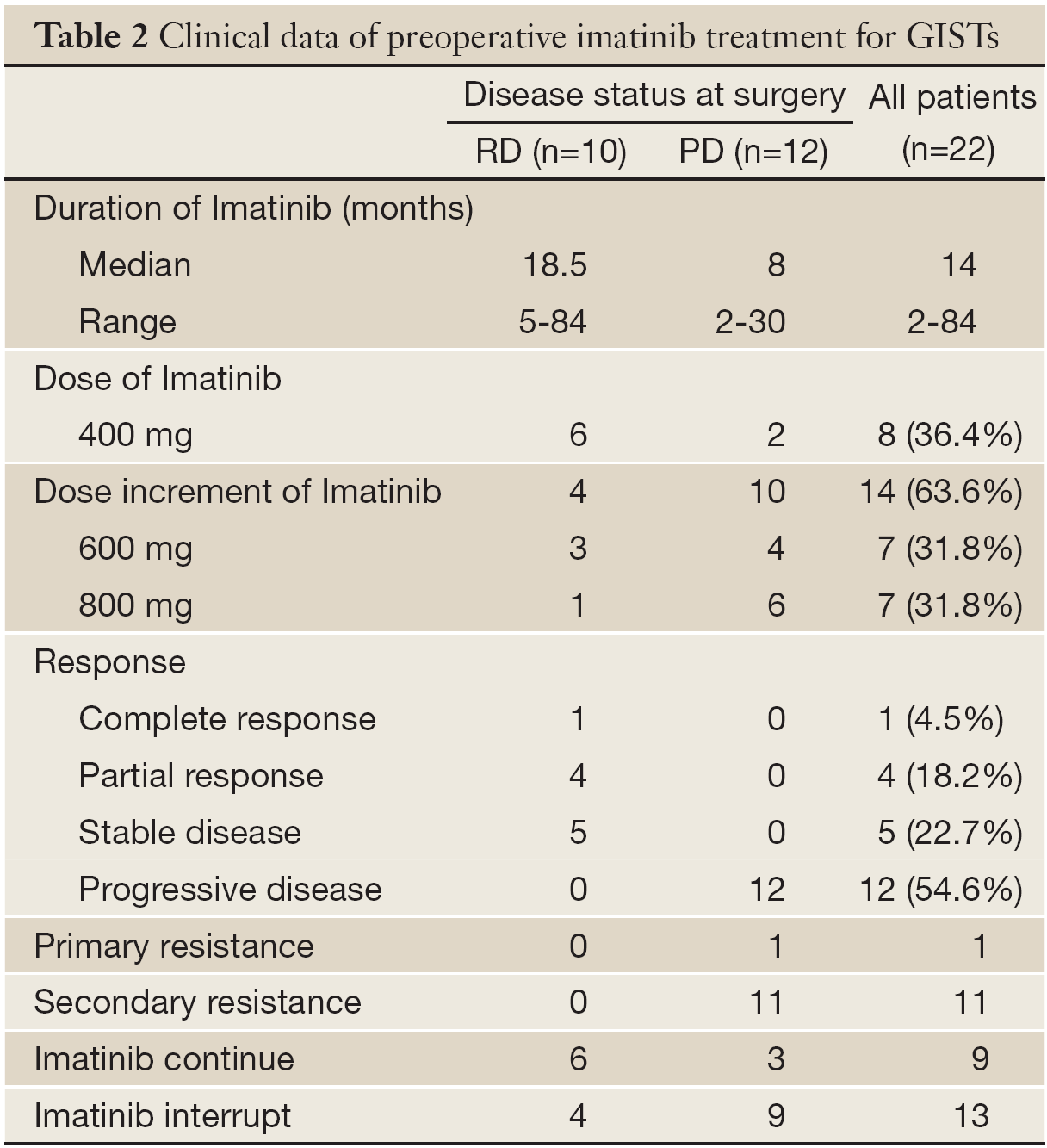
Full table
Surgical intervention
Complete resection was accomplished in 8 of the 10 RD patients (80%). Three of the 12 PD patients (25%), including 1 patient who showed primary resistance to imatinib, achieved complete resection. The other 9 (75%) PD patients had incomplete resections, including 2 patients who underwent palliative resection because of hundreds of tumors covering the entire abdominal cavity. The amount of blood loss during the operation was below 1,000 mL in 6 of 10 RD patients, and none lost over 5,000 mL. On the contrary, in the PD patients, except for 3 (25%) patients who lost less than 1,000 mL, 3 (25%) patients lost more than 5,000 mL, and 1 patient lost over 10,000 mL. There was one hospital death related to surgery, and 2 patients experienced postoperative complications. One patient died 2 days after operation due to dessiminated intravascular coagulation. One PD patient lost over 5,000 mL of blood during surgery, was in shock during the operation, and recovered after extensive effort treatment. One patient experienced postoperative acute renal failure, but recovered with conservative therapy because the complication was comparatively mild.
KIT/PDGFRA genotyping before and after imatinib treatment
For all primary tumors, activating mutations in KIT were seen in 17 (77.3%) patients, including 12 mutations in exon 11, 4 mutations in exon 9 and 1 mutation in exon 13. PDGFRA mutations were seen in 4 (18.2%) patients, while KIT and PDGFRA wild type gene was found in the one (4.5%) patient who showed primary imatinib resistance. After surgery following imatinib treatment, mutation analysis was performed. In addition to the original mutations, 3 of 12 patients with secondary imatinib resistance harbored secondary mutations in KIT exon 17 in the progressive lesion, while the other lesions showed only the original mutations (Table 3).
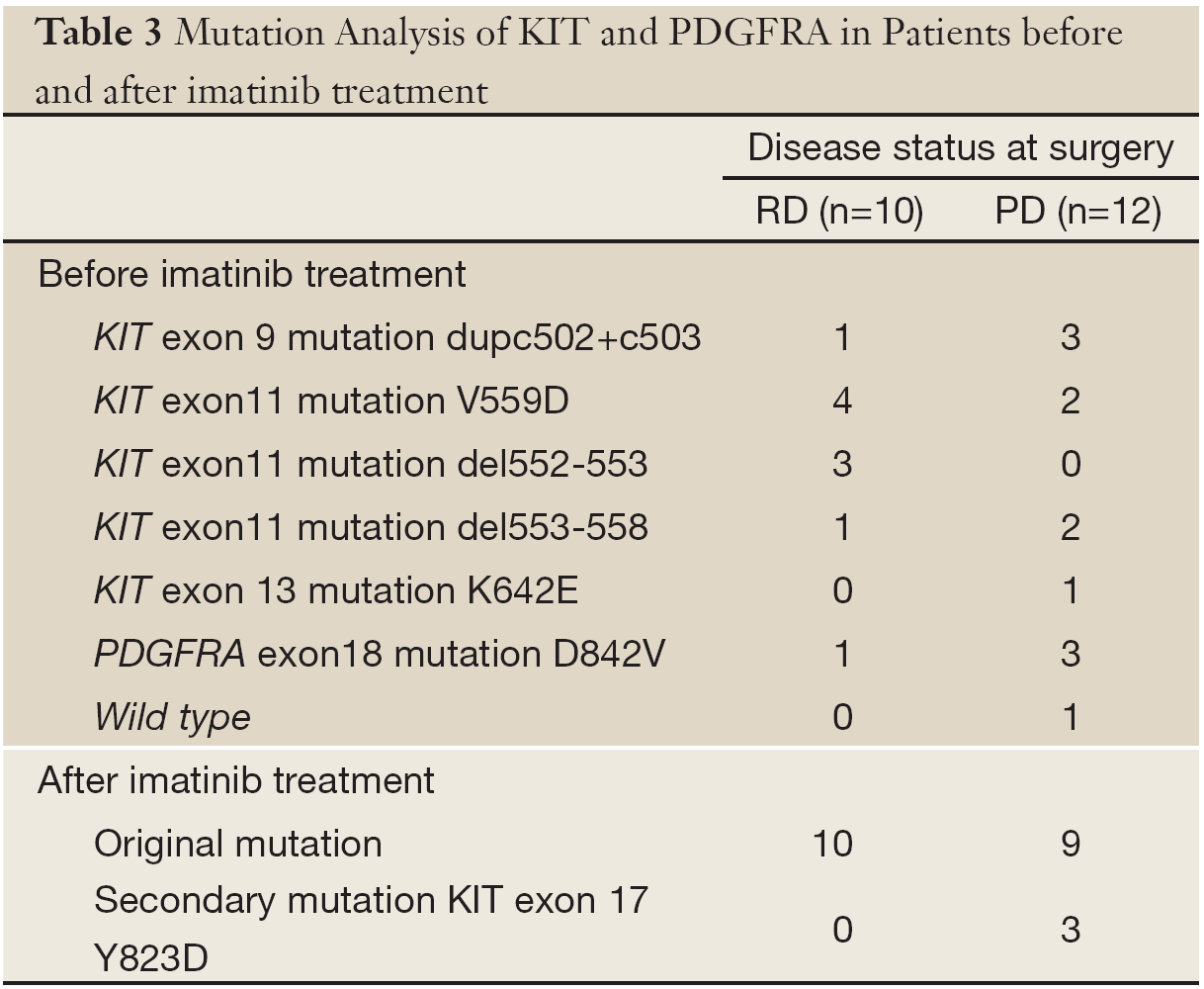
Full table
Progression-free survival and overall survival analysis
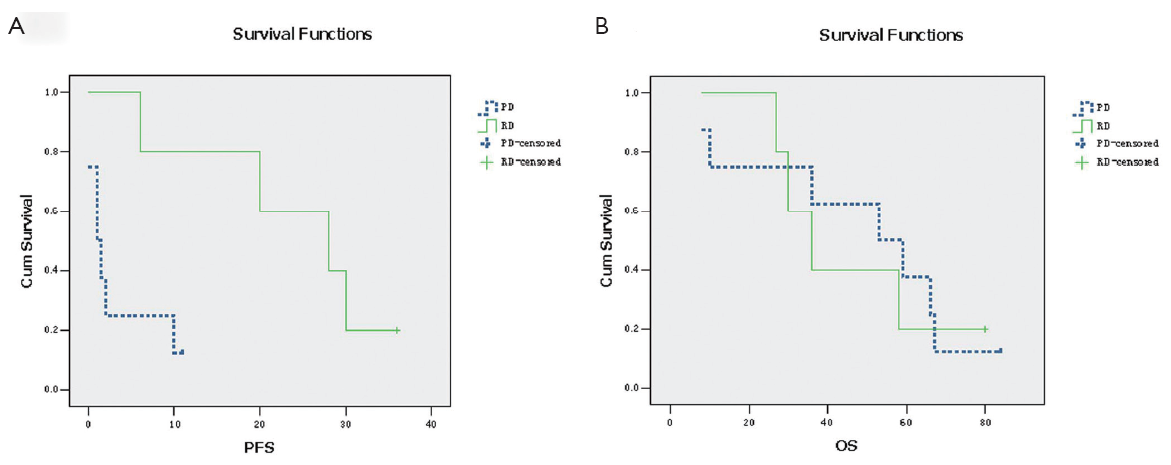
Except for one patient who died while in hospital, all other patients were alive at last followup. After a median follow-up of 53 months after surgery (range, 8-84 months) for the surviving patients, PFS for RD and PD patients was 24.8 and 2.81 months, respectively. The difference in PFS between patients with RD and those with PD was significant (P<0.001). All PD patients experienced disease progression within 11 months after surgery. To evaluate the role of surgery in the setting of recurrent or metastatic GISTs, we reanalyzed the data of 3 patients with recurrent or metastatic GISTs who had RD during imatinib treatment. The median PFS for these 3 patients was 20 months. The difference in PFS between patients with recurrent or metastatic GISTs exhibiting RD and patients with PD was still significant (Figure 1A, P<0.001). In the 21 patients who were still alive, 9 patients remain alive with unresectable GISTs, while the other 13 patients are disease-free. The OS for the 10 patients exhibiting RD on imatinib and for the 11 patients who experienced PD has not yet reached the median. For RD patients, the estimated OS was 100% at 2 years, especially one patient was alive for more than 7 years. For PD patients, OS was 87.5% at 2 years. The difference in OS between RD and PD patients was not significant (Figure 1B, P>0.05).
Discussion
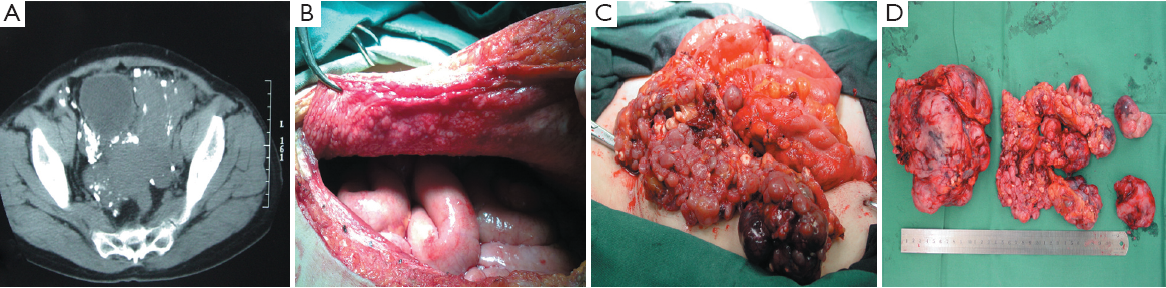
Imatinib has shown considerable clinical efficacy in treating advanced GISTs, and has been shown to prolong OS of patients with recurrent or metastatic disease. Preoperative imatinib therapy for primary or recurrent unresectable GISTs commonly results in tumor downstaging and enables curative surgical resection (11), or allows large tumors to be resected with less blood loss. SYM and his group found that earlier surgical intervention in patients with advanced, imatinib-responsive GIST may be optimal in preventing tumor progression (12). In our investigation, blood loss during surgery and hospital deaths for PD patients were much higher than for RD patients. 75% of PD patients lost more than 5,000 mL of blood, while 3 (25%) PD patients lost over 10,000 mL. One PD patient died of disseminated intravascular coagulation 2 days after surgery, due to much blood loss. Another PD patient went into shock during the operation because of blood loss exceeding 8,000 mL, only to recover after doctors’ effective treatment. In our study, 10 patients developed disease progression even after dose escalation of imatinib. Of these patients, the tumor in one patient grew so quickly that it occupied the entire pelvis (Figure 2A,B), which induced dysuresia and dyschesia. In another patient who show secondary imatinib resistance, after escalating the dose from 400 to 600 mg once daily, tumor necrosis occurred which led to high body temperature for several days before surgery. Since sunitinib at that time had not been approved in China, surgical intervention was the only treatment modality available, and both these patients underwent palliative surgery to reduce tumor size (Figure 2C,D). Over several months of follow up, we found that these palliative operations improved the two patients’ quality of life greatly. Our observation suggests that careful patient selection is required for the performance of surgery in patients with recurrent or multiple metastatic disease after imatinib therapy, because the probability of vascular friability in these patients is greatly elevated, leading to greater blood loss during surgery. Palliative nonresectional surgery should be considered only for specific symptoms just like difficult defecation due to tumor oppression or palliative care, either by focal debulking of symptomatic deposits or using bypass procedures.
In the present study, imatinib treatment was stopped one week before surgical procedures in accordance with the standard of care. In 6 of 10 RD patients, imatinib was reintroduced at the preoperative dose as soon as solid food was started. The median time of imatinib recommencement was 9 days (range, 2-20 days) after surgery. We found that postoperative complications were likely unrelated to imatinib treatment. Some series have shown that continuing imatinib treatment until 1 day before surgery does not increase postoperative complications and that imatinib can be safely restarted within 2 weeks after surgery (13). Four of 22 patients interrupted imatinib treatment for 2-12 months, mostly for financial reasons or drug adverse effects. Imatinib treatment was resumed after these patients developed disease progression, and all 4 patients were still sensitive to imatinib when treatment was resumed. These findings are in agreement with a Korean study, which found that in patients with advanced GISTs controlled with imatinib, imatinib interruption resulted in a high risk of PD within 1 year. However, the majority of disease was controlled with imatinib resumption after PD, and there were no statistically significant differences in PFS and OS between patients who stopped and restarted imatinib and patients who got the drug continuously (14).
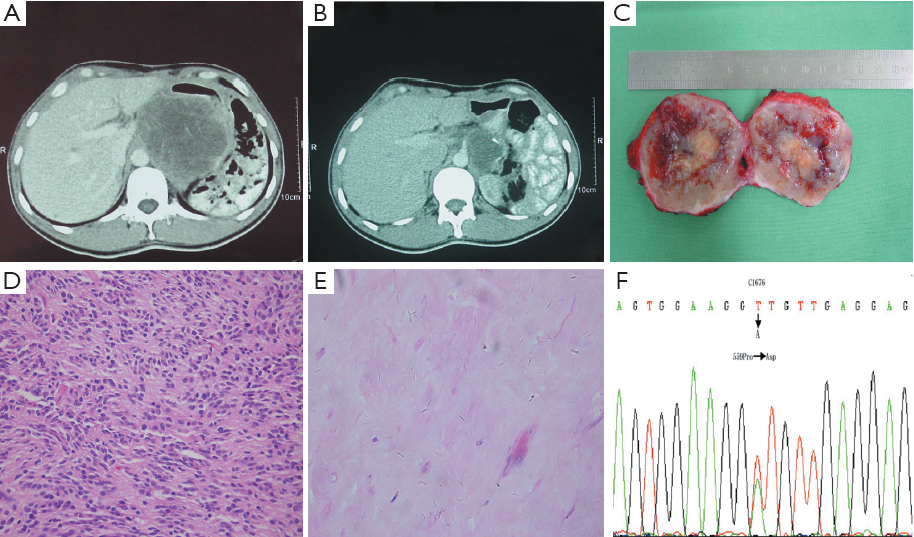
All patients received evaluation for response to imatinib by CT scan based on changes in tumor size, degree, and extent of enhancement. Although imatinib therapy has dramatically improved the prognoses of patients with metastatic GISTs (15), a complete pathologic remission is rarely achieved (16). In the present study, after 6 months of imatinib treatment after recurrence, one patient with a primary gastric GIST which was radically resected but recurred in the retroperioneum three years later achieved a complete pathologic response. Given the fact that the diameter of the recurrent nodule decreased from 10 to 6 cm, the patient was evaluated as a PR by CT scan before surgery. However, the excision sample showed no viable tumor cells and the case was finally confirmed as a CR by pathology examination (Figure 3), which suggests that a CT scan is not a definitive tool in the evaluation of GIST during imatinib therapy. This interesting case was published elsewhere. PET is a sensitive and specific method with which to evaluate tumor response on the basis of changes in tumor metabolism (17), which can demonstrate response before CT and may indicate response or progression when the CT findings are equivocal. However, functional PET scans also may become positive again within weeks of imatinib being stopped. The role for PET in assessing the likely response of patients included in neo-adjuvant trials needs to be further investigated (18).
In our cases, GISTs with mutations of the KIT exon 11 showed a better response compared to GISTs with other mutation such as KIT exon 9, PDGFRA mutation D842V, and wild type tumors; which suggests that mutations status correlates with the grade of initial imatinib response, and that a pre-treatment mutation analysis of the biopsy specimen may facilitate the treatment decision on preoperative imatinib treatment. Resistance to imatinib after initial response, mediated by expansion of therapy resistant and potentially pre-therapeutic clones with secondary mutations has also been recognized in metastatic GISTs (19). In our study, secondary mutation of KIT exon 17 Y823D occurred in 3 of 12 PD patients who showed an initial response to therapy, which indicates that additional mutations occurring in codon 823 of KIT exon 17 may provide a mechanism for secondary imatinib resistance.
In our series, surgical intervention for patients with metastatic and unresectable GISTs who were responsive to imatinib therapy resulted in prolonged PFS. But, for patients with advanced GISTs who had disease progression after achieving SD for some period, (classified as PD patients in our study) the effect of surgical interventions was limited. Although the difference in OS between patients exhibiting RD and those with PD was not significant, the findings were different those reported by SYM et al. (12), in which OS was significantly higher in patients with RD than in those with PD. Shorter follow-up time may account for our different results. Based on our findings, complete surgical resection was achieved in 8 of 10 patients with recurrent or metastatic GISTs exhibiting response to imatinib, and we believe that the quality of life for RD patients was better than for PD patients. Moreover, these patients showed prolonged progression free survival after surgery with mostly mild surgical morbidity. Our study, along with others (20,21), suggests that surgical intervention for GISTs responsive to imatinib may be an important measure of the management strategy. Surgery can be considered a relative indication only for advanced patients in whom a few nodules show resistance to imatinib while most other lesions have not developed resistance and continue to respond, or for specific symptoms, such as gastric outlet obstruction or tumor pressure on the bladder or rectum.
In summary, we suggest that surgical resection following imatinib treatment improves progression free survival for Chinese patients with recurrent or metastatic GISTs responsive to imatinib, but does not prolong overall survival as well as in patients who develop imatinib resistance because of short follow-up time. Surgical resection following imatinib is feasible and can be considered for patients with advanced GISTs responsive to imatinib. In the near future, our findings should be further investigated by more randomized clinical trials.
Acknowledgements
The corresponding author had the final responsibility for the decision to submit the paper for publication and wrote the manuscript in cooperation with all the other authors.
Disclosure: The work described in this article has no financial commitment or obligation with a company. No company played any role in writing or revising the manuscript.
References
- Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol 2000;15:1293-301.
- Zhao XH, Yue CJ. Gastrointestinal stromal tumor. J Gastrointest Oncol 2012;3:189-208.
- Edmonson JH, Marks RS, Buckner JC, et al. Contrast of response to dacarbazine, mitomycin, doxorubicin, and cisplatin (DMAP) plus GM-CSF between patients with advanced malignant gastrointestinal stromal tumors and patients with other advanced leiomyosarcomas. Cancer Invest 2002;20:605-12.
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34.
- Andtbacka RH, Ng CS, Scaife CL, et al. Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol 2007;14:14-24.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16.
- Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs 1992;10:239-53.
- Van Glabbeke M, Verweij J, Casali PG, et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol 2005;23:5795-804.
- Bonvalot S, Eldweny H, Péchoux CL, et al. Impact of surgery on advanced gastrointestinal stromal tumors (GIST) in the imatinib era. Ann Surg Oncol 2006;13:1596-603.
- Haller F, Detken S, Schulten HJ, et al. Surgical management after neoadjuvant imatinib therapy in gastrointestinal stromal tumours (GISTs) with respect to imatinib resistance caused by secondary KIT mutations. Ann Surg Oncol 2007;14:526-32.
- Kitamura Y, Hirota S, Nishida T. Gastrointestinal stromal tumors (GIST): a model for molecule-based diagnosis and treatment of solid tumors. Cancer Sci 2003;94:315-20.
- Sym SJ, Ryu MH, Lee JL, et al. Surgical intervention following imatinib treatment in patients with advanced gastrointestinal stromal tumors (GISTs). J Surg Oncol 2008;98:27-33.
- Barnes G, Bulusu VR, Hardwick RH, et al. A review of the surgical management of metastatic gastrointestinal stromal tumours (GISTs) on imatinib mesylate (Glivec). Int J Surg 2005;3:206-12.
- Lee JL, Ryu MH, Chang HM, et al. Clinical outcome in gastrointestinal stromal tumor patients who interrupted imatinib after achieving stable disease or better response. Jpn J Clin Oncol 2006;36:704-11.
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34.
- Heinrich MC, Corless CL. Gastric GI stromal tumors (GISTs): the role of surgery in the era of targeted therapy. J Surg Oncol 2005;90:195-207; discussion 207.
- Jager PL, Gietema JA, van der Graaf WT. Imatinib mesylate for the treatment of gastrointestinal stromal tumours: best monitored with FDG PET. Nucl Med Commun 2004;25:433-8.
- Neuhaus SJ, Clark MA, Hayes AJ, et al. Surgery for gastrointestinal stromal tumour in the post-imatinib era. ANZ J Surg 2005;75:165-72.
- Wardelmann E, Merkelbach-Bruse S, Pauls K, et al. Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin Cancer Res 2006;12:1743-9.
- Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 2006;24:2325-31.
- Rutkowski P, Nowecki Z, Nyckowski P, et al. Surgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylate. J Surg Oncol 2006;93:304-11.
