Current diagnostic approach to patients with adnexal masses: which tools are relevant in routine praxis?
Introduction
Malignant ovarian tumors are more often diagnosed at an advanced stage and are associated with the highest mortality of all gynecological cancers (1). Nature of adnexal tumors in preoperative evaluation still remains uncertain. However, an accurate diagnosis is essential to provide optimal therapeutic approach. Good preoperative discrimination between benignant and malignant ovarian tumors results in more women being appropriately referred for gynecologic oncology care and more women with benign conditions undergoing conservative surgical treatment (2).
The aim of the study was to investigate which anamnestic, laboratory and ultrasound parameters used in routine practice could predict the nature of adnexal mass, and enable a practitioner to refer a patient to a relevant clinical doctor, either gynecologist or gynecological oncologist.
Methods
Patient selection
The study included all consecutive patients that were treated for adnexal tumors at the Clinic of Gynecology and Obstetrics, Clinical Center of Serbia and Ultramedica Clinic, American Medical Academy Belgrade during the period of 2 years (from July 1, 2009 to June 30, 2011). The study was approved by the Ethic Committees of the respective institutions.
Preoperative diagnostic methods
On admission, after signing informed consent, detailed anamnesis and standard laboratory tests [blood analysis, erythrocyte sedimentation rate (ESR) and tumor marker levels] were taken from all patients. Body mass index (BMI) was calculated using the standard formula: body weight (kg)/[body height (m)]2.
Furthermore, expert clinical and ultrasonographic examinations of pelvic organs (multilocular or bilateral tumor, solid/cystic components/parts, thickness of the capsule and/or septas, metastases and ascites presence) were performed. Finally, risk of malignancy index (RMI) for all the patients was calculated, using the formula: RMI=U×M×CA125. In the formula, “U” represents the ultrasonographic index, “M” is menopausal status, while CA125 is calculated directly into the equation. The patients were divided into three groups according to the RMI values (low risk <25; intermediate risk 25-250; high risk >250).
Moreover, the tumor vascularity index [power Doppler index (PDI)] was determined for all patients by quantification of the number of pixels in a defined region of interest according to the formula: PDI = number of colored pixels/(total number of pixels-number of pixels in the fluid or avascular areas). It was estimated on selected frames of the tumors using an in-house color-quantifying program.
At the end, we calculated sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of PDI, RMI and obtained tumor markers [CA125, carcinoembryonic antigen (CEA), CA19-9 and CA15-3].
Postoperative analysis
Standard operative procedures appropriate for the staging of the tumor were undertaken and all tumors were extracted and sent to histopathological analysis.
Postoperatively, histopathological findings (HP) of tumors were analyzed. Histopathological diagnoses were related to all anamnestic, laboratory and ultrasound parameters, successively.
Moreover, we have divided all examined variables into three groups and evaluated their correlation with tumor type, for the whole group together. The first group regarded anamnestic data (personal and family illness history, employment status, menopausal status menarche time, number of births, miscarriages and abortions, patients’ age, BMI, and presence of symptoms), the second one included ultrasound parameters (tumor dimensions, multilocularity and bilateralism, presence of solid parts, metastases and ascites as well as RMI), and the third consisted of laboratory analyses (tumor marker levels and ESR).
Then, the relevance of these groups was assessed in order to examine which of the standard anamnestic and clinical data can help in differentiating benignant, borderline and malignant tumors.
Statistical analysis
Data were expressed as x̄±s. For statistical analysis, we used standard methods of descriptive and analytical statistics. Tests, such as Kolmogorov-Smirnov Z test, Friedman’s parametric analysis of variance (ANOVA), and χ2 test, were applies for investigation of differences among patients regarding the evaluated parameters collected by preoperative diagnostic methods. Discriminant analysis was performed in order to examine which of the preoperatively obtained parameters, gathered in three groups, could help in differentiation among tumor groups. All the data analyses were performed with SPSS 15.0 software (SPSS Inc., Chicago, IL, USA), and P<0.05 was considered statistically significant.
Results
There were 689 women involved in the study. Out of all cases, adnexal masses were malignant in 112, benignant in 544, and in 33 patients, diagnosis of borderline tumors was established.
Anamnestic data
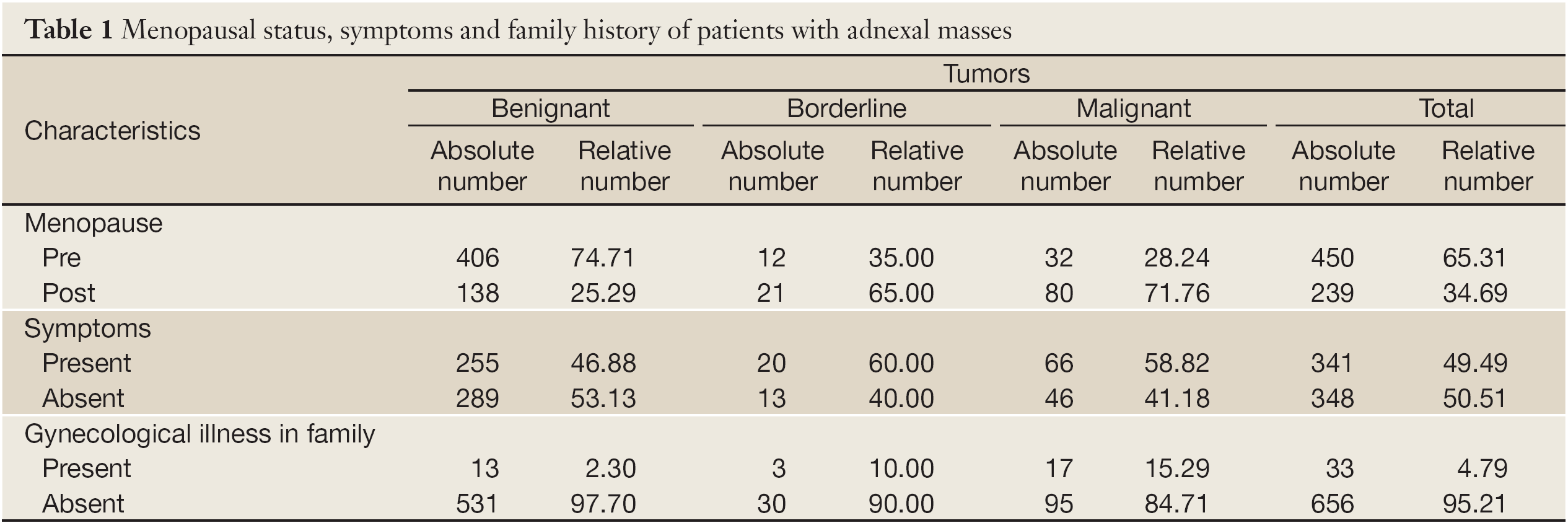
Significant differences were found when only gynecological illnesses among close family members were assessed. There were highly significantly more negative family findings in women with benignant tumors, while family history was mostly positive in women with malignant and borderline tumors (χ2=28.553; P=0.000) (Table 1).
Full table
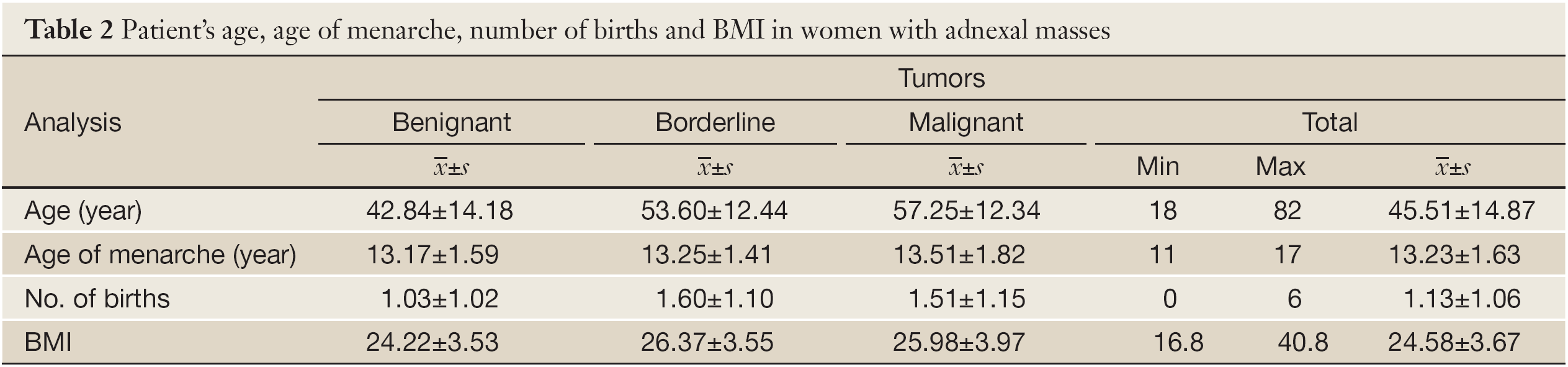
There were no significant differences among tumor types regarding the age in which women had their menarche (F=1.481; P=0.228) (Table 2).
Full table
There were high significant differences among tumor types regarding the number of births that women had (F=9.305; P=0.000). Women who had malignant and borderline tumors had significantly less births than women with benignant adnexal masses. There were no differences between women with malignant and borderline tumors (Table 2).
There were high significant differences among tumor types regarding the women’s BMI (F=11.023; P=0.000). Women who had benignant tumors had the lowest BMI. There were no significant differences between women with malignant and borderline adnexal masses. The majority of women with malignant and borderline tumors were overweight (BMI: 25-29.99), while most women with benignant tumors had normal weight (BMI: 18-24.99) (χ2=33.606; P=0.000). On the other hand, there were the least morbidly obese and underweight women with all three examined tumor type groups. Moreover, there were no cachectic women (Table 2). The cut-off at the level of overweight (BMI=25) has the sensitivity of 60% and specificity of 63%.
There were high significant differences among tumor types regarding the women’s age (F=41.999; P=0.000). The majority of women with benignant tumors were 30-39 years old, while most of the women with malignant and borderline tumors were 50-59 years old. Women with benignant adnexal masses were the youngest, while there were no significant differences between women with malignant and borderline tumors (Table 2).
There were highly significant differences among tumor types (benignant, malignant and borderline) regarding the menopausal status of examined women (χ2=77.219; P=0.000). Malignant tumors were more frequent in post-menopause (Table 1).
Women were either asymptomatic or suffered from pain, abdominal swelling, bleeding, urinary disturbances, etc. There were no significant differences among tumor types regarding the symptoms that examined women suffered from (χ2=5.006; P=0.082) (Table 1).
Ultrasound parameters
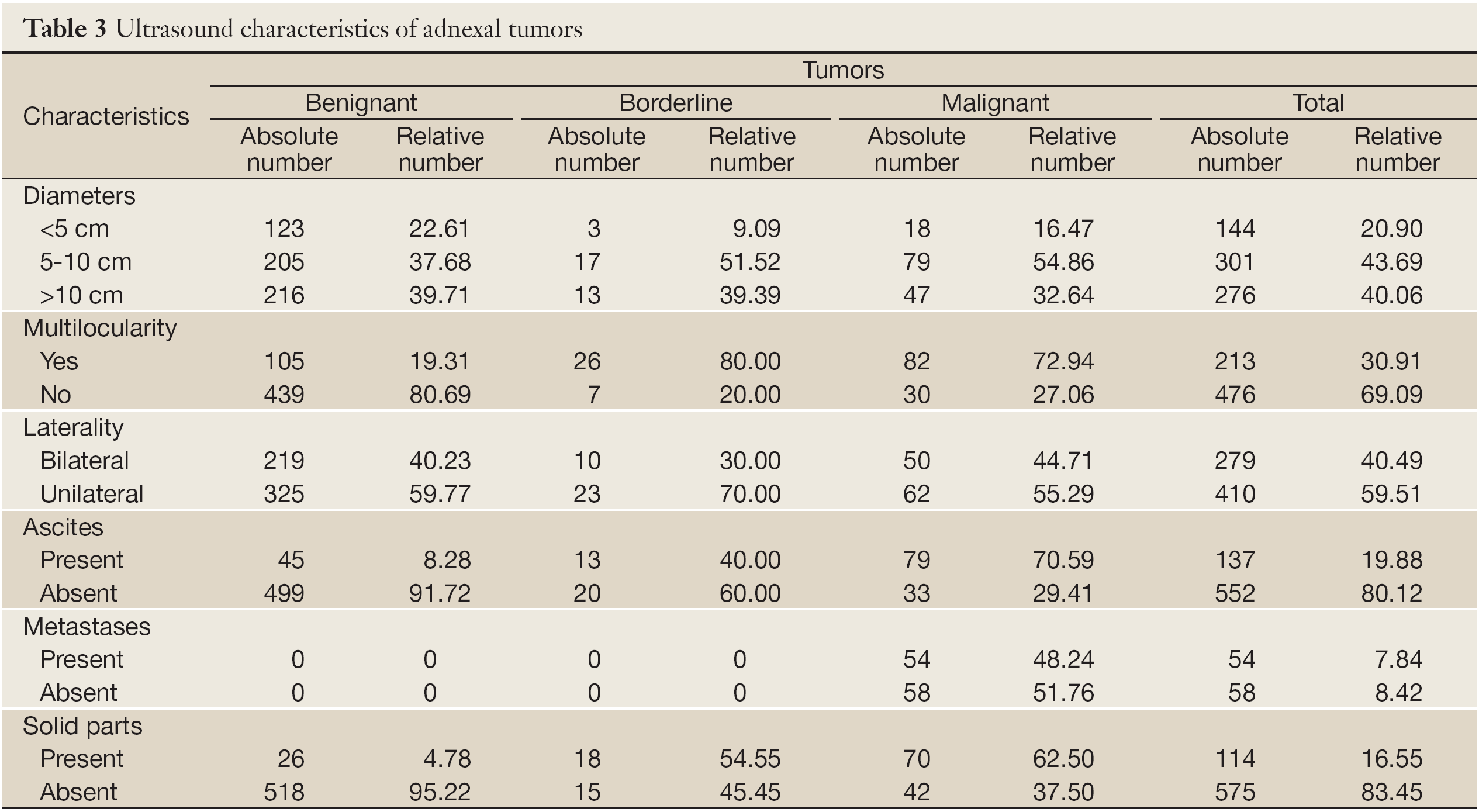
There were highly significant differences among tumor types regarding their diameters (χ2=17.597; P=0.001). Significantly more malignant tumors were 5-10 cm in diameter. Significantly less benignant and borderline tumors were <5 cm in diameter (Table 3).
Full table
There were no significant differences among tumor types regarding their multilocularity (χ2=1.524; P=0.467). Numerous benignant as well as malignant tumors were multilocular (Table 3).
There were no significant differences among tumor types regarding their bilaterality (χ2=1.551; P=0.461). Considerable number of women had pathological changes, benignant or malignant, on both ovaries (Table 3).
There were significantly more malignant and borderline tumors that had solid parts, while more benignant tumors did not have solid parts (χ2=254.283; P=0.000) (Table 3).
Out of 112 malignant tumors, 41 (36.61%) had given metastases. The most metastases women had were registered in pelvic lymph nodes, liver and intestines (81%) (Table 3).
There were significant differences among tumor types regarding the presence of ascites (χ2=183.296; P=0.000). Highly significantly more women with benignant and borderline tumors did not have ascites, while significantly more malignant had (Table 3).
There were significant differences in thickness of tumor capsule and/or septas (χ2=372.144; P=0.000). Benignant tumors mostly had the thickness of capsule and/or septas <5 mm. Although malignant tumors had thicker capsules and/or septas, there were no significant differences between malignant and borderline tumors (malignant: x̄±s=9.34; borderline: x̄±s=7.62).
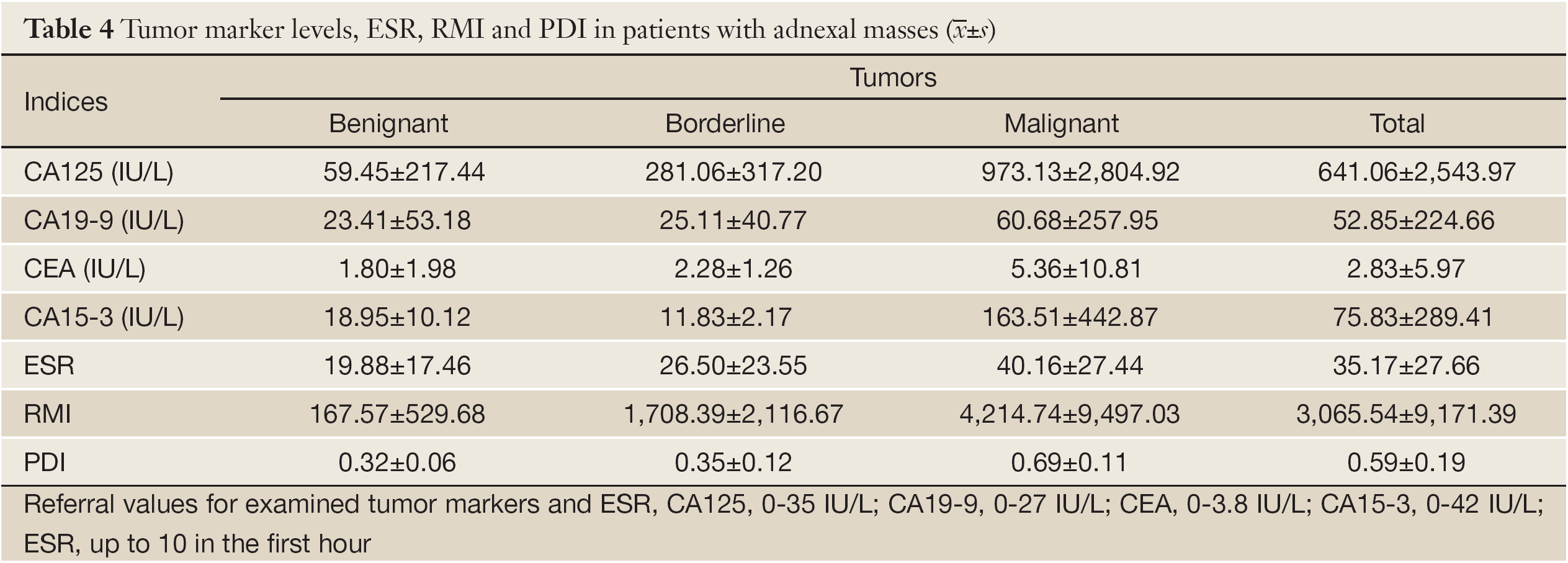
There were significant differences among tumor types and the PDI values (χ2=576.504; P=0.000). All benignant tumors had PDI <0.55 and significantly more malignant tumors had PDI >0.55. Borderline tumors had PDI in both categories (Table 4). Sensitivity of PDI was 65.31%, specificity 76.53%, PPV 24.24% and NPV 95.04%.
Full table
There were 223 cases of low RMI, 290 women had intermediate risk, while 166 women were in high risk regarding their RMI. There were highly significant differences among tumor types regarding the women’s RMI (F=40.692; P=0.000). Significantly higher values of RMI were in women with malignant than in other tumor types. There were no significant differences in RMI between women with benignant and borderline tumors (Table 4). Sensitivity of RMI was 72.38%, specificity 87.13%, PPV 57.58% and NPV 92.89%.
Laboratory analyses
There were highly significant differences among tumor types regarding the level of CA125 (F=23.363; P=0.000). Women with malignant tumors had the highest levels of CA125. There were no significant differences in CA125 level between women with benignant and borderline tumors (Table 4). Sensitivity of CA125 was 85.58%, specificity 56.09%, PPV 31.79% and NPV 94.21%.
There were highly significant differences among tumor types regarding the level of CEA (F=9.053; P=0.000). Women with malignant tumors had the highest levels of CEA. There were no significant differences in CEA level between women with benignant and borderline tumors (Table 4). Sensitivity of CEA was 18.03%, specificity 95.09%, PPV 57.89% and NPV 75.61%.
There were no significant differences among tumor types regarding the level of CA19-9 (F=1.831; P=0.162) (Table 4). Sensitivity of CA19-9 was 20%, specificity 65.17%, PPV 29.55% and NPV 52.73%.
There were no significant differences among tumor types regarding the level of CA15-3 (F=2.886; P=0.062) (Table 4). Sensitivity of CA15-3 was 53.13%, specificity 96.08%, PPV 89.47% and NPV 76.56%.
There were highly significant differences among tumor types regarding ESR (F=33.704; P=0.000). Significantly higher ESR was in women with malignant tumors than in other tumor types. There were no significant differences in ESR between women with benignant and borderline tumors (Table 4). Sensitivity of ESR was 83.87%, specificity 75.31%, PPV 28.89% and NPV 63.12%.
Factor assesses together in groups
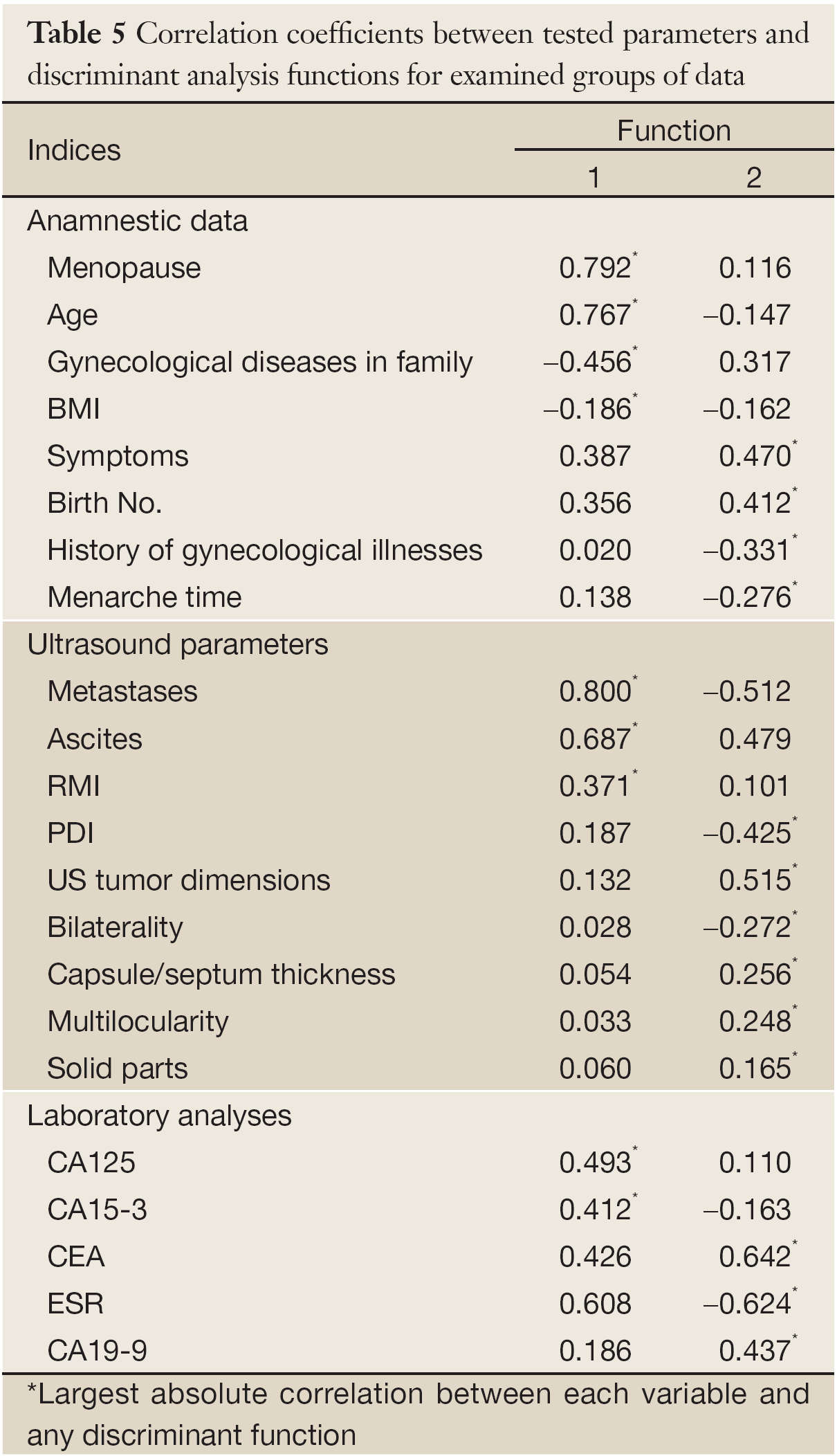
Anamnestic data of women evaluated together were found to be good discriminating factor among malignant, benignant and borderline tumors. We obtained one statistically significant function (eigenvalue =0,266; % of variance =97.8; canonical correlation =0.458; Wilks λ=0.785; χ2=128.973; P=0.000) (Table 5).
Full table
Ultrasound parameters all together were good discriminating factors among malignant, benignant and borderline tumors. We obtained two statistically significant functions (function 1: eigenvalue =1.101; % of variance =97.6; canonical correlation=0.724; Wilks λ=0.464; χ2=409.837; P=0,000; function 2: eigenvalue =0.027; % of variance =2.4; canonical correlation =0.162; Wilks λ=0.974; χ2=14.187; P=0.028) (Table 5).
Laboratory analyses (levels of tumor markers and ESR) were good discriminating factors among malignant, benignant and borderline tumors. We obtained one statistically significant function (eigenvalue =0.411; % of variance =99.3; canonical correlation =0.540; Wilks λ=0.706; χ2=22.985; P=0.011) (Table 5).
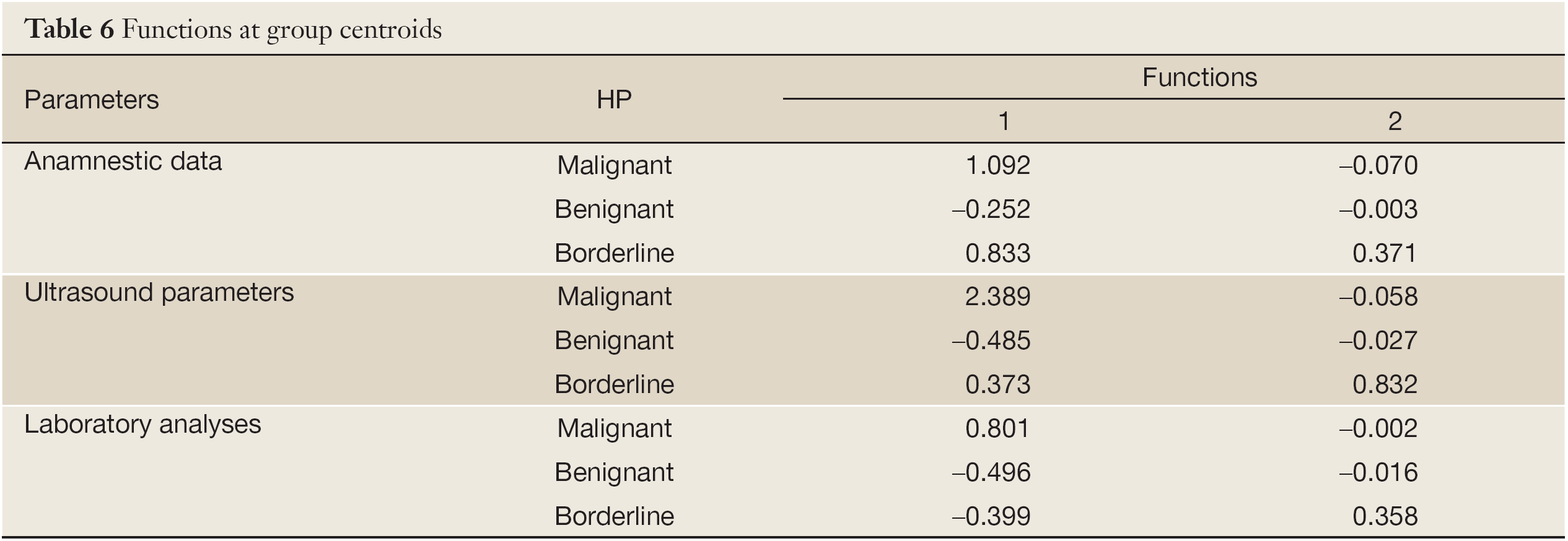
From the largest group centroids for significant functions, it can be concluded that patient’s age and BMI, menopausal status and family history of gynecological diseases can optimally distinguish malignant and somewhat less reliably borderline from other tumor types. All tested ultrasound parameters proved to be significant, but presence of metastases and ascites as well as RMI were of most importance. Presence of metastases and ascites as well as the high RMI point out malignant tumors and vaguely less reliably borderline nature of tumors; other parameters distinguish well borderline tumors and slightly less good malignant from benignant tumors. CA125 and CA15-3 discriminate malignant tumors from other tumor types (Table 6).
Full table
Discussion
Ovarian cancer carries the worst prognosis among all gynecological cancers, mainly due to the lack of effective screening methods for early stage detection of the disease (3). Accurate preoperative prediction of the benign or malignant character of a pelvic tumor is essential for optimal management (4).
Well known risk factors for malignancy are nulliparity, low parity, delayed childbearing, early onset of menses, late menopause, postmenopausal estrogen use for 10 or more years, and family history of ovarian or breast cancer (5). However, when malignant tumors are compared with benignant tumors, there is no significant difference in the onset interval after menopausal (6). The level of CA125 over 35 IU/L, presence of ascites, nodular or fixed pelvic mass, abdominal or distant metastasis and family history of breast or ovarian cancer in a first-degree relative are well known indications for referral to a gynecological oncologist (7). The risk is decreased among women who have used oral contraceptives, have been pregnant, or had a prolonged breast-feeding. Symptoms that are associated with ovarian cancer are typically nonspecific (8). The main clinical feature is abdominal symptoms, such as abdominal pain and distension in the malignant cases (6). Both height and obesity are positively associated with several types of cancer. High BMI can be a risk factor for ovarian cancer, especially in premenopausal women (9,10). The results of our study confirm the mentioned literature data.
Transvaginal ultrasound can discriminate between benign and malignant ovarian tumors better than all other radiological methods (11,12). Combined morphological and vascular imaging obtained by ultrasound appears to further improve the preoperative assessment of adnexal masses, based on the knowledge of the phenomena of neoangiogenesis and extravasations through leaky capillaries in ovarian cancer (5,13). The power Doppler vascularity index has high diagnostic value in discriminating between benign and malignant adnexal masses (14). In some studies, the sensitivity of power Doppler diagnostics for prediction of malignancy was up to 75% (15). But, although ultrasound is considered the gold standard for ovarian imaging, there are numerous false-positive and false-negative findings (16). In our study, ultrasound scan characteristics, especially PDI, are found to be very important for discriminating benign from malignant tumors.
Serum CA125 level is a valuable parameter for both diagnosis and monitoring of epithelial carcinoma. However, CA125 as a single parameter does not distinguish sufficiently benignant from malignant masses preoperatively, as it can be elevated in various benignant diseases and even in physiological conditions (16). A general conclusion is that some additional information can be obtained from combinations of serum tumor markers (4). CA19-9 is more frequently elevated than CA125 and hence a more useful marker in some cases such as mature cystic teratomas (17,18). Abnormal levels of CEA and CA19-9 were found more frequently in stages more advanced stages such as IB-IIIC (19). Researchers have found that in patients with an undiagnosed tumor in the pelvis, the CA125/CEA ratio may be used to preoperatively identify a substantial fraction of patients with non-ovarian malignancies (20). According to our findings, CA125 should be considered together with CEA in preoperative differentiation of adnexal tumors.
RMI, based on menopausal status, ultrasound findings and serum CA125, has already been validated and is widely used in selective referral of women from local units to specialized cancer centers (21,22). Compared with all other diagnostic methods, the most accurate numerical values were obtained with RMI (23). Sensitivity and specificity for ovarian cancer versus benign pelvic mass for RMI ≥200 were 92% and 82%, respectively. Therefore, RMI ≥200 is a reliable tool for identifying patients with ovarian cancer (24). RMI is an easily applicable method in the primary evaluation of patients with adnexal masses, resulting in timely referral to gynecological oncology centers for suitable surgical operations (25,26). According to our results, calculation of RMI in preoperative triage of patients with adnexal tumors is strongly recommended.
When all parameters were assessed together in formed groups, menopausal status, number of births, personal history of gynecological diseases metastases and ascites, RMI, CA125 and CA15-3 are proven to be the most important factors for discriminating malignant tumors from other tumor types. For distinguishing borderline from other tumor types, ultrasound parameters can be of most help.
In conclusion, malignant and borderline tumors are more frequent in postmenopausal women, while benignant tumors are more common in premenopausal women. Women with malignant and borderline tumors have fewer births than women with benignant adnexal masses. Women with benignant tumors have the lowest BMI, while the majority of women with malignant and borderline tumors are overweight. Significantly more malignant tumors are 5-10 cm in diameter. Highly significantly more women with benignant and borderline tumors did not have ascites, while significantly more malignant did. Malignant tumors have significantly higher values of PDI. Significantly higher are values of RMI in women with malignant than in other tumor types (benignant and borderline), while there are no significant differences in RMI between women with benignant and borderline tumors. Women with malignant tumors have the highest levels of CA125, CEA and ESR.
According to our results, patients’ age-menopausal status, BMI, CA125, CA15-3 and CEA levels, ESR, ultrasound scan characteristics, PDI and RMI can predict the nature of adnexal tumors.
Acknowledgements
This work was supported by Grant No 41021 from the Ministry of Science and Technological Development of the Republic of Serbia.
Disclosure: The authors declare no conflicts of interest.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43-66.
- Ameye L, Valentin L, Testa AC, et al. A scoring system to differentiate malignant from benign masses in specific ultrasound-based subgroups of adnexal tumors. Ultrasound Obstet Gynecol 2009;33:92-101.
- Fehm T, Neubauer H, Bräutigam K, et al. Diagnostics and therapy of ovarian cancer. Innovative techniques. Der Gynäkologe 2010;43:586-94.
- Schutter EM, Kenemans P, Sohn C, et al. Diagnostic value of pelvic examination, ultrasound, and serum CA 125 in postmenopausal women with a pelvic mass. An international multicenter study. Cancer 1994;74:1398-406.
- Togashi K. Ovarian cancer: the clinical role of US, CT, and MRI. Eur Radiol 2003;13:L87-104.
- Wu ZM, Di W. Clinical diagnosis and treatment of small ovarian tumor in postmenopausal women. Chin J Cancer Res 2006;18:229-34.
- Chia YN, Marsden DE, Robertson G, et al. Triage of ovarian masses. Aust N Z J Obstet Gynaecol 2008;48:322-8.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009;361:170-7.
- Schouten LJ, Rivera C, Hunter DJ, et al. Height, body mass index, and ovarian cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev 2008;17:902-12.
- Dotlić J, Terzić M, Likić I, et al. Evaluation of adnexal masses: correlation between clinical, ultrasound and histopathological findings. Vojnosanit Pregl 2011;68:861-6.
- Sehouli J, Henrich W, Braicu I, et al. Preoperative diagnostics in ovarian cancer. What do we really need? Der Gynäkologe 2006;39:428-37.
- van Trappen PO, Rufford BD, Mills TD, et al. Differential diagnosis of adnexal masses: risk of malignancy index, ultrasonography, magnetic resonance imaging, and radioimmunoscintigraphy. Int J Gynecol Cancer 2007;17:61-7.
- Ohel I, Sheiner E, Aricha-Tamir B, et al. Three-dimensional power Doppler ultrasound in ovarian cancer and its correlation with histology. Arch Gynecol Obstet 2010;281:919-25.
- Marret H, Sauget S, Giraudeau B, et al. Power Doppler vascularity index for predicting malignancy of adnexal masses. Ultrasound Obstet Gynecol 2005;25:508-13.
- Mansour GM, El-Lamie IK, El-Sayed HM, et al. Adnexal mass vascularity assessed by 3-dimensional power Doppler: does it add to the risk of malignancy index in prediction of ovarian malignancy?: four hundred-case study. Int J Gynecol Cancer 2009;19:867-72.
- Terzić M, Dotlić J, Ladjević IL, et al. Evaluation of the risk malignancy index diagnostic value in patients with adnexal masses. Vojnosanit Pregl 2011;68:589-93.
- Emin U, Tayfun G, Cantekin I, et al. Tumor markers in mature cystic teratomas of the ovary. Arch Gynecol Obstet 2009;279:145-7.
- Ugur MG, Ozturk E, Balat O, et al. Do high levels of CA 19-9 in women with mature cystic teratomas of the ovary warrant further evaluation? Eur J Gynaecol Oncol 2012;33:207-10.
- Nomelini RS, da Silva TM, Tavares Murta BM, et al. Parameters of blood count and tumor markers in patients with borderline ovarian tumors: a retrospective analysis and relation to staging. ISRN Oncol 2012;2012:947831.
- Sørensen SS, Mosgaard BJ. Combination of cancer antigen 125 and carcinoembryonic antigen can improve ovarian cancer diagnosis. Dan Med Bull 2011;58:A4331.
- Pfisterer J, Schmalfeldt B, du Bois A. Ovarian cancer. A challenge for physician and patient. Der Gynäkologe 2006;39:239-50.
- Miller RW, Ueland FR. Risk of malignancy in sonographically confirmed ovarian tumors. Clin Obstet Gynecol 2012;55:52-64.
- Anton C, Carvalho FM, Oliveira EI, et al. A comparison of CA125, HE4, risk ovarian malignancy algorithm (ROMA), and risk malignancy index (RMI) for the classification of ovarian masses. Clinics (Sao Paulo) 2012;67:437-41.
- Håkansson F, Høgdall EV, Nedergaard L, et al. Risk of malignancy index used as a diagnostic tool in a tertiary centre for patients with a pelvic mass. Acta Obstet Gynecol Scand 2012;91:496-502.
- Ashrafgangooei T, Rezaeezadeh M. Risk of malignancy index in preoperative evaluation of pelvic masses. Asian Pac J Cancer Prev 2011;12:1727-30.
- Terzic M, Dotlic J, Berisavac M, et al. Ultrasound findings in postmenopausal women with adnexal masses. Ultrasound Obstet Gynecol 2010;36:251-2.
