Prognostic value of interim 18F-FDG PET/CT in diffuse large B-cell lymphoma
Introduction
Diffuse large B-cell lymphoma (DLBCL), the most common subtype of non-Hodgkin’s lymphoma, is clinically heterogeneous. Approximately 30-40% of patients will relapse after first-line treatment (1-3). Currently, the prognosis of patients with DLBCL is estimated by using the clinical parameters of the International Prognostic Index (IPI) (4). And patients with different lymphoma cell origins have different prognosis (5). The 5-year overall survival (OS) for the germina center B cell origin (GCB) group was 76% compared with only 34% for the non-GCB group (P<0.001). However, the treatment outcomes of individual patients within the same IPI risk group or the same cell origin can be considerably different. So we need to find a new factor to value the prognosis of patients with DLBCL.
Fludeoxyglucose F18 positron emission tomography and computed tomography (18F-FDG PET/CT), providing information about the metabolic activity in patients with lymphoma, is helpful for differentiating viable tumor from post-treatment fibrosis or necrosis. Evidences showed that the post-treatment 18F-FDG PET/CT can predict the outcome of patients with DLBCL (6-9). However, the role of interim PET/CT, after a few cycles of treatment, for predicting patients’ outcome is still controversial. In addition, there is still no universal conclusion on SUVmax cut-off value for interpreting interim PET/CT as positive or negative.
In this retrospective study, we try to find out the SUVmax cut-off value for interpreting interim PET/CT as positive or negative, and to discuss the prognostic value of interim PET/CT in DLBCL.
Materials and methods
Patients
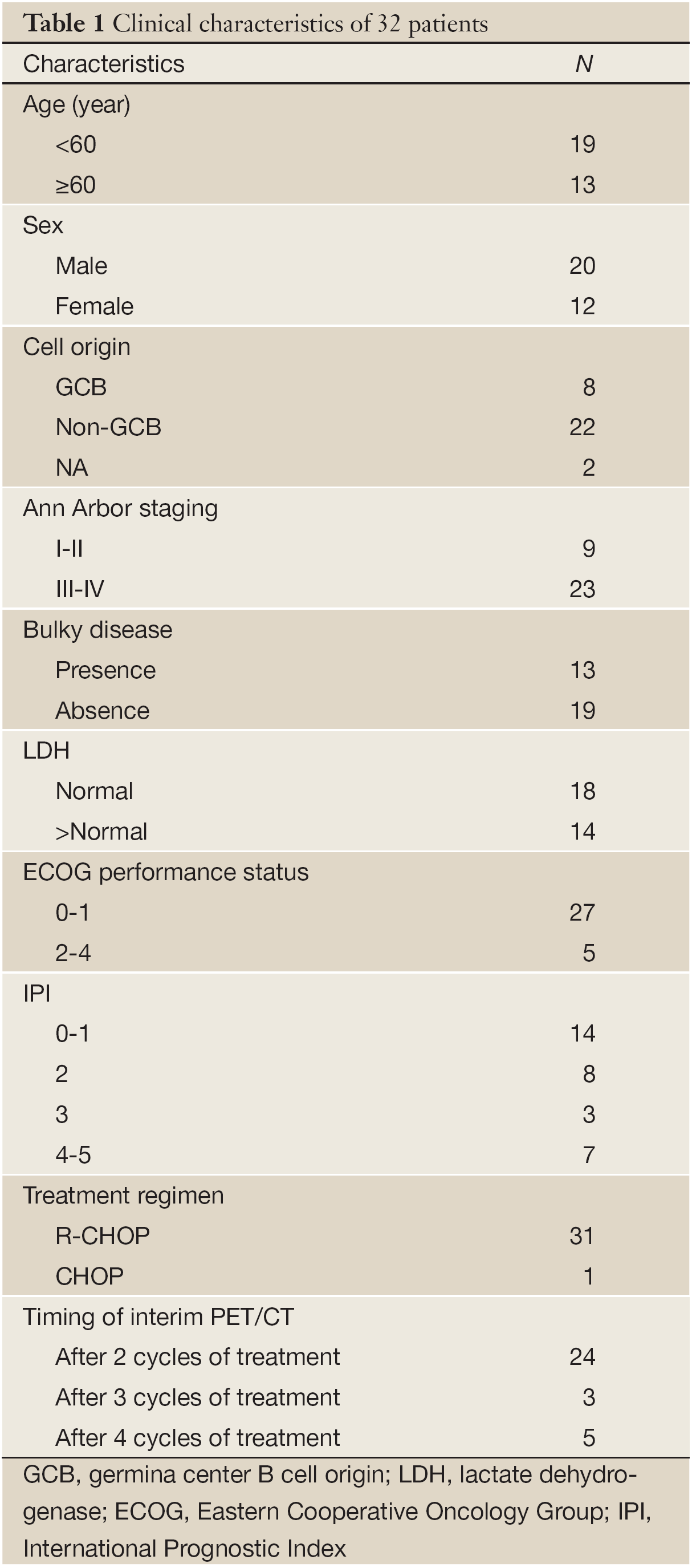
Between September 2009 and November 2011, a total of 32 patients with newly diagnosed DLBCL were enrolled at Peking University Cancer Hospital. All patients were diagnosed according to 2008 WHO classification for Tumors of Hematopoietic and Lymphoid Tissue. All patients underwent baseline, interim (after 2-4 cycles of treatment), and post-treatment PET/CT scans. The treatment efficacy and survival time were retrospectively analyzed.
All patients were treated with R-CHOP regimen [rituximab 375 mg/m2 i.v. on day one (D1), cyclophosphamide 750 mg/m2 i.v. on D1, vincristine 1.4 mg/m2 i.v. on D1, doxorubicin 50 mg/m2 i.v. on D1, and prednisone 100 mg p.o. on D1-5] or CHOP regimen. According to National Comprehensive Cancer Network (NCCN) guideline (10), 1 patient received local residual disease radiation after first-line treatment. Five high-risk patients received consolidative autologous stem cell transplantation with BEAM (Carmustine, etoposide, cytarabine, melphalan) as preparative regimen.
F-FDG PET/CT scans
18F-FDG PET scan (Gemini TF 16 PET/CT, Philips, Netherland) was performed according to standard procedures. PET acquisition was performed in 6-hour fasting patients after intravenous injection of 0.1 mCi/kg of 18F-FDG. We utilized a low-dose spiral mode CT scan (100 mAs, 120 keV; slice thickness 3 mm), covering the area from the base of the skull to the proximal thighs, which was used for attenuation correction and image fusion. PET data were reconstructed iteratively with attenuation correction based on CT data and reoriented in axial, sagittal, and coronal slices.
PET/CT images were interpreted on the basis of visual analysis by 2 experienced nuclear medicine physicians. The examination was considered negative when no pathologic FDG uptake was shown. Focal FDG uptake beyond the physiological uptake areas was interpreted as positive. Lymph nodes with a short axis diameter of <1.0 cm was defined as abnormal.
Statistical analysis
OS was defined as the time from the date of diagnosis until death as a result of any cause. Progression-free survival (PFS) was measured as the time from diagnosis until lymphoma progression, relapse after response, or death as a result of any cause (9). The Kaplan-Meier method with the log-rank test was performed to estimate the PFS rate and to compare survival differences between groups. P<0.05 was considered statistically significant, and all P values were two-sided. All statistical analyses were performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics of patients
Baseline characteristics of 32 patients are listed in Table 1. Twenty-three patients (71.9%) presented with advanced stage disease and 27 (84.4%) had good performance status. Cases were subclassified using CD10, bcl-6, and MUM1 expression (11), 8 cases (25%) were considered GCB, and 22 cases (68.8%) non-GCB. Two cases cannot determine cell origin because of limited sample or technology reason. Thirty-one patients received R-CHOP regimen, and 1 patient CHOP regimen. Most of the patients (75%) underwent PET/CT scans after 2 cycles of treatment.
Full table
Correlation of interim PET/CT results with PFS
After a median follow-up period of 16.7 months (9.1-35.5 months), 4 patients showed progressive disease.
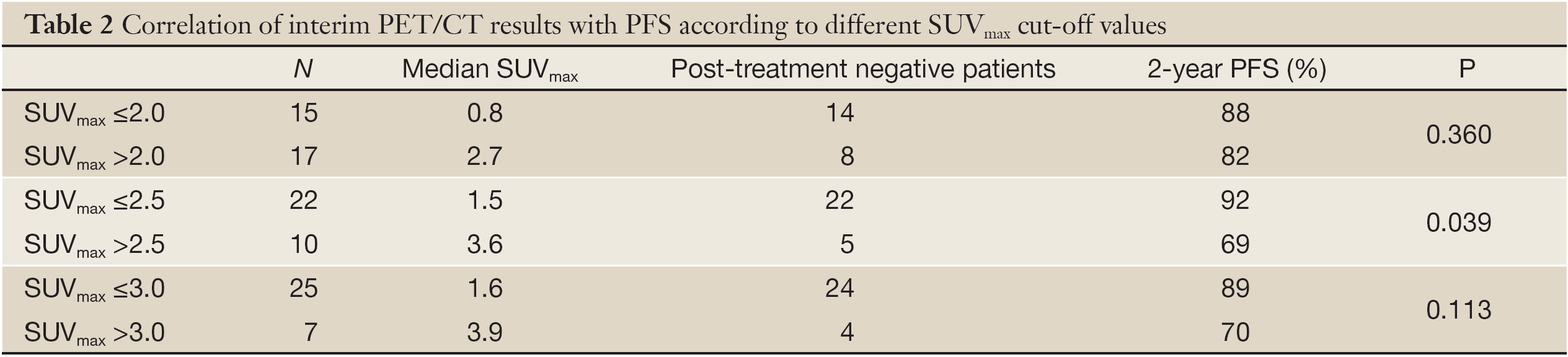
We set 2.0, 2.5 and 3.0 as the SUVmax cut-off value of interim PET/CT, respectively. PET/CT was defined as negative or positive according to the cut-off value.
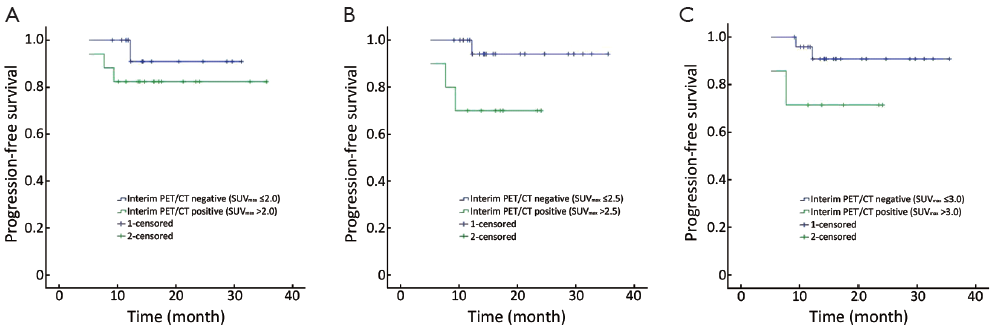
If SUVmax 2.0 was applied as the cut-off value, the median SUVmax of interim PET/CT negative patients was 0.8 (0-1.8, n=15), and 14 patients maintained negative after completion of first-line treatment. The median SUVmax of interim PET/CT positive patients was 2.7 (2.2-15.3, n=17), 8 patients (41.7%) turned to negative at the end of the treatment, and these 8 patients are still in remission now. There was no difference in 2-year PFS rates between interim PET/CT negative and positive patients (88% vs. 82%, P=0.360) (Table 2, Figure 1A).
Full table
If SUVmax 2.5 was applied as the cut-off value, the median SUVmax of interim PET/CT negative patients was 1.5 (0-2.4, n=22), and all of these patients maintained negative after completion of first-line treatment. The median SUVmax of interim PET/CT positive patients was 3.6 (2.6-15.3, n=10), 5 patients (50%) turned to negative at the end of the treatment, and these 5 patients are still in remission now. The 2-year PFS rates differed between the interim PET/CT negative and positive patients (92% vs. 69%, P=0.039) (Table 2, Figure 1B).
If SUVmax 3.0 was applied as the cut-off value, the median SUVmax of interim PET/CT negative patients was 1.6 (0-2.7, n=25), and 24 patients maintained negative after completion of first-line treatment. The median SUVmax of interim PET/CT positive patients was 3.9 (3.3-15.3, n=7), 4 patients (57.1%) turned to negative at the end of the treatment, and these 4 patients are still in remission now. There was no difference in 2-year PFS rates between interim PET/CT negative and positive patients (89% vs. 70%, P=0.113) (Table 2, Figure 1C).
Correlation of SUV reduction with PFS
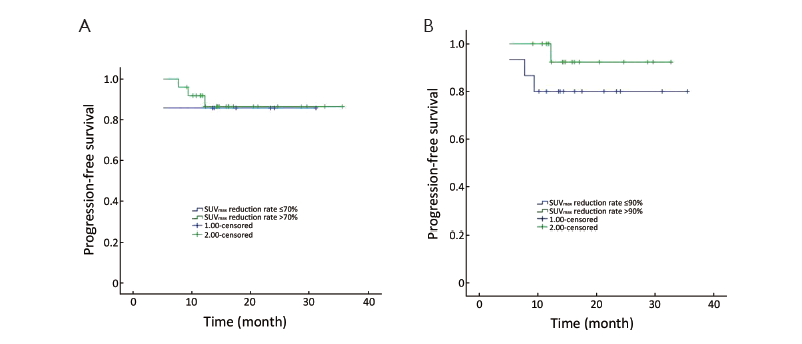
This study calculated the SUVmax reduction rate between baseline and interim PET/CT, and we defined a reduction rate of 70% and 90% as cut-off value, respectively. A cut-off of 70% SUVmax reduction yielded a 2-year PFS rate of 86% in patients with reduction of more than 70% vs. 84% with reduction of 70% or less (P=0.885) (Figure 2A). The 2-year PFS rate was 89% in patients with reduction of more than 90%, and 79% in those with reduction of 90% or less (P=0.229) (Figure 2B).
Correlation of PET/CT results with OS
There was only 1 patient died until the last follow-up time (August, 2012). We cannot analyze the correlation of interim PET/CT with OS.
Discussion
This study aims to determine the prognostic value of interim 18F-FDG PET/CT in DLBCL. Actually, there are many controversies until now.
Multiple studies have proved that interim PET/CT can predict the outcomes of patients with DLBCL (12,13). Yang DH et al. evaluated the prognostic significance of interim PET/CT in the treatment of DLBCL. SUVmax 3.0 was defined as the cut-off value of interim PET/CT. Patients who continued to have positive interim PET/CT showed a significantly high relapse rate (62.8%) compared to those with a negative PET/CT (12.1%) (P<0.01). After a median follow-up period of 30.8 months, the positivity of interim PET/CT was found to be a prognostic factor for both OS and PFS. This study concluded interim PET/CT scanning had a significant predictive value for disease progression and survival of DLBCL (12). However, other studies have drawn different conclusions (14,15). The study performed by Yoo C et al. demonstrated that there was no difference in PFS (P=0.07) and OS (P=0.24) between interim PET/CT positive and negative groups (14).
In the present study, we also evaluated the correlation of interim PET/CT with the outcomes of patients with DLBCL. PET/CT results were affected by different machines, observers, and interpretation criteria. A more direct index in PET/CT is urgently needed to guide clinical treatment. A study has applied SUVmax cut-off value to define PET/CT as negative or positive (12), but there is still controversy. In our study, we set SUVmax 2.0, 2.5 and 3.0 as cut-off value of interim PET/CT, respectively. If SUVmax 2.0 and 3.0 were applied as cut-off value, there was no difference in 2-year PFS rates between interim PET/CT negative and positive patients (P=0.360; P=0.113). However, if we applied SUVmax 2.5 as the cut-off value, the 2-year PFS rates differed between the negative and positive patients (P=0.039). Unfortunately, we cannot analyze patients’ OS because of the small sample size and short follow-up time.
Recently, the role of SUVmax reduction becomes more important in predicting outcomes of patients with DLBCL (16,17). Casasnovas RO et al. assessed whether SUVmax can predict the survival of DLBCL. Eighty-five high-risk patients underwent PET/CT scans at baseline (PET0), after 2 (PET2) and 4 cycles (PET4) chemotherapy, respectively. ΔSUVmax was calculated between PET2 and PET0 (ΔSUVmaxPET0-2) or PET4 (ΔSUVmaxPET0-4), ΔSUVmaxPET0-2 (>66% vs. ≤66%) analysis identified patients with significantly different 2-year PFS and OS rates (P=0.0282; P<0.0001). ΔSUVmaxPET0-4 (>70% vs. ≤70%) seemed even more predictive for 2-year PFS and OS rates (P<0.0001; P<0.0001) (16). In this study, we also calculated SUVmax reduction of interim PET/CT scan compared to the baseline result, and applied 70% and 90% as the cut-off value of SUVmax reduction. But we did not find the correlation of SUVmax reduction and the outcomes of DLBCL, and this may be attributed to the small sample size and short follow-up time.
The interim PET/CT cannot predict outcomes of DLBCL accurately, and the reasons are as following:
Difficulty in determining the exact timing of interim PET/CT scan
If patients underwent interim PET/CT too early (after 1-2 cycles of treatment), 2/3 interim positive patients will be turned to negative after the following treatment (15), and it has reached agreement that post-treatment PET/CT negative patients have a good prognosis (6-8). The outcomes of this group of interim PET/CT positive patients are similar to that of interim negative patients (14,15). In this study, about half of the interim PET/CT positive patients turned to negative after the following treatment, and these patients did not relapse till now, indicating the treatment after interim PET/CT can affect the prognosis of patients. If patients underwent interim PET/CT too late (after 4 cycles for patients planning for receiving 6 cycles of treatment, after 6 cycles for the patients planning for receiving 8 cycles of treatment), the interim PET/CT loses its significance for predicting prognosis early.
High false-positive rate
FDG as a marker does not have such a high specificity because it is also taken up in infections and inflammatory processes (18-20). Rituximab is a kind of immunotherapy drug, and antibody-mediated cellular cytotoxicity and complement activity are important mechanisms in rituximab’s activity. Both processes are able to attract mediators of inflammation to tumor sites (21,22). Although a study recommended to exclude false-positive cases by biopsy (21), it is hard in a clinical setting to perform biopsies on every lesion showing residual FDG uptake, and these may cause unwanted procedure-related complications or interruptions of treatment.
Absence of uniform interpretation criteria
Currently, there are many PET interpretation criteria, but most of these criteria are defined for post-treatment assessment. Recently, Horning et al. reported only a moderate reproducibility among the observer in interim PET interpretation (23). To minimize the affection of immunochemotherapy on the PET result, PET scans should not be performed for at least 3 weeks, and preferably 6-8 weeks, after completion of therapy (9). This is easy for the post-treatment PET scan. However, it is difficult for the interim PET scan, because the time interval of the regimen for lymphoma is 2-3 weeks. To ensure the following treatment on time, the interim PET is always within 3 weeks. So it is inevitable of the affection of immunochemotherapy on the interim PET result.
In conclusion, using a SUVmax cut-off value of 2.5, interim PET/CT can predict the 2-year PFS rate of patients with DLBCL. But this is a small retrospective study. We should treat this result cautiously, and cannot adjust the following treatment according to the interim PET/CT. In the future, larger prospective trials are needed to assess the real prognostic value of interim PET/CT in patients with DLBCL.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011;12:1013-22.
- Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008;9:105-16.
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010;116:2040-5.
- Sehn LH, Berry B, Chhanabhai M, et al. The revised international prognostic index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007;109:1857-61.
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503-11.
- Thomas A, Gingrich RD, Smith BJ, et al. 18-Fluoro-deoxyglucose positron emission tomography report interpretation as predictor of outcome in diffuse large B-cell lymphoma including analysis of ‘indeterminate’ reports. Leuk Lymphoma 2010;51:439-46.
- Vitolo U, Chiappella A, Bellò M, et al. The outcome of patients with diffuse large B-Cell lymphoma (DLBCL) treated with Rituximab-CHOP (R-CHOP) is not predicted by 18-FDG-Positron Emission Tomography/Computerized Tomography (PET) performed at intermediate in-course evaluation, but only by PET assessed at the end of therapy. Blood (ASH Annual Meeting Abstracts) 2010;116:2819.
- Cashen AF, Dehdashti F, Luo J, et al. 18F-FDG PET/CT for early response assessment in diffuse large B-cell lymphoma: poor predictive value of international harmonization project interpretation. J Nucl Med 2011;52:386-92.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579-86.
- National Comprehensive Cancer Network. [EB/OL] Version 2. [2012-02-23] Available online: http://www.nccn.org/index.asp
- Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275-82.
- Yang DH, Min JJ, Song HC, et al. Prognostic significance of interim 18F-FDG PET/CT after three or four cycles of R-CHOP chemotherapy in the treatment of diffuse large B-cell lymphoma. Eur J Cancer 2011;47:1312-8.
- Delarue R, Meignan M, Fournier M, et al. Predictive value of interim PET in elderly patients with diffuse large B cell lymphoma (DLBCL): A sub-group analysis of the LNH03-6B GELA Study. Ann Oncol 2011;22:159.
- Yoo C, Lee DH, Kim JE, et al. Limited role of interim PET/CT in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol 2011;90:797-802.
- Gigli F, Gardellini A, Bertazzoni P, et al. Positive interim 18F[FDG] positron emission tomography seems not to predict relapse in patients with diffuse large B-Cell lymphoma. Ann Oncol 2011;22:158.
- Casasnovas RO, Meignan M, Berriolo-Riedinger A, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood 2011;118:37-43.
- Itti E, Lin C, Dupuis J, et al. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-cell lymphoma: SUV-based assessment at 4 Cycles of chemotherapy. J Nucl Med 2009;50:527-33.
- Inoue Y, Tamaki H, Yamagami T, et al. False positive FDG-PET findings due to bone metastasis from prostate cancer in staging of non-Hodgkin’s lymphoma. Eur J Haematol 2007;79:88-90.
- Ford CD, Gabor F, Morgan R, et al. False positive restaging PET scans involving the spleen in two patients with aggressive non-Hodgkin lymphoma. Clin Nucl Med 2006;31:391-3.
- Focosi D, Caracciolo F, Galimberti S, et al. False positive PET scanning caused by inactivated influenza virus vaccination during complete remission from anaplastic T-cell lymphoma. Ann Hematol 2008;87:343-4.
- Moskowitz CH, Schöder H, Teruya-Feldtein J, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol 2010;28:1896-903.
- Han HS, Escalón MP, Hsiao B, et al. High incidence of false-positive PET scans in patients with aggressive non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Ann Oncol 2009;20:309-18.
- Horning SJ, Juweid ME, Schöder H, et al. Interim positron emission tomography scans in diffuse large B-cell lymphoma: an independent expert nuclear medicine evaluation of the Eastern Cooperative Oncology Group E3404 study. Blood 2010;115:775-7.
