Intraoperative ultrasound assistance in resection of intracranial meningiomas
Introduction
Intracranial meningiomas are relatively common tumors derived from arachnoidal cells and most frequently occur in association with intracranial meninges. They account about 20% of primary brain tumors (1,2). Most intracranial meningiomas are benign tumors (WHO grade I) with well prognosis after standard treatment (3). Usually, the optimal therapy of intracranial meningioma is surgical removal of the lesion using microsurgical techniques. The precise resection of the tumor is essential. However, there are some problems the neurosurgeon may encounter during the operation. For instance, it’ll be very difficult to remove the tumors completely if they are closely related to the vessels, especially those wrap up the arteries, which will result in tumor residual (4).
Nowadays, many advanced image techniques are applied into intracranial meningioma removal, which greatly improve the tumor resection rate and minimize post-operative neurological deficits (5). Pre-operative CT and MRI scan could reveal the location and volume of the tumor. The magnetic resonance angiography (MRA) could exhibit the vessels surrounding the tumor. The relationship of tumor and its adjacent vessels could be further evaluated by three-dimensional (3D) graphics provided by Virtual Reality. Moreover, the neurosurgeon could even obtain more information about the tumor using intra-operative neuro-navigation system.
Intraoperative ultrasound is also available in modern neurosurgery, which has been applied for brain tumor resection for many years. Though its poor image quality, intraoperative ultrasound is still a less invasive and more convenient approach, especially its ability of displaying blood flow. Researches proved that intraoperative ultrasound could assist in determine the brain tumor vessels in an intraoperative setting. It has been applied in intracranial lesion resection (6-8), such as glioma, meningioma, and intradural spinal tumors, which make the operation more precise.
In the paper, we report our experience with intraoperative ultrasonography as assistance for locating and determining the tumor and its supplying arteries during intracranial meningioma resection, correlate the sonographic images to preoperative CT and MRI images, and analyze the advantages of this technique. It’s proved that intraoperative ultrasonography is a useful technique in intracranial meningioma resection.
Patients and methods
Patient information
20 patients with anterior or middle skull base meningioma were operated under the guidance of intraoperative ultrasound at the Neurosurgery Department of Shanghai Huashan Hospital from December 2011 to January 2013. There were 7 male and 13 female patients, aged from 31 to 66 years old. 10 patients suffered from headache and 6 patients suffered from dizziness; 4 patients presented with visual disorders (3 of vision blur; 1 of vision decrease); 2 patients showed loss of olfactory sensation; 2 patients had aphasia; and 1 patient found intracranial lesion by check up. The duration of initial symptoms prior to admission ranged from 2 weeks to 2 years (Table 1).
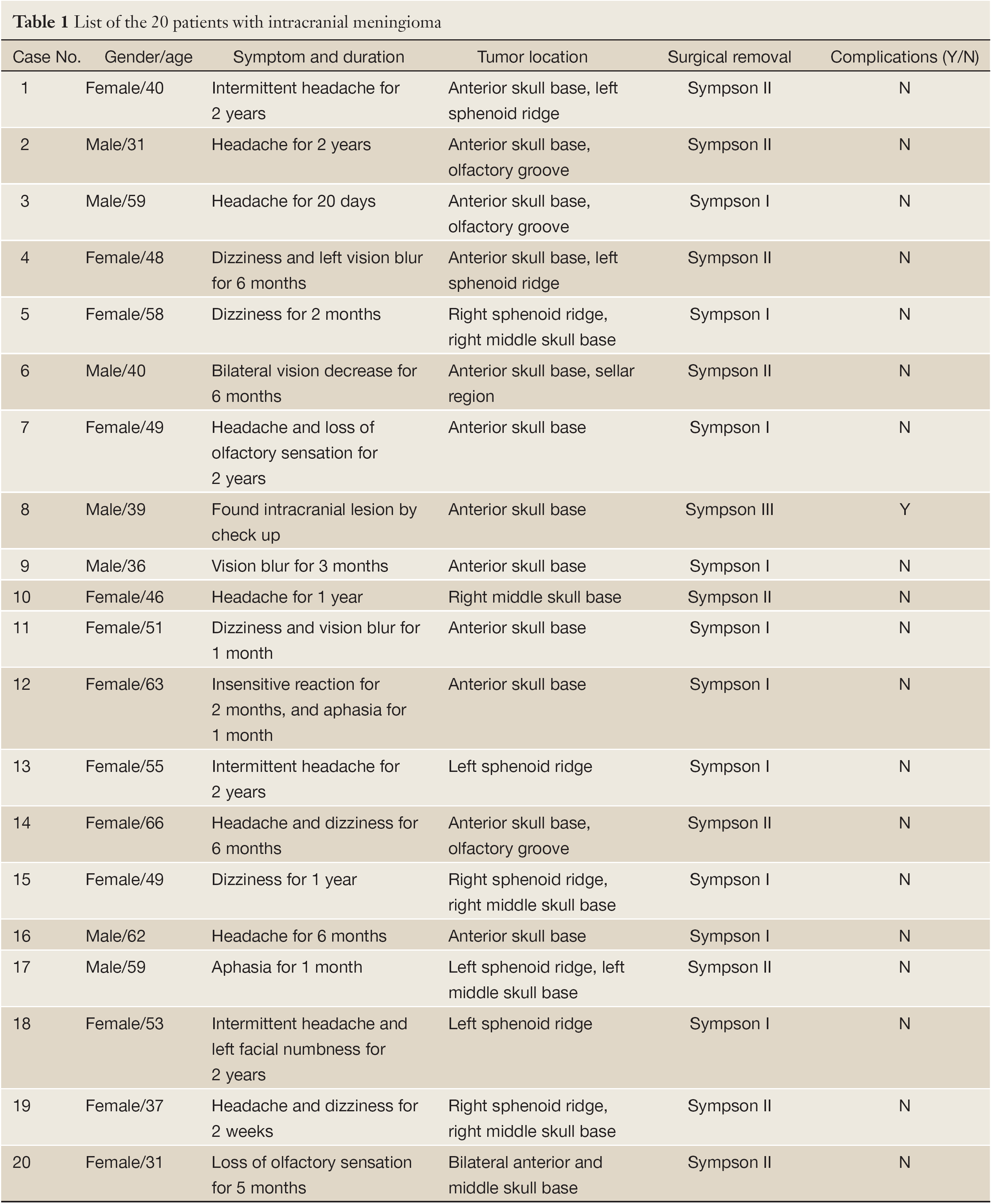
Full Table
Imaging examination
Preoperative CT and MRI scanning (Figure 1A-C) with contrast enhancement were performed in all patients. Brain MRA was also performed by Siemens 3T MRI scanner (Figure 1D). The three dimensional time-of-flight magnetic resonance angiography (3D-TOF MRA) sequence was used and the scanning parameters were as follows: TR =21 ms, TE =3.6 ms, FOV =200 mm, averages =1, slice thickness =0.9 mm. The images data were post-processed by maximum intensity projection (MIP) in the workstation to display the relationships of the tumor and intracranial arteries.

Virtual reality planning
All the patients’ imaging data of brain CT, MRI and MRA were transferred to a virtual reality (VR) workstation (Dextroscope, Volume Interactions, Singapore) (9). The images were processed within the RadioDexter software (Volume Interactions) in order to display the 3D stereoscopic images of tumor and its adjacent supplying arteries, including internal carotid artery (ICA) and its branches such as anterior cerebral artery (ACA) and middle cerebral artery (MCA), which makes the relationship of tumor and vessels more clear and vivid.
Neuronavigation
A Medtronic navigational system (Treon, Medtronic Stealth Station) was used in all surgical manipulations (10). The day before surgery, at least 8-10 fiducials were placed on the patients in a scattered pattern for reference prior to imaging and for viewing on the resulting images. Brain CT, MRI and MRA data of the patients were input into the workstation of the navigation system. Registration was performed in the operating room by touching the markers with the probe while indicating to the surgical workstation where marker was being touched. After registration, the lesion on the scalp projection was localized for surgical incision.
Treatment and intraoperative ultrasound
Prior to the dura was opened and tumor resection, the extent of tumor was detected intraoperatively by ultrasonography (ALOKA, Japan). The ultrasonic probe with sterile package was put directly on the brain surface, using a small amount of sterile saline as the contact agent (11). The tumor appeared high echo, and the surface borderlines of the tumor were confirmed through coronal, sagittal and horizontal scanning. During the operation, the ultrasonography could be used to detect the tumor supplying arteries, which helps to protect these important vessels (Video 1). After the resection, the ultrasonography was also used to figure out the resection of the tumor and other intracranial situations.
Results
Ultrasound imaging
Guided tumor resection with intraoperative ultrasonography was carried out in all operations. The image quality was good in all cases, enabling a clear visualization of tumor borders and its adjacent vessels (Figure 1H-J). The meningiomas are hyperechoic compared to normal brain tissue. During the tumor resection, the intraoperative ultrasonography was used to detect the arteries that surrounding the meningioma, or supplying the meningioma, or wrapped by the meningioma (Figure 1H-J). Intraoperative ultrasonography is very useful and safety for intracranial meningioma resection, especially those closely related to adjacent vessels. After partial tumor resection, the intraoperative ultrasonography could also real-timely track the location of arteries and its shift.
Intraoperative ultrasonography
It’s very important for neurosurgeons to know exactly the vessels that surrounding the tumor during the operation (Video 1). Intraoperative ultrasound angiography could be assessed for visualizing vessels engulfed by the tumor. Also major intracranial arteries and their branches feeding the tumor could be revealed within the tumor (Figure 1H-J). In patient No.17, the left ICA and MCA were partial engulfed in the tumor and the MCA was pushed inferiorly, which could be visualized from the preoperative MRA and VR images (Figure 1D-F). During the operation, the intraoperative neuronavigation also confirmed the situations (Figure 1G). Moreover, the neurosurgeon could easily figure out the tumor borders (Figure 1H), its adjacent vessels and blood flow by using the intraoperative ultrasound angiography before tumor resection, which was indicated by the colorful dots and oscillogram in Figure 1I-J. This intraoperative technique is essential for avoiding those vital arteries and protecting them while safely removing the tumor (Figure 1K).
Overall outcomes
Among the 20 patients, total tumor resection (Simpson grade I) was performed in 10 patients and postoperative MRI showed no sign of residual tumor tissue (Figure 1L); tumor resection (Simpson grade II) was achieved in 9 patients, and subtotal tumor resection (Simpson grade III) was performed in 1 patient whose tumor had extensive extent and invaded to scalp and skull (Table 1). Compared to the preoperative MRI, no new significant diffusion changes that could indicate circulatory changes because of traction or trauma to the adjacent parenchyma were seen after surgery. None of the patients died, and all patient experienced postoperative improvement of their symptoms except the patient of case No.8. The patient had tumor residual because of extensive tumor invading. There were no reoperations due to complications.
Discussion
Meningiomas arise from meningeal arachnoidal cells, and they account for about 20% of all primary brain lesions (1,2). Meningiomas may occur in any location throughout the neuraxis. The optimal therapeutic option of intracranial meningiomas is surgical removal. Except for malignant meningiomas (WHO grade II and III) that may relapse postoperatively, most benign meningiomas (WHO grade I) have good prognoses after surgical resection (3,4,12). However, neurosurgical procedures of anterior and middle skull base meningiomas are extremely difficult due to their complicated anatomy structures and the close relationships of the tumor with its adjacent arteries.
How to radically resect the meningioma while safely preserving the arteries well is becoming the key, which is also the goal that neurosurgeon pursues all the time. With the availability of CT, MRI, vessel-related imaging technique (CTA and MRA), and modern image processing workstation (Virtual Reality), it now becomes possible for neurosurgeons to get detail information about the tumor, its supply arteries and its relationships with adjacent brain tissues (5,9).
Over the past decades, intraoperative imaging techniques such as neuronavigation have been applied for neurological surgery (10,13). In spite of higher resolution, these technologies require complicated procedures, are rigid and difficult to adopt for real-time imaging during surgery. In comparison, intraoperative ultrasonography is of low cost and flexible, different ultrasound probes can be applied relatively easily to adapt to various sizes and shapes to address various applications in brain tumor surgery (14).
Intraoperative ultrasonography could help with surgical planning during the operation. Its capability of continuous visualization of intracranial meningioma provides a valuable tool to define the exact tumor location and size, the shift of tumor supplying vessels, and the relationships of the two parts (Figure 1H-J). Bearing in mind with the knowledge about the tumor and its surrounding important arteries, neurosurgeons could carefully plan and adapt an image-guided optimal removal of the tumor with less injury (Figure 1K).
Intraoperative ultrasonography could help to confirm the tumor location beneath the dura and optimize the dimension of the dura incision. Intraoperative ultrasonography made it possible to identify the entire expansion of the tumor. Meningiomas usually are hard, and should be removed little by little so as to not cause brain tissue trauma, and complete resection of the adherent part are also essential to prevent tumor recurrence (15) (Figure 1L).
The displaying of tumor vessels is essential to preserve the normal brain tissue functions, and might prevent brain damage and minimize bleeding (Video 1) (16). High image quality and resolution are crucial for successful image-guided surgery. Higher frequency in intraoperative ultrasonography produced improved resolution but reduced tissue penetration. Early studies employed ultrasound probes with a frequency of 5 and 7.5 MHz. Now the 10 MHz probes are frequently used (17), which is suited for spinal cord tumor resection (11). In our experience, we employed multi-frequency probes according to the tumor volume for an increased resolution for tumor vessels.
The advantages of intraoperative ultrasonography application in intracranial meningioma resection should be assessed in larger series studies in the future (18). Furthermore, ultrasound image quality may decrease during the operation because of blood and air bubbles in the surgical area. It’s true that current imaging techniques may not fully reflect the biological extent of the tumor. However, the aim of intraoperative ultrasonography guidance would be to achieve the optimal extent of intracranial meningioma resection without vessels injury (19,20).
Conclusions
Our experiences in this series of intracranial meningiomas indicated that intraoperative ultrasonography is really a sensitive tool in intracranial meningioma resection. The application of intraoperative ultrasonography in detecting tumor border and its supplying arteries during tumor resection helps to plan the operation and decrease severe post-operative neurological deficits. Intraoperative ultrasonography guidance in anterior and middle skull base meningioma resection could establish clear anatomical landmarks to guide the tumor resection, protect the vital structures, especially the important surrounding arteries, and eventually reduce the surgical complications.
Acknowledgments
The study was supported by National Natural Science Foundation of China (81200936, 30872675, 30901549), Shanghai Committee of Science and Technology (12JC1401800), and 2011 Shanghai Medical College Young Scientist Fund of Fudan University (11L-24).
Disclosure: The authors declare no conflict of interest.
References
- Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol 2006;5:1045-54. [PubMed]
- Mawrin C, Perry A.. Pathological classification and molecular genetics of meningiomas. J Neurooncol 2010;99:379-91. [PubMed]
- Perry A, Louis DN, Scheithauer BW, et al. Meningiomas. In: Louis DN, Ohgaki H, Wiestler OD, et al. eds. WHO Classification of Tumours of the Nervous System. Lyon: IARC, 2007:163-72.
- Alexiou GA, Gogou P, Markoula S, et al. Management of meningiomas. Clin Neurol Neurosurg 2010;112:177-82. [PubMed]
- Saloner D, Uzelac A, Hetts S, et al. Modern meningioma imaging techniques. J Neurooncol 2010;99:333-40. [PubMed]
- Miller D, Heinze S, Tirakotai W, et al. Is the image guidance of ultrasonography beneficial for neurosurgical routine? Surg Neurol 2007;67:579-87; discussion 587-8. [PubMed]
- Sure U, Benes L, Bozinov O, et al. Intraoperative landmarking of vascular anatomy by integration of duplex and Doppler ultrasonography in image-guided surgery. Technical note. Surg Neurol 2005;63:133-41; discussion 141-2. [PubMed]
- Tirakotai W, Miller D, Heinze S, et al. A novel platform for image-guided ultrasound. Neurosurgery 2006;58:710-8; discussion 710-8. [PubMed]
- Tang HL, Sun HP, Gong Y, et al. Preoperative surgical planning for intracranial meningioma resection by virtual reality. Chin Med J (Engl) 2012;125:2057-61. [PubMed]
- Mao Y, Zhou L, Du G, et al. Image-guided resection of cerebral cavernous malformations. Chin Med J (Engl) 2003;116:1480-3. [PubMed]
- Zhou H, Miller D, Schulte DM, et al. Intraoperative ultrasound assistance in treatment of intradural spinal tumours. Clin Neurol Neurosurg 2011;113:531-7. [PubMed]
- Tang H, Sun H, Chen H, et al. Clinicopathological analysis of metaplastic meningioma: report of 15 cases in Huashan Hospital. Chin J Cancer Res 2013;25:112-8. [PubMed]
- Bernays RL. Intraoperative imaging in neurosurgery. MRI, CT, ultrasound. Introduction. Acta Neurochir Suppl 2003;85:1-3. [PubMed]
- Unsgaard G, Ommedal S, Muller T, et al. Neuronavigation by intraoperative three-dimensional ultrasound: initial experience during brain tumor resection. Neurosurgery 2002;50:804-12; discussion 812. [PubMed]
- Li F, Lin J, Zhu G, et al. Neuroimaging and functional navigation as potential tools to reduce the incidence of surgical complications of lateral ventricular meningiomas. Clin Neurol Neurosurg 2011;113:564-9. [PubMed]
- Solheim O, Selbekk T, Lindseth F, et al. Navigated resection of giant intracranial meningiomas based on intraoperative 3D ultrasound. Acta Neurochir (Wien) 2009;151:1143-51. [PubMed]
- Reinacher PC, van Velthoven V. Intraoperative ultrasound imaging: practical applicability as a real-time navigation system. Acta Neurochir Suppl 2003;85:89-93. [PubMed]
- Bozinov O, Burkhardt JK. Intra-operative computed-tomography-like real-time three-dimensional ultrasound in neurosurgery. World Neurosurg 2012;78:5-7. [PubMed]
- Chacko AG, Kumar NK, Chacko G, et al. Intraoperative ultrasound in determining the extent of resection of parenchymal brain tumours--a comparative study with computed tomography and histopathology. Acta Neurochir (Wien) 2003;145:743-8; discussion 748. [PubMed]
- Bai HM, Wang WM, Li TD, et al. Three core techniques in surgery of neuroepithelial tumors in eloquent areas: awake anaesthesia, intraoperative direct electrical stimulation and ultrasonography. Chin Med J (Engl) 2011;124:3035-41. [PubMed]
