Recurrent orbital space-occupying lesions: a clinicopathologic study of 253 cases
Introduction
Although orbital lesions are rare, these sight-threatening and possibly life-threatening disorders consist of a broad disease spectrum. Familiarity with the incidence of various etiologies can be useful for correct diagnosis (1). Similarly, to summary and recognize the categories, features and frequencies of recurrent orbital diseases after operation may contribute to taking effective approaches to reduce the relapse. The risks of postoperative recurrence are often correlated with the preoperative misdiagnostic conditions (2).
In this paper, we retrospectively studied the clinical features, histopathologic classification and frequencies in a group of patients with recurrent orbital space-occupying lesions after operation.
Materials and methods
This is a retrospective study of 253 consecutive patients with recurrent orbital diseases after operation. They were admitted to the Institute of Orbital Diseases, the General Hospital of the Armed Police Force, Beijing, China, from January 2009 to December 2010. Orbital computed tomography (CT) and magnetic resonance Imaging (MRI) scans confirming the local recurrence were required for all cases, and patients with excisional biopsy for diagnostic purpose in the last operation were excluded. The time span between the time of admission into our wards and the last operation was measured as the last recurrence interval.
Our series included 123 males and 130 females aged from 2 to 78 years (mean age, 36.2±15.1 years). All patients had undergone surgical removal of their recurrent mass or the exenteration of orbit, and some of them were also referred for external radiotherapy or/and chemotherapy postoperatively. Specimens were obtained and the paraffin-embedded sections were then examined by routine hematoxylin-eosin (H&E) staining or a further immunohistochemical staining. Two pathologists gave the pathologic diagnosis. An accurate diagnosis was validated by both clinical and imaging investigations as well as histological data, and the incidence and composition of various recurrent lesions after operation were then calculated. A literature review was also conducted to probe the possible causes for patients with a higher risk of relapse in this series. The secondary orbital meningioma of intracranial origin had been classified into neurogenic tumors, not of secondary orbital tumors in our study, for the differentiation from the primary intra-orbital meningioma is often difficult on image (3).
Results
Clinical features
In this series, 31 (12.3%) cases of recurrence developed with symptom-free and CT or/and MRI documented recurrent mass lesions (Figure 1). Respectively, 159, 65, 20, 8 cases and 1 case had previously experienced once, twice, three, four and six times of surgeries. The four most frequently observed manifestations of 222 (87.7%) cases were exophthalmos (139 cases), periorbital masses or ptosis (79 cases), orbital pain (39 cases) and loss of visual acuity (35 cases). For the treatment, 231 (91.3%)cases underwent surgical removal of the recurrent orbital lesions, and 22 (8.7%) cases had to receive the exenteration of orbit.
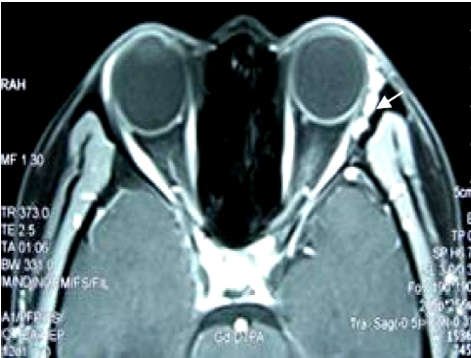
Histopathologic classification
According to the histogenesis, 253 cases of recurrent orbital lesions were classified into 11 categories (Table 1). There were 65 (25.7%) cases of lacrimal gland tumors, 54 (21.3%) cases of vasogenic diseases, 42 (16.6%) cases of neurogenic tumors, 24 (9.5%) cases of secondary tumors, 21 (8.3%) cases of orbital inflammation, 14 (5.5%) cases of myogenic tumors, 12 (4.7%) cases of fibrous and adipose tumors, 7 (2.8%) cases of lympho-hematopoietic tumors, 7 (2.8%) cases of bone or cartilage tumors, 6 (2.4%) cases of orbital cysts, and 1 (0.4%) case of Indefinitely differentiated tumor. Special note is that the secondary orbital meningioma of intracranial origin in this series had not been classified as the orbital secondary tumors, but the neurogenic tumors.
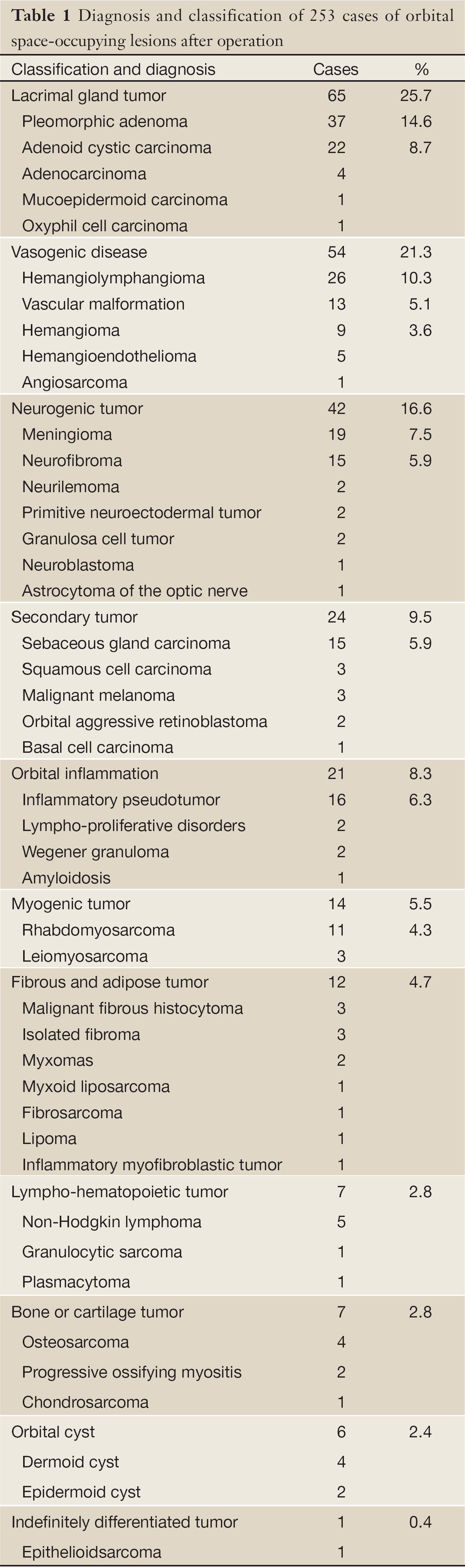
Full Table
Pathologic diagnosis
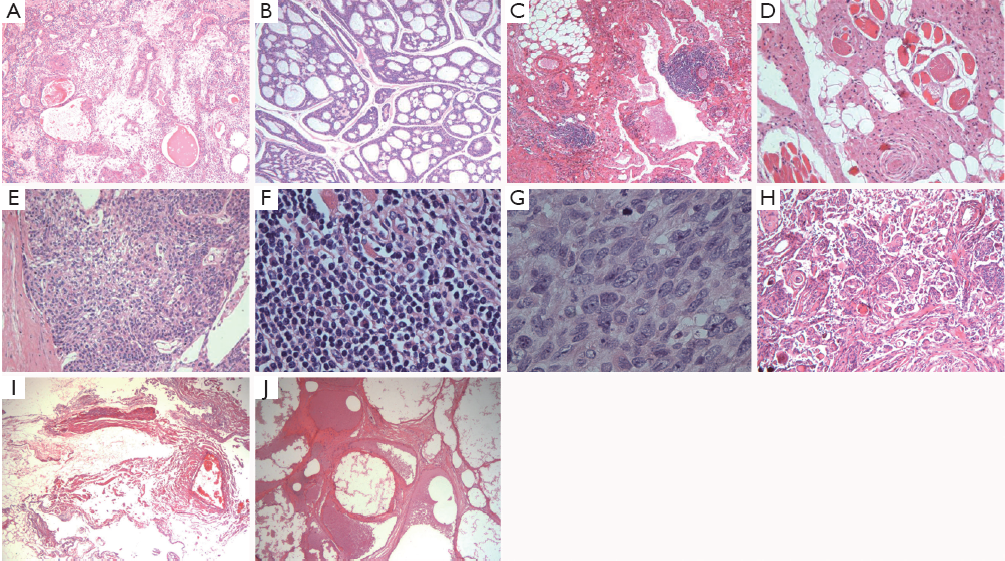
The first 10 kinds of recurrent orbital diseases are as follows (Figure 2): 37 (14.6%) cases of lacrimal gland pleomorphic adenomas, 26 (10.3%) cases of hemangiolymphangiomas, 22 (8.7%) cases of lacrimal gland adenoid cystic carcinomas, 19 (7.5%) cases of meningiomas, 16 (6.3%) cases of inflammatory pseudotumors, 15 (5.9%) cases of neurofibromas, 15 (5.9%) cases of sebaceous gland carcinomas, 13 (5.1%) cases of vascular malformations, 11 (4.3%) cases of rhabdomyosarcomas, and 9 (3.6%) cases of hemangiomas.
Twenty-nine (11.5%) cases had recurred 3 times or over, and they are as follows (Table 2): lacrimal gland tumors, 10 cases; orbital secondary tumors, 7 cases; vasogenic disease, 4 cases; neurogenic tumors, 3 cases; myogenic tumor, 2 cases; fibrous and adipose tumor, 1 case; lympho-hematopoietic tumor, 1 case; and bone or cartilage tumor, 1 case. The last recurrence interval ranged from 1 month to 40 years with the median of 4.75 years, and 37 (14.6%) cases getting recurrence in 10 or more years postoperatively were as follows: lacrimal gland pleomorphic adenoma, 16 case; hemangiolymphangioma, 8 cases; neurofibroma, 6 cases; vascular malformation, 2 case; and others including one each of hemangioma, lacrimal gland adenoid cystic carcinoma, dermoid cyst, hemangioendothelioma, and meningioma of the optic nerve sheath.
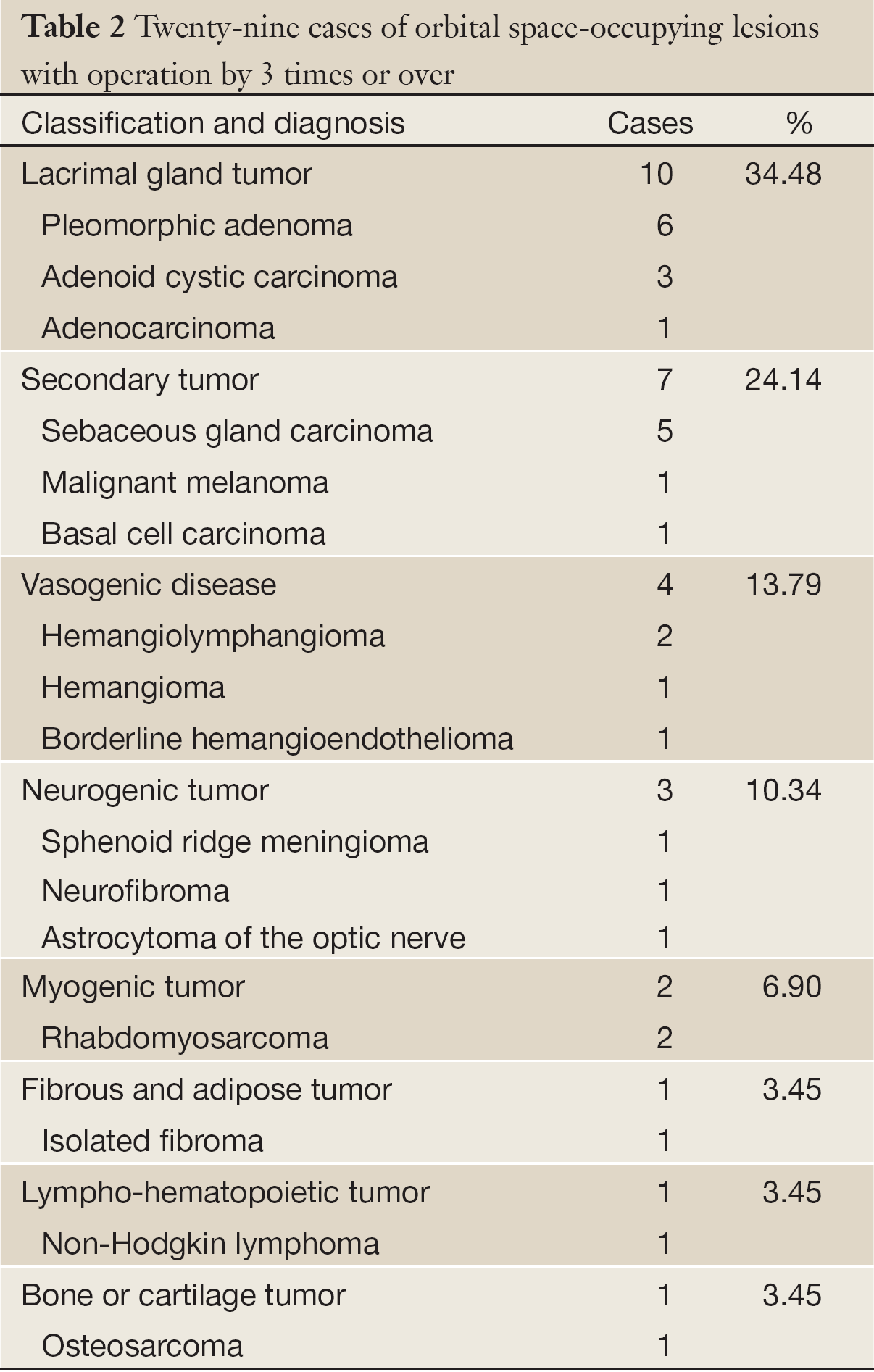
Full Table
Discussion
In the present study, most patients showed obvious signs and symptoms of relapse and underwent surgical removal of the recurrent lesions, and minority had to receive the exenteration of orbit due to the delay in diagnosis. Because the recurrent space-occupying lesions were of relatively small size at the time of early postoperative stage, recurrence was often discovered only by imaging examination. Thus, with a general understanding of those orbital diseases prone to relapse after operation and an early handling, we are able to avoid enlarging the scope of surgical resection or the exenteration of orbit. Soysal retrospectively reviewed 68 cases of the exenteration of orbit in the management of orbital tumors over a ten-year period, and disclosed that the great majority of the patients had undergone a long duration of disease (4).
This study showed that the most frequent orbital lesions were lacrimal gland tumors accounting for 25.7% of the series. The vasogenic disease (21.3%) comprised the second most common, which included vascular malformation and neoplasm. The neurogenic tumor (16.6%) ranked as the third leading category. Besides of vascular malformation and orbital inflammatory lesion, the majority of our cases were orbital neoplasm of different origin (Table 1). Kim UR et al. revealed that the most common lesions of 6,328 individuals with orbital disorders in South India were inflammatory and systemic diseases involving the orbit, followed by 20.1% of neoplasm (1). That the result was not consistent with our findings may due to the different research objects. Our series were especially limited to those suffered postoperative recurrence of their orbital lesions. There are many other reports on the incidence of various orbital disease, and the results vary widely. The possible explanations for this may be the different classification criteria and varying sources of patients. Interestingly, major series had all confirmed the relative rarity of primary epithelial tumors of the lacrimal gland (1,5-7). Lacrimal gland tumors were the commonest category in our series (Table 1), which mainly include pleomorphic adenoma and adenoid cystic carcinoma. This inconsistency most likely stems from the fact that the lacrimal gland epithelial tumor after resection is most prone to recur.
The first 10 orbital diseases in our series can be divided into two major types according to the surgical strategies. One is that the space-occupying lesion had to be partially removed for avoiding the risk of sacrificing visual function, the other is that the lesion had been attempted complete surgical excision. Hemangiolymphangioma, vascular malformation and hemangioma were categorized as vasogenic in origin. Hemangiolymphangioma was the second commonest lesion in our series, which is thought to be a variant of lymphangioma showing vascular component (8).
Lymphangioma can be responsible for sudden proptosis due to orbital hemorrhage, and timely surgical intervention is sometimes needed (9). But a complete resection is rarely possible for its infiltrative and diffuse nature of these vasogenic disorders (10-12).
Neurofibroma constitutes 5.9% of our cases and comes right after meningioma in the neurogenic tumor, which has the similar characteristics of the anatomy of the hemangiolymphangioma with the extensive and infiltrative nature. A complete resection is also very difficult, thus the surgical excision often be undertaken just for cosmetic or functional purposes (13-15). Inflammatory pseudotumor was seen in 16 of 21 patients with orbital inflammation in our series, which is usually referred to the idiopathic inflammation with a pleomorphic cellular response and a fibrovascular tissue reaction (16). During the tumor-like inflammation, all orbital structures may be diffusely involved. When cortisone therapy showed to be a low response, a partial excision could be effective in alleviating the symptoms and preventing the complications (17,18).
The above orbital diseases arranged for partially excising were inevitably to recur, and repeated surgery is required when threatening complications reappeared. In contrast to this type of lesions, the recurrence of other 5 top orbital diseases was more likely based upon subjective factors. Firstly, pleomorphic adenoma and adenoid cystic carcinoma of lacrimal gland accounted for a major proportion of lacrimal gland tumors in our study. In addition, the lacrimal gland epithelial tumor is of repeated and lifelong risking of recurrence. It was thought that the process of stripping the neoplasm off the lacrimal gland tissue is easy to induce orbital tumor cells dissemination. McNab et al. found that recurrent lacrimal gland pleomorphic adenoma often displayed multifocal and widespread tumor nodules in the operative field (19). Familiar with this anatomical character, we can thus determine appropriate management to reduce the recurrence. Secondly, the most common neurogenic tumor in our study was orbital meningioma, followed by neurofibroma. Meningioma constitutes approximately 4% of all orbital tumors, which mainly includes primary intraorbital meningioma and secondary orbital meningioma of intracranial origin (20). In the practice of Oya et al., although the orbital/sphenoid intraosseous, intraorbital and intradural tumor components were treated surgically as complete as possible, it still amounted to 17.9% of the recurrence rate (21). A study by Saeed et al. revealed the close recurrence rate of 17% in 66 patients with sphenoorbital meningioma, but only 1 out of 15 patients who underwent radiotherapy showed signs of recurrence (22). Therefore, an attempted complete resection combined with adjuvant postoperative radiation or chemical treatment can be used as an effective measure to control the recurrence rate of orbital meningioma. Thirdly, secondary orbital tumors are referred to those extending to the orbit from neighboring structures (23).
Sebaceous gland carcinoma had the highest incidence of secondary orbital tumors, accounting for 15 of 25 cases in our series. Early recognition of sebaceous carcinoma is often challenging, for it always masqueraded as chalazion (24). A biopsy should be performed to provide an accurate diagnosis. Otherwise, the delayed treatment may lead to high rate of local recurrence (25). Lastly, rhabdomyosarcoma was found to be the most common myogenic tumor of the orbit. It is known that rhabdomyosarcoma is the most common soft tissue sarcoma in children with a relative predominance for head and neck region, and the orbital rhabdomyosarcoma accounts for approximately 10% of these tumors (26,27). Recurrence or metastatic spread of rhabdomyosarcoma usually occurred within three years of treatment (28). Treatment for rhabdomyosarcoma involved chemotherapy, radiotherapy and surgery, and how to achieve the optimum use of these three treatments to reduce the relapse is proved to be debatable (29).
In summary, our series could be divided into 2 main categories according to the surgical strategies. One is that the treatment was to attempt complete resection of the orbital disease, the other is that the lesions had to be partially removed for avoiding the risk of sacrificing visual function. According to their histological origin, they were classified as lacrimal gland tumor, vasogenic orbital disease, neurogenic tumor, orbital secondary tumor, orbital inflammation, myogenic tumor, fibrous and adipose tumor, lympho-hematopoietic tumor, bone or cartilage tumor, orbital cysts, and indefinitely differentiated tumor. Besides of vascular malformation and orbital inflammatory lesion, the majority of our cases were orbital neoplasm of different origin. Lacrimal gland epithelial tumor is the first leading recurrent orbital disease after operation, and early and longer-term postoperative follow-up is needed.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kim UR, Khazaei H, Stewart WB, et al. Spectrum of orbital disease in south india: an aravind study of 6328 consecutive patients. Ophthal Plast Reconstr Surg 2010;26:315-22. [PubMed]
- Wylegała E, Orzechowska-Wylegała B. Foreign bodies in the orbit misdiagnosed as fracture of the orbital lateral wall. Klin Oczna 2003;105:197-9. [PubMed]
- Yu HJ, Wu YT, Chen HK, et al. Primary orbital meningioma: a study of six cases at a single institution. APMIS 2011;119:36-43. [PubMed]
- Soysal HG. Orbital exenteration: a 10-year experience of a general oncology hospital. Orbit 2010;29:135-9. [PubMed]
- Henderson J. Orbital Tumors. 3rd ed. New York, NY: Brian C. Decker, 1994:43-51.
- Wilson MW, Grossniklaus HE. Orbital disease in North America. Ophthalmol Clinic North Am 1996;9:539-47.
- Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 Montgomery Lecture, part 1. Ophthalmology 2004;111:997-1008. [PubMed]
- Shetty DC, Urs AB, Rai HC, et al. Case series on vascular malformation and their review with regard to terminology and categorization. Contemp Clin Dent 2010;1:259-62. [PubMed]
- Morax S, Desjardins L. Orbital tumor emergencies in childhood. J Fr Ophtalmol 2009;32:357-67. [PubMed]
- Berthout A, Jacomet PV, Putterman M, et al. Surgical treatment of diffuse adult orbital lymphangioma: two case studies. J Fr Ophtalmol 2008;31:1006-17. [PubMed]
- Choudhri O, Chang SD. Images in clinical medicine. Orbital and cerebral arteriovenous malformations. N Engl J Med 2011;365:e28. [PubMed]
- Ko F, DiBernardo CW, Oak J, et al. Confirmation of and differentiation among primary vascular lesions using ultrasonography. Ophthal Plast Reconstr Surg 2011;27:431-5. [PubMed]
- Rilliet B, Pittet B, Montandon D, et al. Orbitotemporal facial involvement in type 1 neurofibromatosis (NF1). Neurochirurgie 2010;56:257-70. [PubMed]
- Fan XQ, Lin M, Li J, et al. Removal and plastic reconstructive operation of orbital neurofibroma. Zhonghua Yi Xue Za Zhi 2007;87:3264-7. [PubMed]
- Trăistaru R, Rogoveanu O, Popescu R, et al. Periarticular diffuse neurofibroma of the upper limb. Rom J Morphol Embryol 2011;52:1377-83. [PubMed]
- Espinoza GM. Orbital inflammatory pseudotumors: etiology, differential diagnosis, and management. Curr Rheumatol Rep 2010;12:443-7. [PubMed]
- Szabo B, Szabo I, Crişan D, et al. Idiopathic orbital inflammatory pseudotumor: case report and review of the literature. Rom J Morphol Embryol 2011;52:927-30. [PubMed]
- Orgaz MS, Grabowska A, Bellido EC, et al. Idiopathic sclerosing orbital inflammation with intranasal extension. Orbit 2010;29:106-9. [PubMed]
- McNab AA, Satchi K. Recurrent lacrimal gland pleomorphic adenoma: clinical and computed tomography features. Ophthalmology 2011;118:2088-92. [PubMed]
- Jain D, Ebrahimi KB, Miller NR, et al. Intraorbital meningiomas: a pathologic review using current World Health Organization criteria. Arch Pathol Lab Med 2010;134:766-70. [PubMed]
- Oya S, Sade B, Lee JH. Sphenoorbital meningioma: surgical technique and outcome. J Neurosurg 2011;114:1241-9. [PubMed]
- Saeed P, van Furth WR, Tanck M, et al. Surgical treatment of sphenoorbital meningiomas. Br J Ophthalmol 2011;95:996-1000. [PubMed]
- Poloschek CM, Lagrèze WA, Ridder GJ, et al. Clinical and neuroradiological diagnostics of orbital tumors. Ophthalmologe 2011;108:510-8. [PubMed]
- Sung D, Kaltreider SA, Gonzalez-Fernandez F. Early onset sebaceous carcinoma. Diagn Pathol 2011;6:81. [PubMed]
- Wali UK, Al-Mujaini A. Sebaceous gland carcinoma of the eyelid. Oman J Ophthalmol 2010;3:117-21. [PubMed]
- Arya K, Vij H, Vij R, et al. Rhabdomyosarcoma of mandible: a diagnostic predicament. J Oral Maxillofac Pathol 2011;15:320-5. [PubMed]
- Karcioglu ZA, Hadjistilianou D, Rozans M, et al. Orbital rhabdomyosarcoma. Cancer Control 2004;11:328-33. [PubMed]
- Das JK, Tiwary BK, Paul SB, et al. Primary orbital rhabdomyosarcoma with skeletal muscle metastasis. Oman J Ophthalmol 2010;3:91-3. [PubMed]
- Gradoni P, Giordano D, Oretti G, et al. Clinical outcomes of rhabdomyosarcoma and Ewing’s sarcoma of the head and neck in children. Auris Nasus Larynx 2011;38:480-6. [PubMed]
