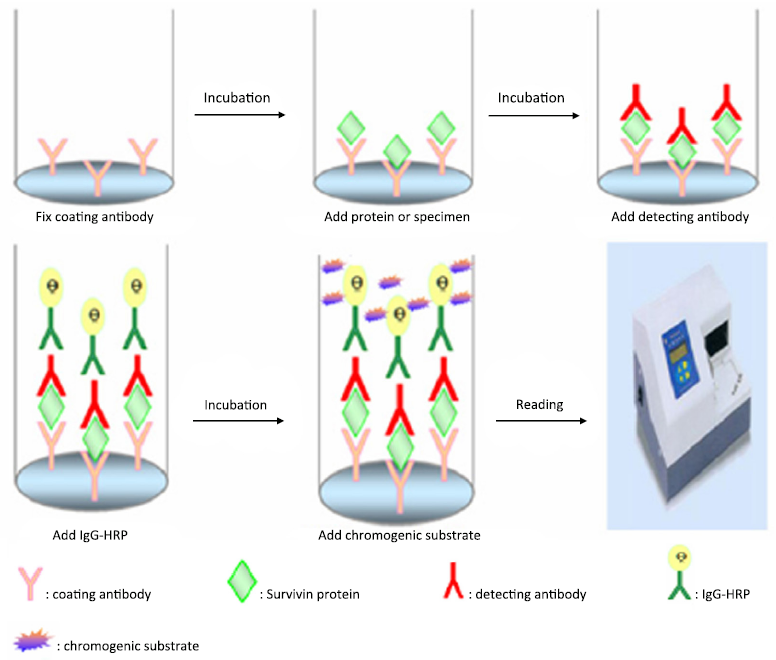
Sandwich ELISA for detecting urinary Survivin in bladder cancer
Introduction
Bladder cancer is the ninth common cancer worldwide and it accounts for 3% of the global cancer incidence (1). More than 90% of the diagnosed cases are transitional cell carcinomas (TCC) and 75% are superficial tumors. Although it is easy to excise these superficial tumors by surgery, 50-70% will be recurrent in the next five years and 15-25% of the recurrent tumors progress into higher grades and stages (2). Currently the gold standard for detecting bladder cancer is cystoscopy and cytology. Cystoscopy is highly sensitive and specific for the detection and surveillance of bladder cancer after therapy. However, it is invasive and expensive so that there is low prevalence in the general patients. Cytology as an adjuvant to cyctoscopy is highly specific to detect progressed diseases, but it is lack of sensitivity for low-grade tumors (3,4).
Bladder cancer is easy to relapse and it is necessary for long-term follow-up. Because of the low sensitivity of cytology and invasiveness of urethra cystoscopy, it is urgent for a detection method which has the characterization of convenience, cheap and no pain. With the progress of cancer research in the field of molecular biology, some tumor markers have shown high sensitivity to detect primary tumor and others can detect recurrence with reasonable sensitivity in bladder cancer (5).
Recently there were some reports about Survivin as an excellent tumor marker in the diagnosis of bladder cancer (6,7). But it has not been completely testified. The Clinical Laboratory of Beijing Cancer hospital had prepared the monoclonal antibody (McAb) and polyclonal antibody (PcAb) of Survivin in precious experiments. Here, we report the development of a Survivin sandwich enzyme-linked immunosorbent assay (ELISA) and its application for the quantitative detection in the urine of bladder cancer.
Materials and methods
ELISA reagents
Recombinant human sequence Survivin protein was produced by our laboratory. McAb-C8 and McAb-D10 were raised by immunizing BALB/c mice with recombined protein and purified by Protein G immuno-affinity chromatography. PcAbs against Survivin were gotten by immunizing New Zealand White (NZW) rabbits and then purified by Protein A affinity chromatography (8). Goat anti-rabbit IgG-horseradish peroxidase (IgG-HRP) as second antibody was purchased from Proteintech Company. 3,3',5,5'-Tetramethyl benzidine (TMB) was obtained from Ameresco and 96-well immunoassay plates from Axygen biotehcnology company. Skimmed milk powder (SMP) was bought from Yili industrial group. Other reagents comprise: 0.05 mol/L carbonate bicarbonate buffer (CBB), pH 9.6 (15 mmol/L Na2CO3, 30 mmol/L NaHCO3); 10 mmol/L phosphate buffer (PBS), pH 7.4 (137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 2 mmol/L KH2PO4); 10 mmol/L PBST (PBS with 0.05% Tween-20); 5% PBS-SMP (PBS with 5% SMP); chromogenic substrate solution containing TMB, hydrogen peroxide and buffer (25 mmol/L citric acid and 50 mmol/L disodium hydrogen phosphate, pH 5.0).
Clinical materials
All studies were approved by the Institutional Review Boards for the Protection of Human Subjects and informed consent was obtained from each participant. A total of 102 bladder cancer patients, including 78 males and 24 females with a mean age of 62.5 years (range from 31 to 90 years), were admitted to Beijing Cancer Hospital from Mar 2009 to October 2010. They were testified by pathology and there were no any low urinary tract operation before samples collection. The controls were 102 people who came from physical examination population in Beijing cancer Hospital from Jun 2009 to July 2009, including 64 males and 38 females with mean age of 54.3 years (range from 39 to 75 years).
Samples collection
When the patients and healthy people underwent routine urine test in Beijing Cancer Hospital, 10 mL medistream urine of subjects was collected at the day and centrifuged at 3,000 r/min for 5 min. The supernatant was acquired and aliquotted, then the samples were frozen at –20 °C until detection.
Procedure of sandwich ELISA
Recombinant MS-Survivin protein as standard substance was utilized to explore diverse conditions in the reaction of Survivin ELISA. Different combinations of McAb and PcAb as coating antibody or detecting antibody were tried. It was also optimized by using different reaction time, incubation temperature and various antibodies’ concentration. This ELISA was evaluated according to assay sensitivity, intra-assay precision, inter-assay precision and minimum detectable dose (MDD) before Survivin levels were detected in urine samples of subjects. Schematic diagram of the sandwich ELISA is shown as Figure 1. The specific process was as follows: (I) 96-well microplates were coated with PcAb or McAb as capturing antibody at 4 °C for 16 h or 37 °C for 2 h, and their concentration ranged from 1 to 5 µg/mL in the buffer of CBB; (II) after washing with PBST three times, the plates were blocked by 100 µL 5% PBS-SMP at 37 °C for 60 min; (III) add 100 µL MS-Survivin protein or samples diluted in PBS after washing again and incubate at 37 °C for 90 min; (IV) repeatedly wash, add 100 µL detecting antibody with different concentrations of McAb or PcAb to the wells and incubate at 37 °C for 90 min; (V) add 100 µL IgG-HRP that was diluted into 5% PBS-DMP with the concentration of 1/2,000-1/4,000 and incubate at 37 °C for 90 min; (VI) after washing five times, add 100 µL of chromogenic substrate and incubate at 37 °C for 10 min, and then it was stopped by adding 12.5% H2SO4 (50 mL/well); and (VII) the absorbance was measured using a microplate reader at 450 nm (Model 680 Microplate Reader, Bio-Rad Laboratories) and the data were recorded.
Statistical analysis
Statistical analysis was carried out by the SPSS for windows release 16.0 package program (SPSS Inc., Chicago, IL, USA). The optical density at 450 nm (OD450) of patients and healthy people was compared by Student’s t-test and assessed using the area under a receiver operating characteristic (AUROC) curve. Cutoff value was determined by the optimal Youden’s index (sensitivity + specificity -1). According to this value, the following calculations will be made: sensitivity and specificity. The mean expression level with different clinical parameters was also calculated among bladder cancer patients and a t-test was performed. P<0.05 was considered statistically significant.
Results
Selection of capturing antibody and detecting antibody
Proper combination of McAb-C8, McAb-D10 and PcAb was conducted. The result showed that McAb-D10 as coating antibody and PcAb as detecting antibody could get MDD, which reached 0.0625 ng/mL in the determination of standard protein. However, it was only 0.25 ng/mL in turn. While McAb-C8 was chosen as coating antibody and PcAb as detecting antibody, the MDD was 0.12 ng/mL. So it could find that choosing McAb-D10 to coat was better than others.
Selection of coating temperature
The coating conditions of 4 °C for 16 h or 37 °C for 2 h were compared. The former MDD was 0.0625 ng/mL, while the latter was 0.125 ng/mL. Therefore, the temperature of 4 °C for coating was better than 37 °C.
Square matrix titration of detecting antibody and IgG-HRP
First of all, 2.5 µg/mL McAb-D10 as capturing antibody was fixed and 1 ng/mL Survivin protein was added as standard substance. Meanwhile, PBS was chosen as the control at every assay. Subsequently detecting antibody whose concentration was 5, 2.5 and 1.25 µg/mL respectively were selected; and IgG-HRP may be diluted into 1/2,000, 1/3,000 and 1/4,000. At last, according to the highest absorbance of specimen and the negative control value of not more than 0.1, we could choose the best combination of them. Specific values of OD450 are shown in Table 1. The optimized result was that the dilution of IgG-HRP was 1/3,000 and the concentration of detecting antibody was 2.5 µg/mL could get the highest OD value.
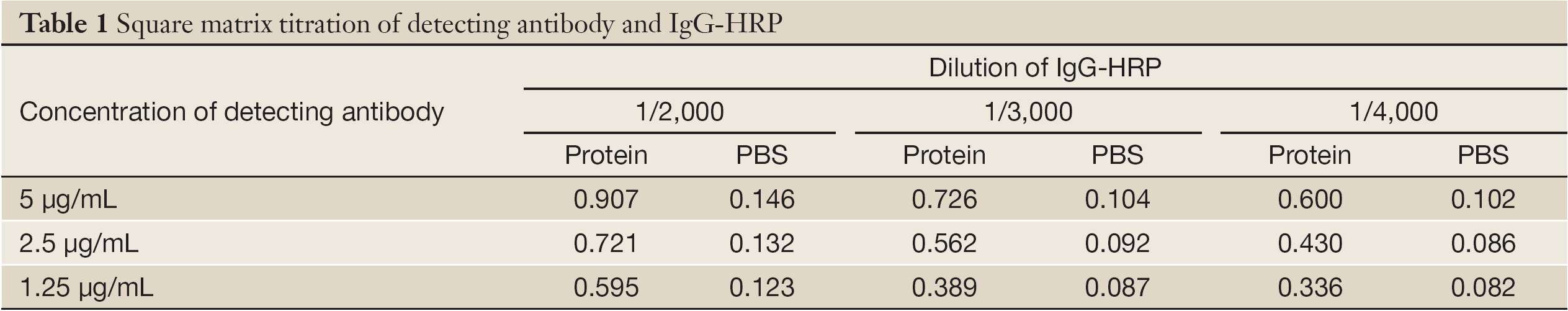
Full Table
Assay precision and reproducibility
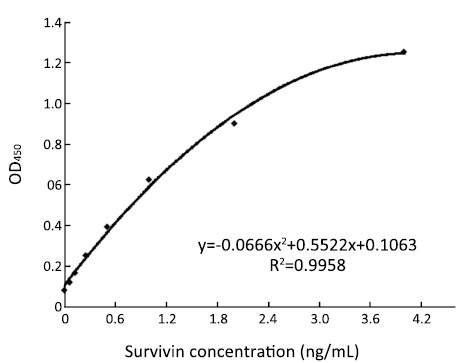
It could be found that the MDD of standard protein is 0.0625 ng/mL from Figure 2. Through replicate assays in 28 well with 1 ng/mL Survivin protein as standard substance, it showed the mean and standard deviation of values were 0.564 and 0.0473, respectively. Therefore the intra-assay CV was 8.39%. Through detecting the same concentration of Survivin protein in ten times, it could get that inter-assay CV was 8.57%.
Establishment of standard curve
Under the optimized condition we started to detect standard protein, which was serially diluted into 2, 1, 0.5, 0.25, 0.125, 0.0625 and 0 ng/mL. Followed the process mentioned above and established standard curve (Figure 2), the equation of regression was y=–0.0666 x2+0.5522 x +0.1063, R2=0.9958.
Survivin levels in the urine of patients and healthy people
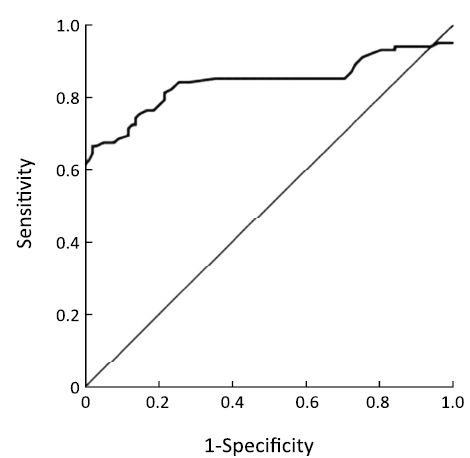
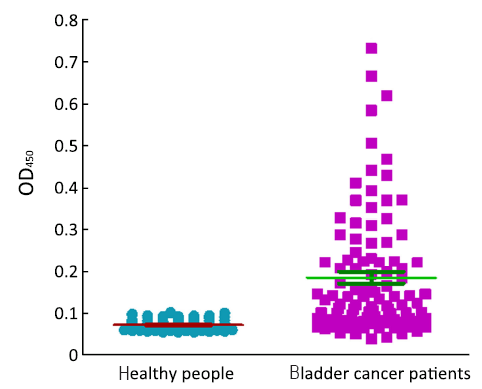
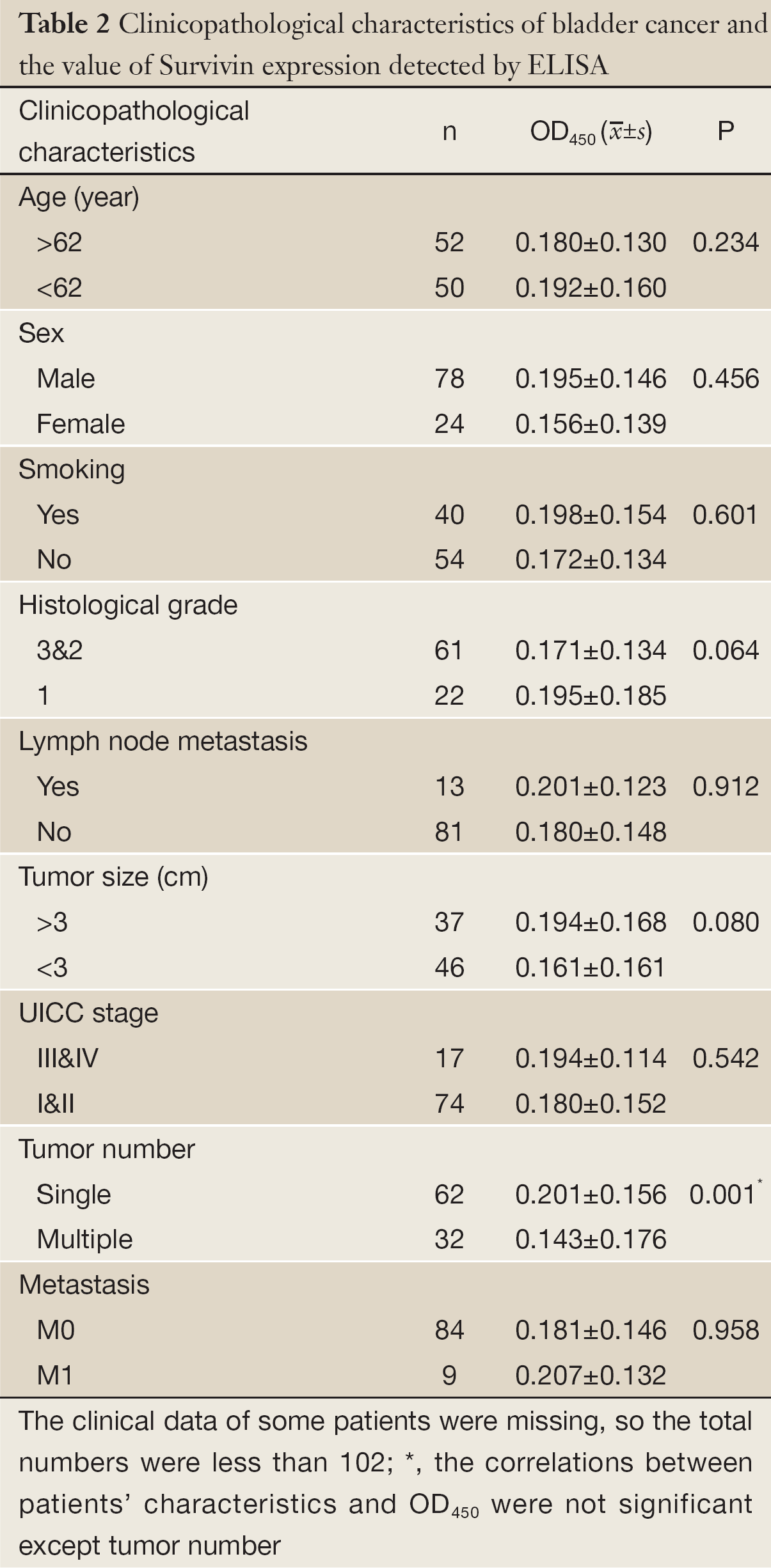
ROC based on the detection of 102 bladder cancer patients and 102 healthy people is shown in Figure 3. The cutoff value was determined by optimal Youden’s index through analyzing the Survivin ROC among patients and healthy people. When OD450 was 0.09, it can get that sensitivity, specificity and Youden’s index were 70.6%, 89.2% and 0.598 respectively. The scatter plot of absorbance in the detection of samples of 102 bladder cancer patients and 102 healthy people is shown in Figure 4 (P<0.0001). Furthermore, the expressions of urine Survivin in bladder cancer patients with various clinical parameters were analyzed, as shown in Table 2. The associations between those clinicopathological characteristics and OD450 were not significant except tumor number, the P value of which was 0.001.
Full Table
Discussion
Survivin is a member of the inhibitor of apoptosis proteins (IAP) family of molecules, a group of proteins involved inhibition of caspases 3, 7 and 9 (9). It is expressed during human fetal development, but is not detectable in normal adult tissues, except for the thymus and placenta. It expressed in most malignant cells, including lymphomas (10), gastric (11), lung (12), bladder cancers (7), pancreatic (13), breast (14) and prostatic cancer (15).
The reasons of Survivin as a significant marker in malignancy is based on its ability to inhibit apoptosis, promote proliferation and enhance angiogenesis (16,17). And it is likely to be involved in tumor development and progression. Therefore, its increased expression would be expected to predict aggressive diseases (18,19). It indicates that the detection of Survivin could be a tumor marker to diagnose and/or prognose cancers.
Currently there are several methods to detect Survivin including reverse transcription-polymerase chain reaction (RT-PCR), real time-PCR, immunohistochemistry (IHC) and ELISA (6,20,21). RT-PCR and real time-PCR have relatively high sensitivity. However, those are time-consuming and costly. IHC is regarded as gold standard in diagnosis of cancers, but there is an apparent drawback that is not easy to get tissue specimens. ELISA was introduced in the 1970s (22). In the typical double antibody sandwich ELISA, antibody attached to the bottom of a well provides both antigen capture and immune specificity, while another antibody linked to an enzyme provides detection and an amplification factor. This approach enables accurate and sensitive detection of the antigen. Because of these desirable features, ELISA has been considered the standard method of cytokine measurement and is widely utilized in clinical laboratories and biomedical research. And ELISA kits easy for commercial availability are also common to measure tumor markers in body fluids such as serum, urine, hydrothorax, etc. (23).
Utilizing the previously prepared protein and antibody in our laboratory, we developed a Survivin ELISA. Through optimization of different conditions, intra-assay precision is 8.39%, inter-assay precision is 8.57% and MDD is 0.0625 ng/mL. The level of Survivin in bladder cancer patients is apparently higher than healthy people. When OD450 is 0.09, it can get the optimized cutoff and the sensitivity and specificity were 70.6% and 89.2% respectively. These data are similar to others reports (3,21). So these results confirmed that Survivin has good value in the diagnosis of bladder cancer. However, urine Survivin may not be a good marker for bladder cancer prognosis from the correlation analysis of clinicopathological characteristics and OD450 detected by Survivin ELISA. More samples and further follow-up are still needed to explore its prognosis value. In summary, our experiment supplied a base to develop a commercial usage of Survivin ELISA for detecting bladder cancer in the next years.
Acknowledgements
This work was supported by the National High Technology Research Development Plan (No.2012AA02A504), the Capital Healthy Development Special Fund (No.2011-1009-03) and the Capital Laboratory Medicine Clinical Characteristic Fund (No.Z121107005112004).
Disclosure: The authors declare no conflict of interest.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Droller MJ. Bladder cancer: state-of-the-art care. CA Cancer J Clin 1998;48:269-84. [PubMed]
- Shariat SF, Casella R, Khoddami SM, et al. Urine detection of survivin is a sensitive marker for the noninvasive diagnosis of bladder cancer. J Urol 2004;171:626-30. [PubMed]
- Lahme S, Bichler KH, Feil G, et al. Comparison of cytology and nuclear matrix protein 22 (NMP 22) for the detection and follow-up of bladder-cancer. Adv Exp Med Biol 2003;539:111-9. [PubMed]
- Konety BR. Molecular markers in bladder cancer: a critical appraisal. Urol Oncol 2006;24:326-37. [PubMed]
- Kenney DM, Geschwindt RD, Kary MR, et al. Detection of newly diagnosed bladder cancer, bladder cancer recurrence and bladder cancer in patients with hematuria using quantitative rt-PCR of urinary survivin. Tumour Biol 2007;28:57-62. [PubMed]
- Margulis V, Lotan Y, Shariat SF. Survivin: a promising biomarker for detection and prognosis of bladder cancer. World J Urol 2008;26:59-65. [PubMed]
- Wang Y, Zhang QY, Wang YM, et al. Cloning of Survivin gene and preparation its monoclonal antibodies as well as checking Survivin expression in liver carcinoma cells. Zhonghua Jian Yan Xue Za Zhi 2006;29:258-62.
- Altieri DC. Molecular circuits of apoptosis regulation and cell division control: the survivin paradigm. J Cell Biochem 2004;92:656-63. [PubMed]
- Sugahara K, Uemura A, Harasawa H, et al. Clinical relevance of survivin as a biomarker in neoplasms, especially in adult T-cell leukemias and acute leukemias. Int J Hematol 2004;80:52-8. [PubMed]
- Wang TT, Qian XP, Liu BR. Survivin: potential role in diagnosis, prognosis and targeted therapy of gastric cancer. World J Gastroenterol 2007;13:2784-90. [PubMed]
- Derin D, Soydinç HO, Guney N, et al. Serum levels of apoptosis biomarkers, survivin and TNF-alpha in nonsmall cell lung cancer. Lung Cancer 2008;59:240-5. [PubMed]
- Kami K, Doi R, Koizumi M, et al. Survivin expression is a prognostic marker in pancreatic cancer patients. Surgery 2004;136:443-8. [PubMed]
- Ryan BM, Konecny GE, Kahlert S, et al. Survivin expression in breast cancer predicts clinical outcome and is associated with HER2, VEGF, urokinase plasminogen activator and PAI-1. Ann Oncol 2006;17:597-604. [PubMed]
- Kishi H, Igawa M, Kikuno N, et al. Expression of the survivin gene in prostate cancer: correlation with clinicopathological characteristics, proliferative activity and apoptosis. J Urol 2004;171:1855-60. [PubMed]
- Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene 2003;22:8581-9. [PubMed]
- Yamamoto H, Ngan CY, Monden M. Cancer cells survive with survivin. Cancer Sci 2008;99:1709-14. [PubMed]
- Wu YK, Chen KT, Kuo YB, et al. Quantitative detection of survivin in malignant pleural effusion for the diagnosis and prognosis of lung cancer. Cancer Lett 2009;273:331-5. [PubMed]
- Kleinberg L, Flørenes VA, Silins I, et al. Nuclear expression of survivin is associated with improved survival in metastatic ovarian carcinoma. Cancer 2007;109:228-38. [PubMed]
- Moussa O, Abol-Enein H, Bissada NK, et al. Evaluation of survivin reverse transcriptase-polymerase chain reaction for noninvasive detection of bladder cancer. J Urol 2006;175:2312-6. [PubMed]
- Wang H, Xi X, Kong X, et al. The expression and significance of survivin mRNA in urinary bladder carcinomas. J Cancer Res Clin Oncol 2004;130:487-90. [PubMed]
- Wild DG. The immunoassay handbook. New Jersey (USA): Humana publishers, 2005.
- Leng SX, McElhaney JE, Walston JD, et al. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci 2008;63:879-84. [PubMed]