Value of pre-treatment biomarkers in prediction of response to neoadjuvant endocrine therapy for hormone receptor-positive postmenopausal breast cancer
Introduction
Endocrine therapy is a standard adjuvant treatment for endocrine-responsive breast cancer. Estrogen receptor (ER) positivity and/or progesterone receptor (PgR) positivity are indications for endocrine therapy (1,2). However, there are factors other than receptor status that can influence endocrine responsiveness. In the P024 trial, the clinical response rate was less than 70% in strongly hormone receptor (HR)-positive breast cancers with 4 months of neoadjuvant letrozole treatment (3); the IMPACT trial had similar results (4). Recent studies have demonstrated that a four-marker immunohistochemical (IHC) panel [ER, PR, human epidermal growth factor receptor 2 (Her-2), and Ki67], may have the ability to guide adjuvant therapy and predict long-term outcomes for HR-positive breast cancers (5). And the four markers were used to surrogate definitions of intrinsic subtypes of breast cancer in recent guidelines (2). However, the correlations between the four markers and responses to neoadjuvant endocrine therapy (NET) were still controversial.
NET provides a unique opportunity to assess tumor responses to treatment and to select molecular biomarkers that may be able to predict responses. However, there remains uncertainty regarding the standard classification of responses to NET.
The aim of this study was to determine the predictive ability of ER, PgR, Her-2 and Ki67 for responses to NET for HR-positive postmenopausal breast cancer. Furthermore, the associations between ER, PgR, Her-2 and Ki67 expression levels and responses to NET as well as the correlation between the responses classified in different systems were investigated.
Patients and methods
Patients
Eligible cases were retrospectively screened from the Breast Cancer Center of the Peking University Cancer Hospital & Institute, database using the following criteria: postmenopausal T1-3N0-1M0 invasive breast cancer, strongly HR-positive cancer (more than 50% of tumor cells positive for ER or PgR), and intention to receive endocrine therapy alone as the adjuvant treatment (Arimidex 1 mg/d for 16 weeks) followed by surgery.
IHC analysis of ER, PR, Her-2 and Ki67
IHC analyses of pre- and post-treatment biomarkers were centrally repeated. Four- percent-formalin-fixed, paraffin-embedded pre-treatment needle biopsy and post- treatment surgical specimens were collected and used to prepare 4 μm sections. Whole tumor sections were incubated with the specific primary mouse monoclonal antibodies to ER (clone SP1), PgR (clone 1E2) or Her-2 (clone 4B5) (all from Ventana, Arizona, USA), while Ki67 labeling index was assessed using mouse monoclonal antibody MIB-1 (Dako, Glostrup, Denmark). Avidin-biotin complex was also purchased from Ventana Medical Systems (Arizona, USA). A Benchmark XT Staining Instrument (Ventana Medical Systems, Inc., Arizona, USA) was used for automated immunostaining.
Stained slides were digitized with an Ariol MB-8 automatic image analysis system (Applied Imaging Inc., San-Jose, California, USA). All slides were scanned at 5× objective magnification, and representative areas of invasive tumors were selected by an experienced pathologist. Then, all selected areas were scanned once more at 20× objective magnification, and the intensity of positively stained nuclei and membranes, the completeness of the positively stained membranes and the percentage of positive cells of all stained sections were obtained automatically.
ER and PgR were scored as percentages of stained tumor cells (6,7). The cutoff for ER or PgR positivity was ≥10% positive cells. Her-2 IHC was scored by the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) criteria, which assess the intensity and completeness of membrane staining (7).
A score of 0/+ was considered negative, and +++ was considered over-expression. The Ki67 index was the percentage of Ki67-positive cancer nuclei. Low proliferation was defined as Ki67 staining of <15% tumor cells (8).
Evaluation of response
The pathological responses of primary tumors were intensively evaluated by two dedicated pathologists according the Miller and Payne grading system (9). The grades were defined as follows: Grade 1 (G1): no change or some alteration to individual malignant cells, but no reduction in overall cellularity; Grade 2 (G2): a minor loss of tumor cells, but the overall cellularity is still high, up to 30% loss; Grade 3 (G3): an estimated reduction in tumor cells between 30% and 90%; Grade 4 (G4): a marked disappearance of tumor cells such that only small clusters or widely dispersed individual cells remain; greater than 90% loss of tumor cells; Grade 5 (G5): no malignant cells identifiable in sections from the site of the tumor, only vascular fibroelastotic stroma remains, often containing macrophages. Ductal carcinoma in situ (DCIS) may be present. In this study, G5 was defined as pathological complete response (pCR), G3/4 were defined as pathological partial response, and G1/2 were defined as no response. A post-treatment Ki67 index value of ≤1% was defined as cell cycle complete response (cell cycle CR), a decrease in the Ki67 index with an index value of >1% after therapy was defined as cell cycle partial response, and no decrease was defined as no response (10).
Statistical analysis
Pearson’s chi-square test was used to test the associations between pre-treatment ER/PgR/Her-2/Ki67 status and response. Spearman correlations and Kappa tests were used to analyze the correlation between the responses classified using different systems. Spearman correlation coefficient <0.4 was considered a low correlation, ≥0.4 and <0.7 was considered a moderate correlation, and ≥0.7 was considered a high correlation. Kappa value <0.4 was considered a low consistency, ≥0.4 and <0.7 was considered a moderate consistency, and ≥0.7 was considered a high consistency. Multivariate logistic regression analysis was used to calculate the combined index including ER, PR, Ki67 (as continuous variables) and Her-2 expression (0, +, ++ vs. +++). Receiver operating characteristic (ROC) curves were used to determine whether parameters can predict responses, and the area under curves (AUC) >0.75 was considered as the parameter capable of predicting the response.
Results
Patients characteristics
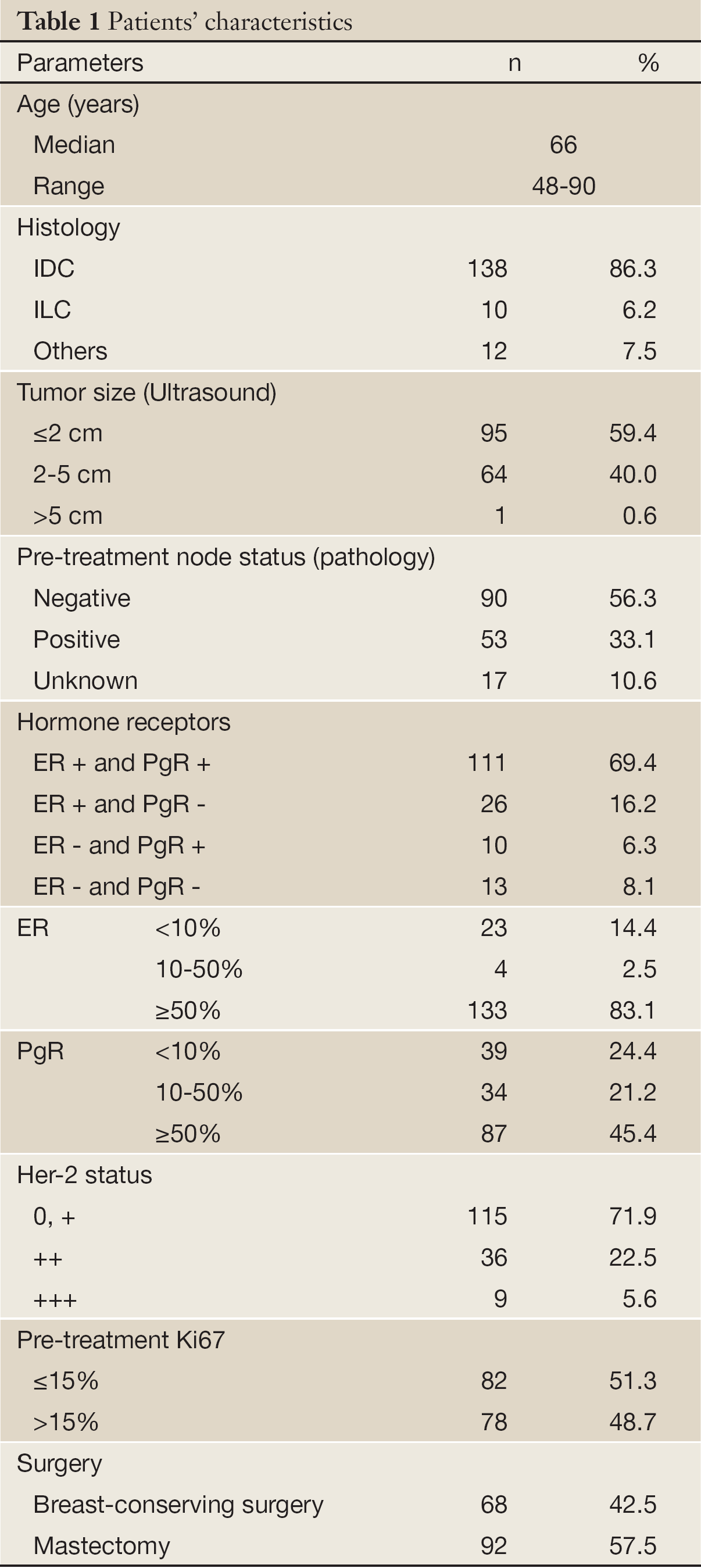
From October 2004 to March 2010, a consecutive cohort of 174 T1-3N0-1M0 primary breast cancer cases met the selection criteria. Fourteen cases were excluded from analysis because the specimens were not large enough to prepare new slides. Finally, data for 160 patients were analyzed retrospectively. The characteristics of the patients are summarized in Table 1.
Full Table
Evaluation of response
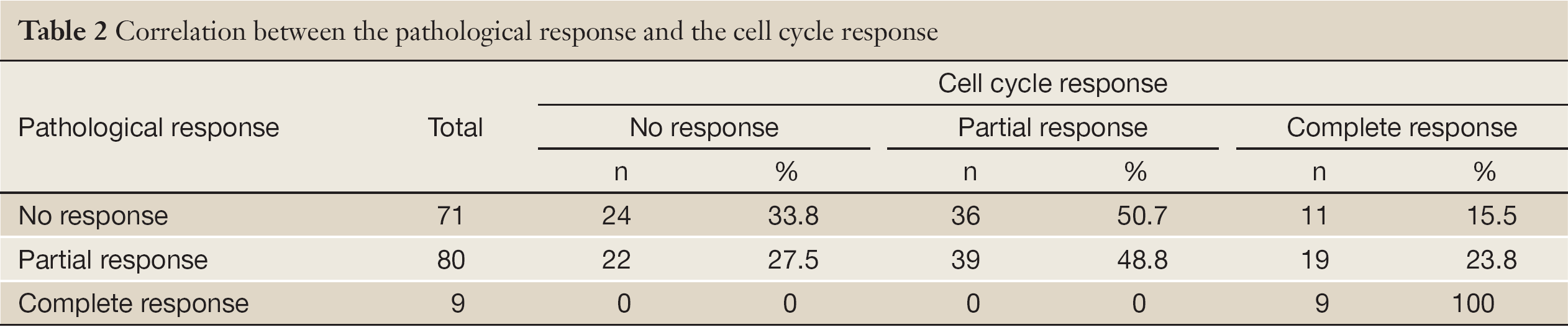
The pCR rate was 5.6% (9/160), the pathological partial response rate was 50.0% (80/160), and the pathological no response rate was 44.4% (71/180). The cell cycle complete response rate was 24.4% (39/160), the cell cycle partial response rate was 46.9% (75/160), and the cell cycle no response rate was 28.7% (46/160, Table 2).
Full Table
The correlation between the pathological response and cell cycle response was low (Spearman correlation coefficient =0.241, P<0.001; Kappa value =0.119, P=0.032; Table 2).
Associations between pre-treatment ER/PgR/Her-2/Ki67 status and responses
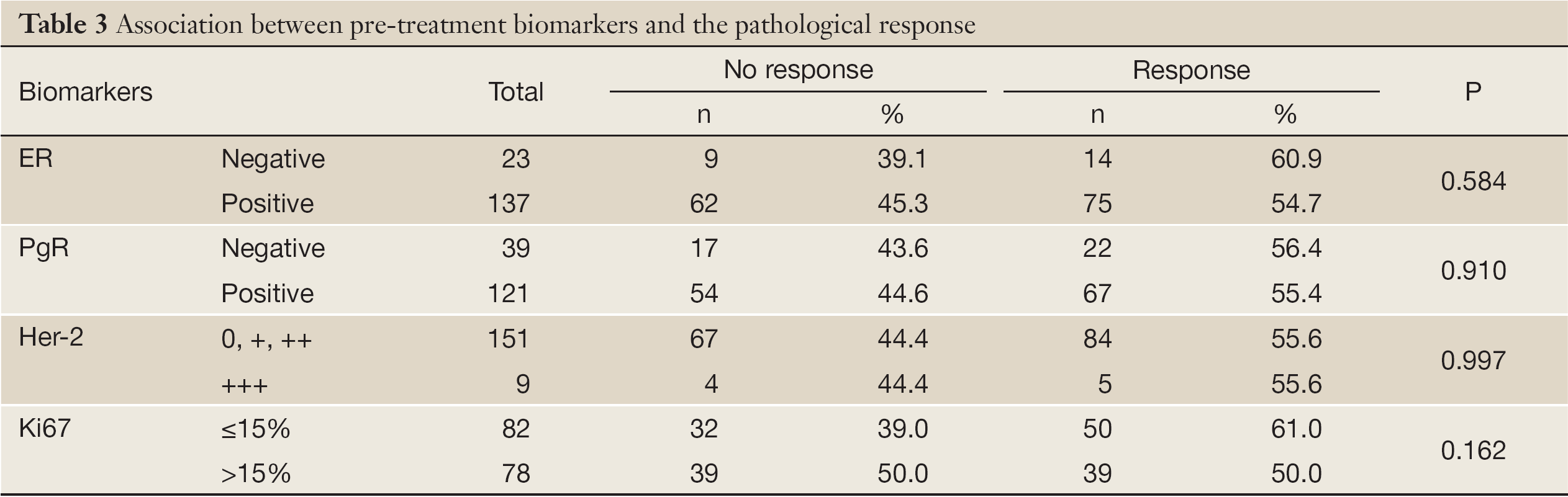
The pathological response was not significantly associated with pre-treatment ER status, PgR status, Her-2 status, and Ki67 index (Table 3). The cell cycle response was significantly associated with pre-treatment ER status, PgR status, Her-2 status, and the Ki67 index (Table 4).
Full Table
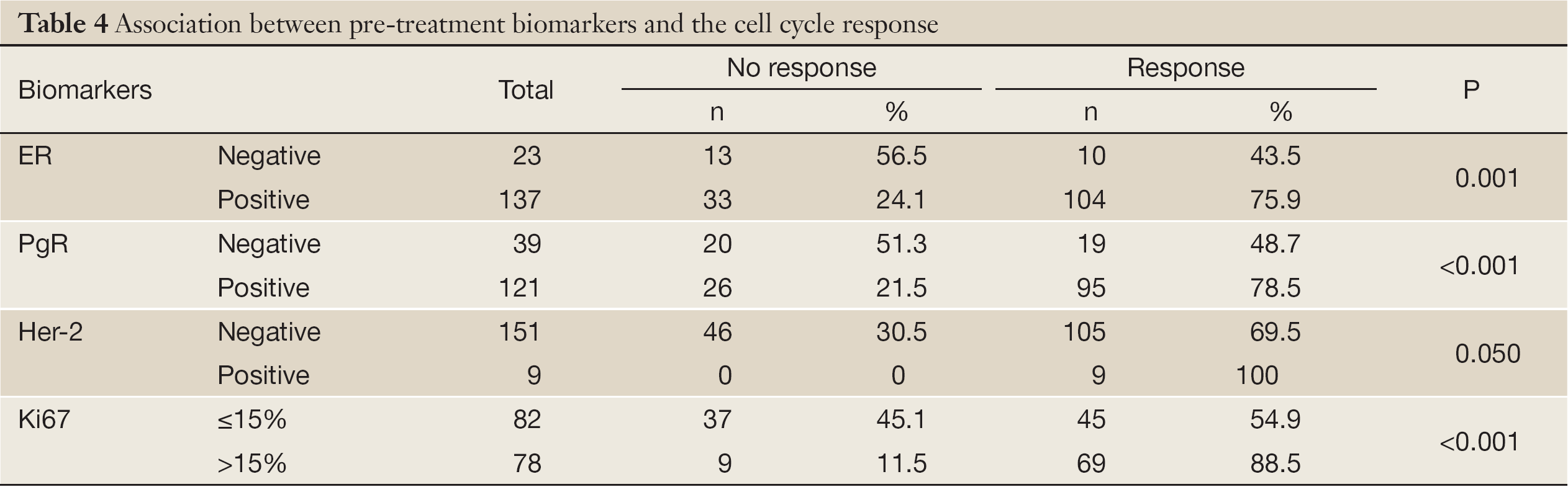
Full Table
The PgR status was significantly associated with the cell cycle response in ER-positive patients (P=0.016). There were more cases with decreases in the Ki67 index among PgR-positive tumors than among PgR-negative tumors. Among ER-negative patients, there were more cases with decreases in the Ki67 index than among ER-positive patients, but this difference was not statistically significant (57.1% vs. 30.8%, P=0.251, Table 5).
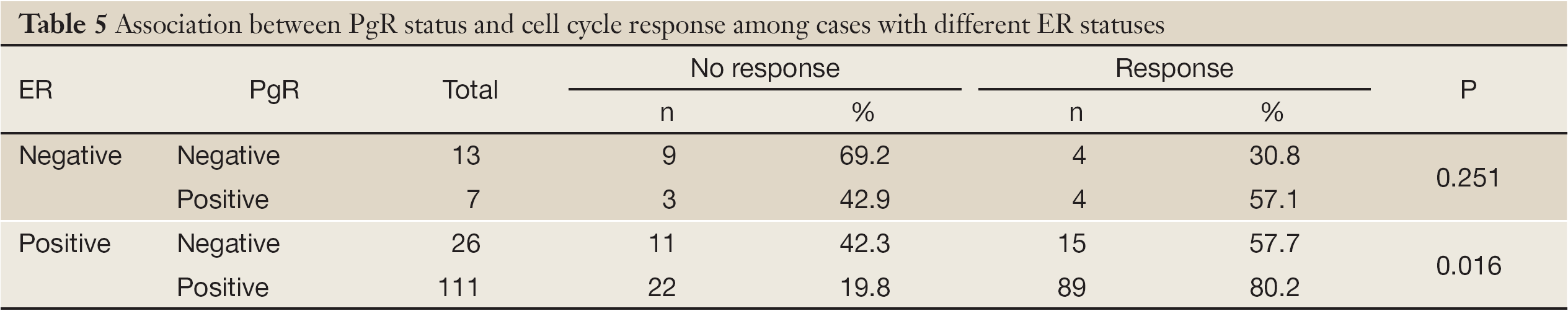
Full Table
Predictive value of pre-treatment ER/PgR/Her-2/Ki67 expression levels for the cell cycle response
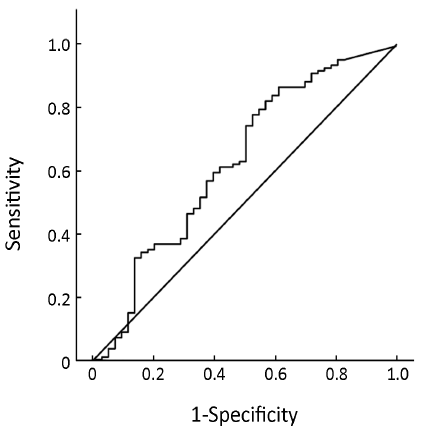
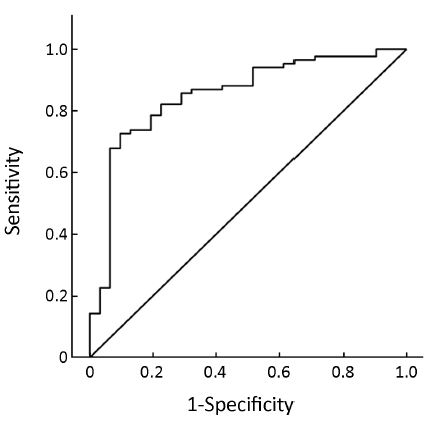
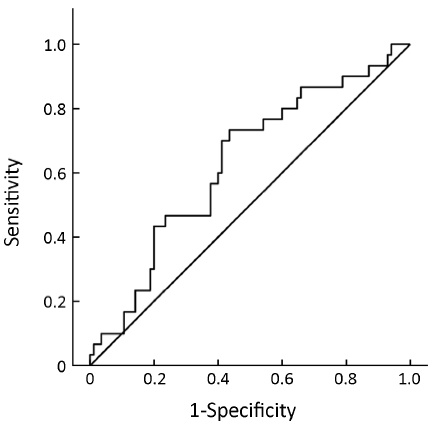
Pre-treatment ER expression was not able to predict the cell cycle response after 16 weeks of neoadjuvant treatment with anastrozole for postmenopausal breast cancer [AUC =0.634, 95% confidence interval (95% CI), 0.534-0.735, P=0.008, Figure 1] or the combined index of pre-treatment ER and PgR (AUC =0.684, 95% CI, 0.591-0.776, P<0.001, Figure 2). However, the combined index including pre-treatment ER/PgR/Her-2/Ki67 expression levels may predict cell cycle responses (AUC =0.830, 95% CI, 0.759-0.902, P<0.001, Figure 3). In Her-2-negative tumors (0 or +, n=115), the combined index including pre-treatment ER/PgR/Ki67 levels may predict the cell cycle response (AUC =0.850, 95% CI, 0.786-0.932, P<0.001, Figure 4) but was unable to predict the cell cycle CR (AUC =0.628, 95% CI, 0.515-0.743, P=0.036, Figure 5).
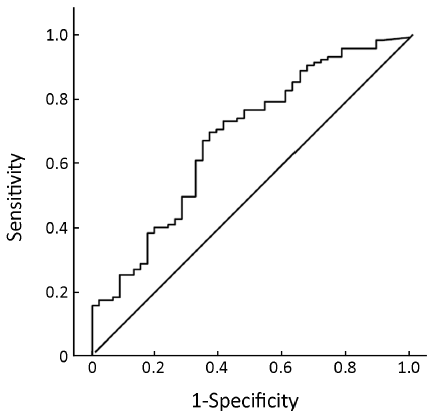
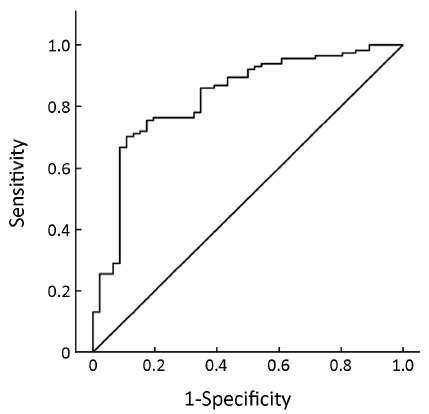
Discussion
NET is an excellent platform for predictive factor study, but a standard classification of responses has not been established. In previous studies, the clinical response has been most commonly used. However, the clinical response has the potential for factitious bias and is not reproducible. In addition, the clinical response is difficult to assess accurately, especially in small cancers (11). Finally, the clinical response might not be related to survival (12).
Recent studies suggested that the pathological response may be a predictive factor for the long-term outcome following NET (13). And pathological response was generally used as an evaluation of response to primary endocrine therapy (14,15). Because pCR, a validated surrogate endpoint for neoadjuvant chemotherapy study, was uncommon after NET (3,4,16), we choose the Miller and Payne system to grade the residual invasive tumor and classify the pathological response to NET in this study.
Previous studies have suggested that changes in the Ki67 index after a period of endocrine therapy may be associated with treatment benefit and long-term outcomes (10,17,18). These data suggested that decrease of in the Ki67 index after a period of anti-hormone treatment could, at least to some extent, reflect the anti-proliferative effects of endocrine therapy and might be a candidate indicator of benefit from endocrine therapy. Furthermore, cell cycle CR, which represents a superior suppression of proliferation, may be able to determine if a tumor is highly sensitive to endocrine therapy and to select eligible patients to safely avoid the use of adjuvant chemotherapy (10,12).
In this study, the correlation between the pathological response and the cell cycle response was low. In addition, the pre-treatment ER status, PgR status, Her-2 status, and Ki67 index were significantly associated with the cell cycle response but not the pathological response. This difference may be the result of the different mechanisms of these two evaluations. The pathological response was graded according to the changes in tumor cellularity after treatment, but the cell cycle response was assessed according to the inhibition of tumor proliferation. The main mechanism of breast cancer chemotherapy is cytotoxic action on tumor cells, and its key feature is a reduction of tumor cellularity (10). This fact may be the reason why the pathological response was validated to reflect the efficacy of neoadjuvant chemotherapy. However, the mechanism of endocrine therapy for breast cancer is primarily the inhibition of tumor cell proliferation without a cytotoxic effect. It is reasonable to hypothesize that the cell cycle response may be the better way to evaluate the efficacy of NET than the pathological response is.
The cutoff point for ER positivity has long been considered 10% positive cells (1). Recently, a 1% cutoff for ER positivity was set by the ASCO/CAP guidelines (6,19). In this study, the ER status was significantly associated with the cell cycle response, and a greater number of ER-positive (≥10% cells positive) cases showed cell cycle responses than ER-negative cases. However, 30.8% (4/13) of ER-negative tumors showed a cell cycle response after 16 weeks of NET in PgR-negative cases. This result implies that the 10% cutoff might not be suitable for ER positivity. Our cohort was not large enough to address in this issue.
In the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) overview analysis, PgR played no role in ER-positive tumors in predicting the benefit of adjuvant tamoxifen therapy (20). In the ATAC trial, PgR may not be a predictive factor of the efficacy of endocrine therapy but was a prognostic factor for breast cancer (21,22). In the IMPACT study, Ki67 expression was reduced to a greater extent in PgR-positive tumors than in PgR-negative tumors after 2 weeks and 12 weeks of anastrozole neoadjuvant treatment (23). Our data demonstrate that the pre-treatment PgR status was significantly associated with the cell cycle response in our study group. There were more PgR-positive cases than PgR-negative cases that showed decreases in the Ki67 index after treatment, regardless of ER status, but our cohort was too small to observe a significant difference in ER-negative cases.
Although HR positivity (ER and/or PgR-positive) is indications for endocrine therapy (1,2,24), factors other than receptor status can influence endocrine responsiveness. Maggie et al. demonstrated that a four-marker surrogate IHC panel (ER, PR, HER2, and Ki67) can independently predict long-team outcomes for HR-positive breast cancers (5). Cuzick J et al. also suggested that an amount of prognostic information contained in the four IHC assays (25). In this study, neither pre-treatment ER expression nor the combined index of pre-treatment ER and PgR were able to predict the cell cycle response to NET, but the combined index of pre-treatment ER, PgR, Her-2 and Ki67 expression levels could predict this response. This result implies that the combined use of the pre-treatment four biomarker expression levels, instead of pre-treatment HR expression levels alone, may predict which patients could benefit from endocrine therapy.
Currently, it is difficult in clinical practice to distinguish which HR-positive, Her-2-negative breast cancers can be treated successfully with endocrine therapy, obviating the need for adjuvant chemotherapy (26). In this study, although the combined index of pre-treatment ER, PgR and Ki67 could predict the cell cycle response in HR-positive, Her-2-negative tumors, it was difficult to predict cell cycle CR, which identified tumors as highly sensitive to endocrine therapy in this sub-group. This result suggested that combined IHC analysis of ER, PgR, Her-2 and Ki67 may identify breast cancer patients who would benefit from endocrine therapy as mentioned above, but it is difficult to identify patients who could benefit sufficiently from endocrine therapy to obviate the need for chemotherapy.
In summary, our study demonstrated that the correlation between the pathological response and the cell cycle response to NET was low; the combined use of pre-treatment ER, PgR, Her-2 and Ki67 expression levels, instead of pre-treatment HR expression levels alone, may predict the cell cycle response after 16 weeks of neoadjuvant anastrozole treatment. In contrast, it was difficult to predict cell cycle CR in HR-positive, Her-2-negative tumors.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: International expert consensus on the primary therapy of early breast cancer. Ann Oncol 2005;16:1569-83. [PubMed]
- Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Thearpy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47. [PubMed]
- Ellis MJ, Coop A, Singh B, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: Evidence from a phase III randomized trial. J Clin Oncol 2001;19:3808-16. [PubMed]
- Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108-16. [PubMed]
- Cheang MC, Chia SK, Voduc D, et al. Ki67 index, Her2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736-50. [PubMed]
- Hammond ME, Hayes DF, Wolff AC, et al. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract 2010;6:195-7. [PubMed]
- Hammond ME, Hayes DF, Wolff AC, et al. Clinical Notice for American Society of Clinical Oncology-College of American Pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. J Clin Oncol 2011;29:e458. [PubMed]
- Jalava P, Kuopio T, Juntti-Patinen L, et al. Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology 2006;48:674-82. [PubMed]
- Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. The Breast 2003;12:320-7. [PubMed]
- Ellis MJ, Coop A, Singh B, et al. Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res 2003;63:6523-31. [PubMed]
- Ishikawa T, Shimizu D, Kito A, et al. Breast cancer manifested by hematologic disorders. J Thorac Dis 2012;4:650-4. [PubMed]
- Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380-8. [PubMed]
- Akashi-Tanaka S, Omatsu M, Shimizu C, et al. Favorable outcome in patients with breast cancer in the presence of pathological response after neoadjuvant endocrine therapy. Breast 2007;16:482-8. [PubMed]
- Tan MC, Al Mushawah F, Gao F, et al. Predictors of complete pathological response after neoadjuvant systemic therapy for breast cancer. Am J Surg 2009;198:520-5. [PubMed]
- Toi M, Saji S, Masuda N, et al. Ki67 index changes, pathological response and clinical benefits in primary breast cancer patients treated with 24 weeks of aromatase inhibition. Cancer Sci 2011;102:858-65. [PubMed]
- Mlineritsch B, Tausch C, Singer C, et al. Exemestane as primary systemic treatment for hormone receptor positive post-menopausal breast cancer patients: a phase II trial of the Austrian Breast and Colorectal Cancer Study Group (ABCSG-17). Breast Cancer Res Treat 2008;112:203-13. [PubMed]
- Dowsett M, Smith IE, Ebbs SR, et al. Short-Term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res 2005;11:951s-8s. [PubMed]
- Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 2007;99:167-70. [PubMed]
- Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 2010;134:907-22. [PubMed]
- Dowsett M, Houghton J, Iden C, et al. Benefit from adjuvant tamoxifen therapy in primary breast cancer according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol 2006;17:818-26. [PubMed]
- Dowsett M, Cuzick J, Wale C, et al. Retrospective analysis of time to recurrence in the ATAC trial according to hormone receptor status: an hypothesis-generating study. J Clin Oncol 2005;23:7512-7. [PubMed]
- Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135-41. [PubMed]
- Dowsett M, Ebbs SR, Dixon JM, et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: Influence of hormonal status and HER-2 in breast cancer—A study from the IMPACT trialists. J Clin Oncol 2005;23:2477-92. [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [PubMed]
- Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011;29:4273-8. [PubMed]
- Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann Oncol 2009;20:1319-29. [PubMed]