Retreatment of a patient who presented with synchronous multiple primary colorectal carcinoma: report of a case
Background
Multiple primary cancers are defined as two or more cancers detected in the same or other organs occurring either synchronously or metachronoulsy (1). Synchronous multiple primary colorectal cancers are those that follow a previous cancer within a definite interval of usually 6 months or 1 year. Preoperative detection of synchronous multiple primary cancers is very important when planning treatments. Along with increasing incidence of colorectal carcinomas and prolonged survival after radical resection, the number of patients with multiple primary colorectal cancer have increased gradually. Generally, synchronous carcinoma does not affect prognosis if it is recognized and treated in time, but if ignored, it may turn into more advanced metachronous cancer. Therefore, after colorectal cancer surgery, occurrence of metachronous carcinoma is regarded as an serious problem (2). Due to influence of doctor’s recognition degree, missed and error diagnosis or inaccurate treatment appears frequently. We report a case of synchronous multiple primary colorectal cancer who had undergone multiple operations that was treated in our center.
Case presentation
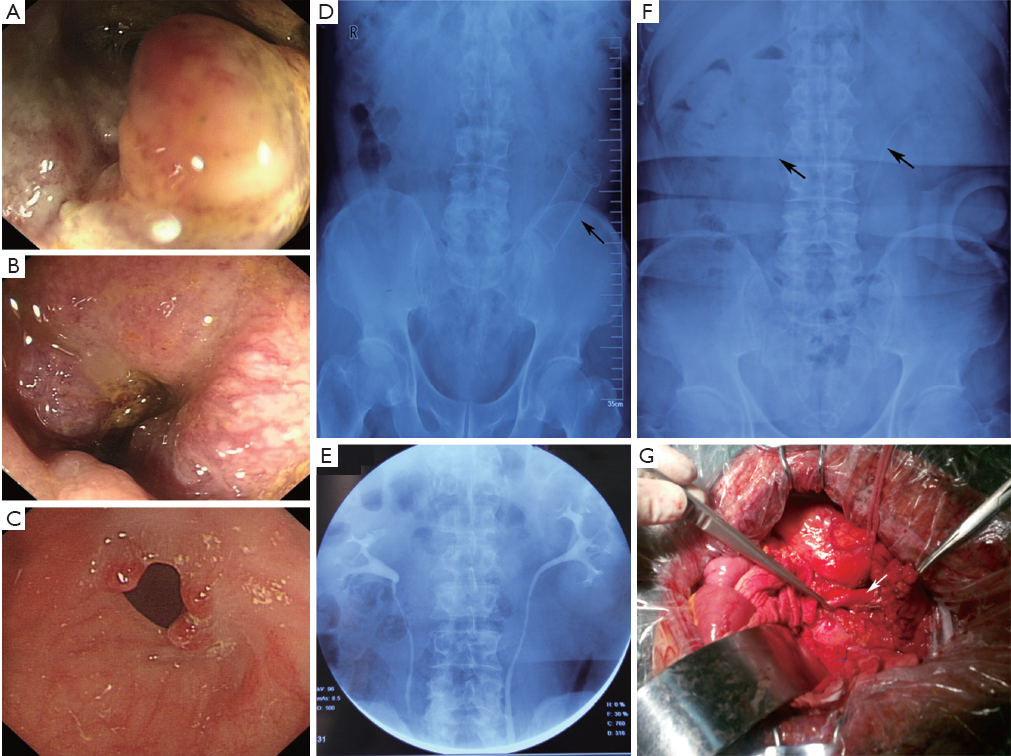
A 60-year-old-man was admitted to the general surgery department on November 1, 2010 due to changes in bowel habits with no personal or familial history of any specific disease. No loss of appetite and general weakness. No fever was noted at home. Physical examination showed no remarkable abdominal abnormality. Laboratory test or chest and abdominal radiography displayed an intrasigmoid occupied lesion and pelvic lymphadenectasis. Colonoscopy found that a lesion located in rectum within 9 cm of the anal verge, occupied four-fifths of the rectal circumference and the endoscope was unable to pass through. Pathologic specimens were obtained in colonoscopy procedure, and the histologic report showed adenocarcinoma. He underwent radical resection of rectal and sigmoid carcinoma on November 5, 2010 as intraoperative palpation discovered a new lesion located in sigmoid. Additionally, prophylactic ileostomy and appendectomy were also carried out. Postoperative pathology showed rectal adenocarcinoma, stage was T4aN0M0 (staging by the American Joint Committee on Cancer staging system, 7th edition); mucinous carcinoma of the sigmoid colon, T4aN0M0; chronic appendicitis.
From November 20, 2010 to February 2011, the patient had received three cycles of FOLFOX6 chemotherapy. Then, adjuvant radiotherapy [conventional radiotherapy: 40 Gy in 20 fractions; three dimensional conformal radiotherapy (3DCRT): 20 Gy in 10 fractions] were administered. Treatment was well tolerated.
Colonoscopy was administered again on March 22, 2011, and found that anastomotic inflammation in the rectum, a new obstructive lesion was located in the descending colon and the endoscope was unable to pass through, which was proved pathologically to be colonic mucinous carcinomas. The patient underwent left hemicolectomy and microadenomectomy of two cecum adenomas were detected by intraoperative colonscopy on the same day. The diagnosis of histopathology examination was colonic mucinous carcinomas (T4aN0M0) and colonic tubular adenoma with low-grade intraepithelial neoplasia. Subsequently, the patient received three cycles of FOLFOX6 chemotherapy again.
The patient got a colonoscopy for the third time for closure of loop ileostomy on July 2011, both anastomotic stenoses were found, and the endoscope was unable to pass through the proximal anastomosis. Metallic stent implantation and cecum polyp electrosection via enteroscope were performed on July 29. One month later, a colonoscopy was carried out and found that the stent migrated upward, it was removed then. After that, the endoscope was unable to pass through the proximal anastomosis again.
On November 1, 2011, the patient was admitted to our center in order to closure of loop ileostomy after establishment of the diagnosis as synchronous multiple primary colorectal carcinoma after operation and postoperative radiotherapy and chemotherapy, anastomotic stenosis, stomal prolapse, peristomal hernia and dermatitis. Two days before surgery, bilateral ureteral catheters were implanted. The patient was taken to the operating room for closure of loop ileostomy, descending colostomy and resection of the proximal anastomosis on November 10. During operation, extensive intraperitoneal adhesions and significant changes in anatomic structure were noted. The bilateral ureteral were attached to adjacent organs so tightly that surgeons only depend on ureteral stents to identify them. The patient recovered without incident and was discharged on postoperative day 7 (Figure 1).
Conclusions
The incidence of synchronous cancers are well known (0.6-1.4%) in patients with colorectal carcinoma (3). It being overlooked can lead to increased morbidity and the possibility of advanced staging of the cancer. Heald et al. (4) found that intraoperative palpation can miss up to 69% of the synchronous cancers. Therefore, full preoperative colonic evaluation with colonoscopy as recommended by several studies (5,6) is warranted. However, approximately 8% to 29% of patients with colorectal cancer present with acute malignant colonic obstruction (7), which disenable colonoscopist to examine the whole large bowel. The recent advances in imaging technology provide greater accuracy for the preoperative evaluation of colorectal cancer (8). Computed tomography (CT), magnetic resonance imaging, and ultrasound have played an important role in the preoperative staging and postoperative surveillance of colon cancer. However, the unique contribution of intraoperative enteroscopy is irreplaceable, especially for those with malignancy intestinal obstruction. We utilize a variety of techniques in combination in order to prevent the missing of synchronous cancers. In our center, colonoscopy combined with CT three-dimensional imaging is a routine examination for preoperative patient, which could improve the accuracy of diagnosis.
It is still the subject of controversy that the proximal fecal diversion—achieved either by a loop colostomy or a loop ileostomy—is performed for prevention of an anastomotic leakage after low anterior resection for rectal cancer, which is one of the most important surgical complications causing morbidity and mortality. It is not clear whether fecal diversion has any impact on anastomotic leakage rate in general (9), although it is widely performed in China, especially in southern China. Because construction of a stoma was largely left to the discretion of the surgeon, there is a general patient selection bias favoring surgery with a stoma. In fact, an anastomotic dehiscence is associated with identified general risk factors such as male gender, malnutrition, cardiovascular disease, steroid use, advanced age of the patient, obesity, and previous irradiation (10,11). We believe that strictly control its indication is the key to perform prophylactic ileostomy, because a number of points as following remain to be evaluated critically: First, a stoma will reduce the quality of life. Second, stoma placement itself is a source of morbidity (12), these complications may even lead to mortality after elective reversal of the stoma (13,14). Furthermore, the closure of a diverting stoma requires a second hospital stay and additional surgery and is accompanied by considerable patient management costs (15).
The updated National Comprehensive Cancer Network (NCCN) Guidelines recommend that after 45 Gy a tumor bed boost with a 2 cm of margin of 5.4-9.0 Gy in 3-5 fractions for postoperative radiation. This case received 20 Gy in 10 fractions 3D-CRT at a high dose, which may increase the risk of presenting anastomotic stricture. Radiation therapy also can cause massive intraperitoneal adhesion formation that increase operative difficulty. Moreover, peritoneal adhesions are almost inevitable after major abdominal operations. Ellis (16) found that the incidence increases up to 93% after multiple operations. Based on above reasons, we speculated that the dissection plane might be difficult to identify due to severe adhesion of the ureter and colon with the peripheral tissues. Preoperative placement of ureteral catheters has been recommended in this case. Although William et al. (17) found that prophylactic ureteral catheters do not assure the prevention of transmural ureteral injuries, it may assist in their immediate recognition, which is in accordance with our view.
In this case report, we described a mistake in diagnosis of synchronous multiple colorectal carcinoma, which resulted in repeat resection. It implies that the value of the pre- or intra-operative total colonoscopy in cases with colorectal cancer. In addition, whether subtotal proctocolectomy is also a choice, how intensive strategies of follow-up should be pursued and what surveillance means can be used for those patients at high risk of intestinal tumor worthy of further reflection.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ueno M, Muto T, Oya M, et al. Multiple primary cancer: an experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int J Clin Oncol 2003;8:162-7. [PubMed]
- Cali RL, Pitsch RM, Thorson AG, et al. Cumulative incidence of metachronous colorectal cancer. Dis Colon Rectum 1993;36:388-93. [PubMed]
- Benedetti M, Tinozzi FP, Dini S, et al. Synchronous and metachronous tumours of colon cancer. A review of 5 years of experience (1999-2004). Ann Ital Chir 2006;77:233-9. [PubMed]
- Heald RJ, Bussey HJ. Clinical experiences at St. Mark’s Hospital with multiple synchronous cancers of the colon and rectum. Dis Colon Rectum 1975;18:6-10. [PubMed]
- Maxfield RG. Colonoscopy as a routine preoperative procedure for carcinoma of the colon. Am J Surg 1984;147:477-80. [PubMed]
- Askew A, Ward M, Cowen A. The influence of colonoscopy on the operative management of colorectal cancer. Med J Aust 1986;145:254-5. [PubMed]
- Deans GT, Krukowski ZH, Irwin ST. Malignant obstruction of the left colon. Br J Surg 1994;81:1270-6. [PubMed]
- Filippone A, Ambrosini R, Fuschi M, et al. Preoperative T and N staging of colorectal cancer: accuracy of contrast-enhanced multi-detector row CT colonography--initial experience. Radiology 2004;231:83-90. [PubMed]
- Karanjia ND, Corder AP, Bearn P, et al. Leakage from stapled low anastomosis after total mesorectal excision for carcinoma of the rectum. Br J Surg 1994;81:1224-6. [PubMed]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- Chen F, Stuart M. The morbidity of defunctioning stomata. Aust N Z J Surg 1996;66:218-21. [PubMed]
- Wheeler MH, Barker J. Closure of colostomy--a safe procedure? Dis Colon Rectum 1977;20:29-32. [PubMed]
- Hallböök O, Matthiessen P, Leinsköld T, et al. Safety of the temporary loop ileostomy. Colorectal Dis 2002;4:361-4. [PubMed]
- Koperna T. Cost-effectiveness of defunctioning stomas in low anterior resections for rectal cancer: a call for benchmarking. Arch Surg 2003;138:1334-8; discussion 1339. [PubMed]
- Ellis H. The causes and prevention of intestinal adhesions. Br J Surg 1982;69:241-3. [PubMed]
- Bothwell WN, Bleicher RJ, Dent TL. Prophylactic ureteral catheterization in colon surgery. A five-year review. Dis Colon Rectum 1994;37:330-4. [PubMed]
