Recurrence risk model for esophageal cancer after radical surgery
Introduction
Although preoperative chemoradiotherapy for esophageal cancer has been regarded as a standard treatment (1), this approach at some centers is not always feasible. These patients who had undergone only radical surgery developed a high frequency of recurrence within one year after operation (2-4), and 3-year overall survival rate is less than 5% after salvage therapy (5). Therefore, the urgent problem was to identify what kind of clinicopathological information would present high risk of recurrence for these patients.
Rizk et al. (6) has recommended that to maximize 5-year survival, a minimum of 10 nodes be resected for T1 cancer, 20 nodes for T2 cancer, and 30 or more nodes for T3/T4 cancers. However, a retrospective observational study demonstrated that fewer than one-third of patients and fewer than 1 in 10 hospitals met the benchmark of examining at least 15 lymph nodes (7). The aim of the present study was, if the lymph node resection number cannot approach to the above requirements, which kind of patients should be considered to be given adjuvant therapy in case of high recurrence risk. Accordingly, a simple model has been tentatively presented to assess recurrence risk after only radical surgery for esophageal cancer.
Patients and methods
Construction of model
Three essential variables related to postoperative recurrence risk for esophageal cancer are the number of lymph node pathologically positive (n) (8,9), the number of lymph node removed (N) (10-12), and the value of pT stage (T) (13). As reported, the bigger the n or T is, the higher the risk of recurrence, while it’s the opposite for the N. For this reason, we take (n+T) and N as numerator and denominator, respectively, in terms of which we could calculate the value at risk (VaR) as follows: VaR = (n+T)/N.
Data collection
Approval was obtained from the Institutional Ethical Board to collect and review data. Patients were enrolled in the study just when they met all the following criteria: patients with middle and lower thoracic esophageal squamous cell carcinoma had undergone R0 (no residual microscopic disease) resection through a left thoracotomy, via 2-field lymphadenectomy in the mediastinum and upper abdomen between January 2005 and December 2006; no evidence of supraclavicular lymph node or distant organ metastases on preoperative evaluation; and no history of radiotherapy or chemotherapy before the first relapse.
Follow-up and definition of recurrence pattern
Clinical follow-up data were obtained by reviewing the patient clinical charts and by phone call contact with patients or their families. Discharged patients were followed up at the outpatient clinic at the 1st, 3rd and 6th months after the operation and every six months afterwards until the present review period or the date of the death if occurred. Lymph node recurrence was clinically diagnosed when finding enlarged supraclavicular or mediastinal lymph node compared with preoperative image, and some patients obtained histological confirmation by supraclavicular lymph node biopsy. Barium swallow examination was given to all follow-up patients in the first year, but routine fiberoptic endoscopy was not performed on asymptomatic patients. Only the patients who reported of dysphagia were investigated with fiberoptic endoscopy.
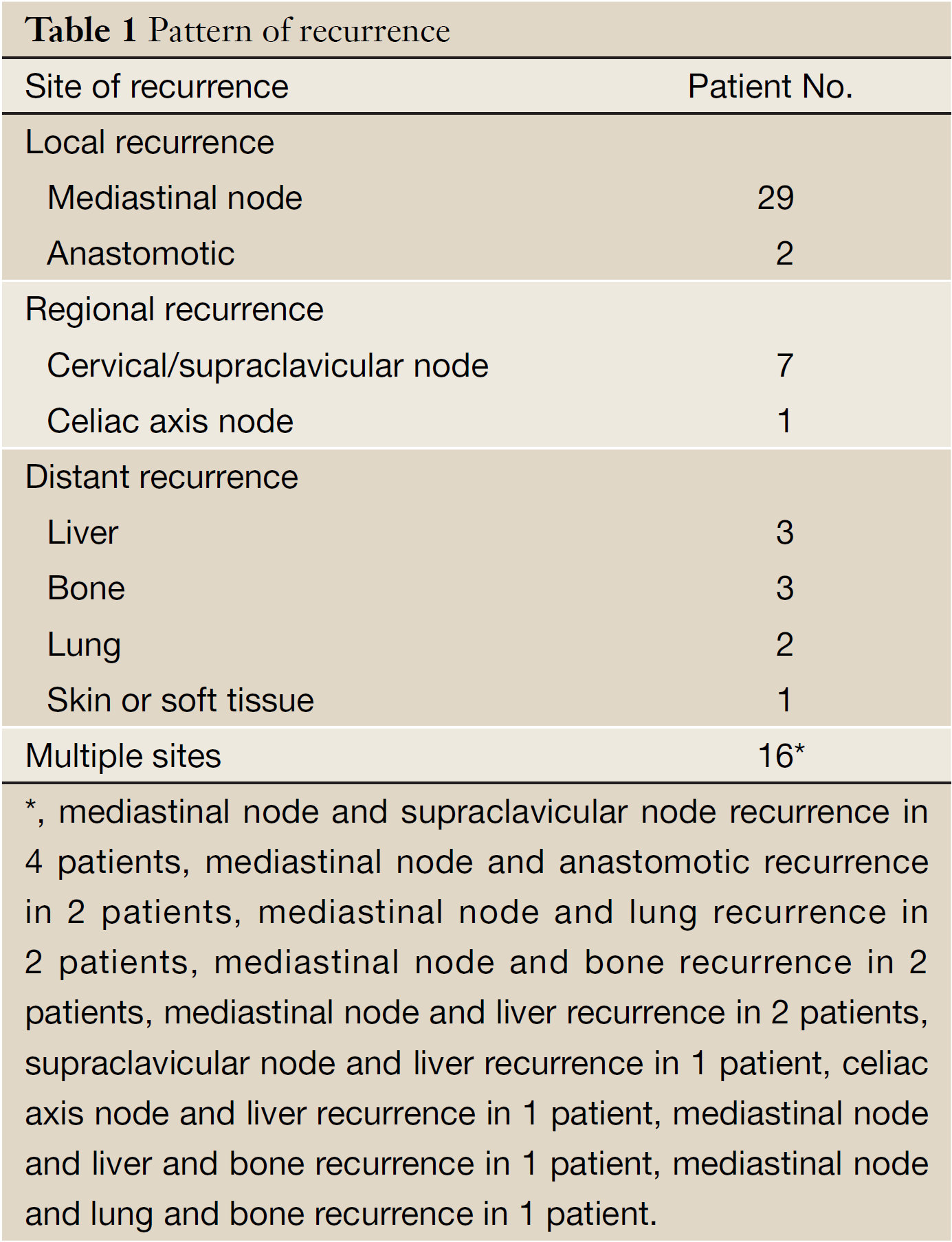
Local recurrence was defined as recurrence at an anastomotic site or within the mediastinum area. Regional recurrence was defined as recurrence at cervical/supraclavicular node or celiac axis node. Distant recurrence was defined as hematogenous metastasis to solid organs, pleura or peritoneum. Combined recurrence was defined as the simultaneous or more than one relapse.
Statistical analysis
Statistical analysis was performed by using SPSS version 9.0 (SPSS Inc., Chicago, IL, USA). Because the final outcome was unavailable after recurrence in some patients, the end point was disease-free survival (DFS, failure included local, regional, and distant failure, as well as death due to any cause), defined as the time from surgery to the first recurrence of esophageal cancer or censored at date of last follow-up. The cutpoint of VaR was inferred by stem-and-leaf plot, as well as by independent-samples t-test for recurrence-free time, further confirmed by crosstab chi-square test, univariate analysis and Cox regression analysis. The DFS was calculated using the Kaplan-Meier method and groups were compared with the log-rank test. Cox regression analysis was performed to judge independent prognostic factors. All statistical tests were performed two-sided, and P<0.05 was considered statistically significant.
Results
Clinicopathologic data at operation
A total of 164 eligible patients were extracted on the basis of the criteria mentioned above, of whom 128 patients were male and 36 were female, with age ranging from 41 to 82 years (median, 59 years). According to the 7th edition of the American Joint Committee on Cancer (AJCC) (13), 45 cases were staged as T1, 50 as T2, 65 as T3, and 4 as T4, respectively. The thoracic esophagus was divided into two portions: middle, 138 cases, and lower, 26 cases. In total 121 lymph nodes were pathologically positive (range, 1 to 8 nodes; positive ratio, 6.8%) among 1,770 resected lymph nodes (mean, 10.8 nodes per patient; range, 2 to 23). The cases with pathologically positive lymph nodes accounted for 26.2% (43/164). Pathologists in our hospital routinely examined dissected lymph nodes by one maximum cross-section with hematoxylin and eosin staining.
Pattern of recurrence
The median time of follow-up was 54.6 (range, 1 to 75) months. During the follow-up period, 64 patients (39.2%) had recurrence, leading to 57 patients dying of cancer and 7 patients living with recurrent disease. The pattern of recurrence is summary in Table 1. The 1-, 3-, and 5-year DFS after radical surgery was 82.9%, 66.9%, and 58.4%, respectively for all patients. The mean time of recurrence was 20.7 (range, 1 to 64) months.
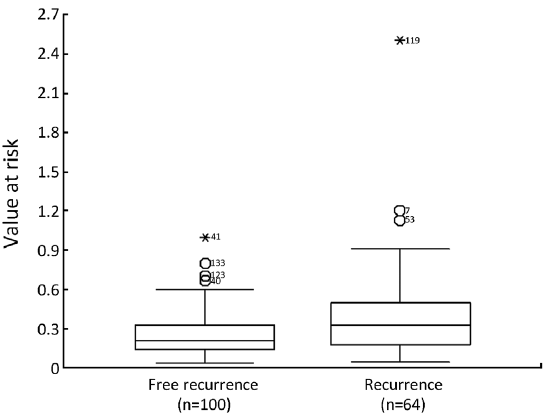
Cutpoint of VaR
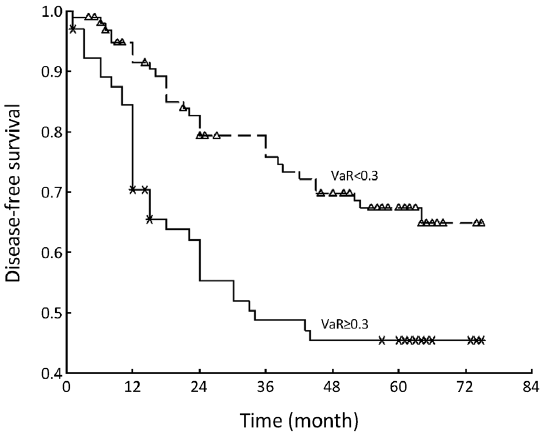
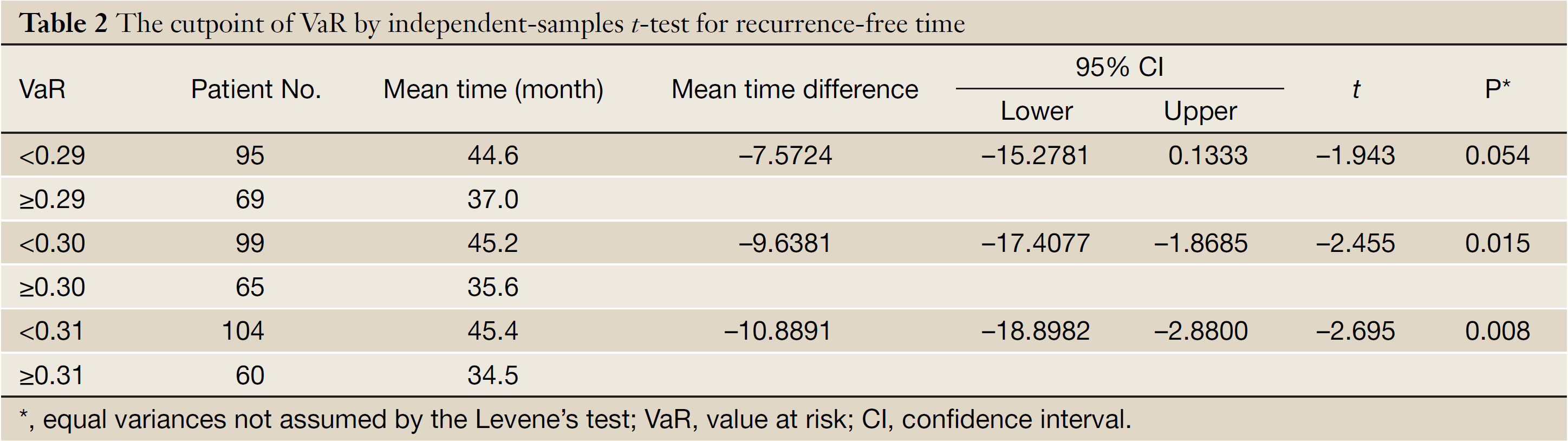
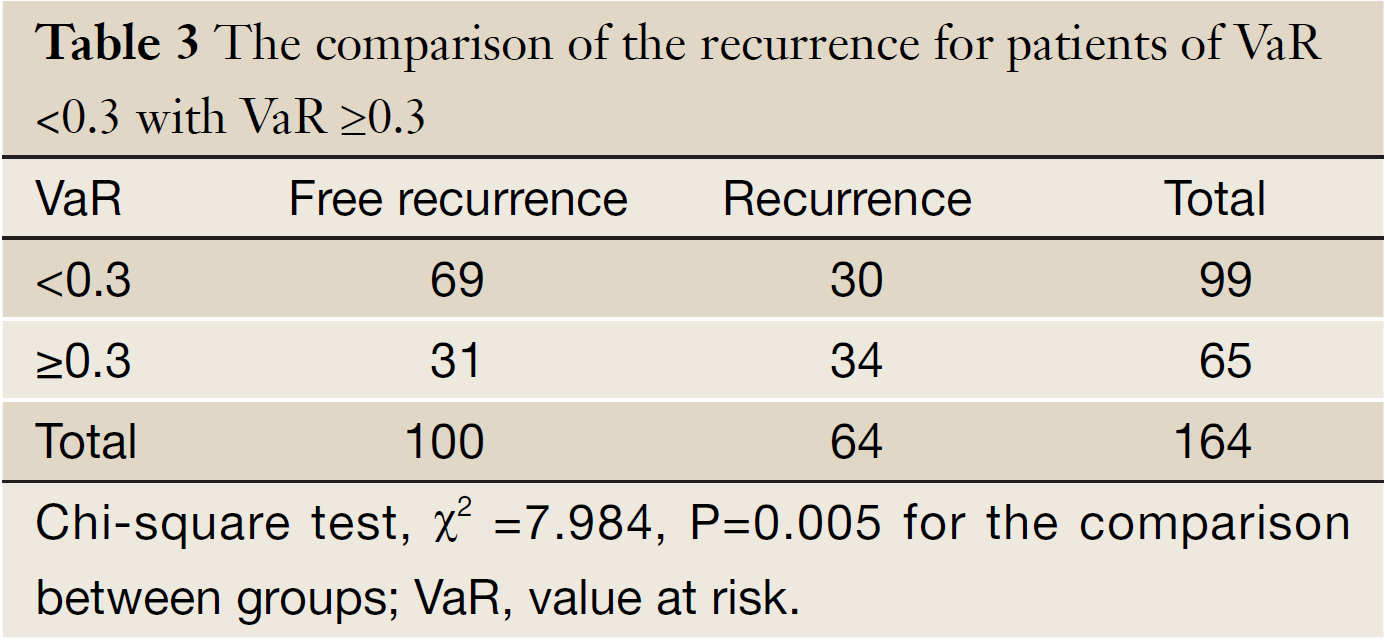
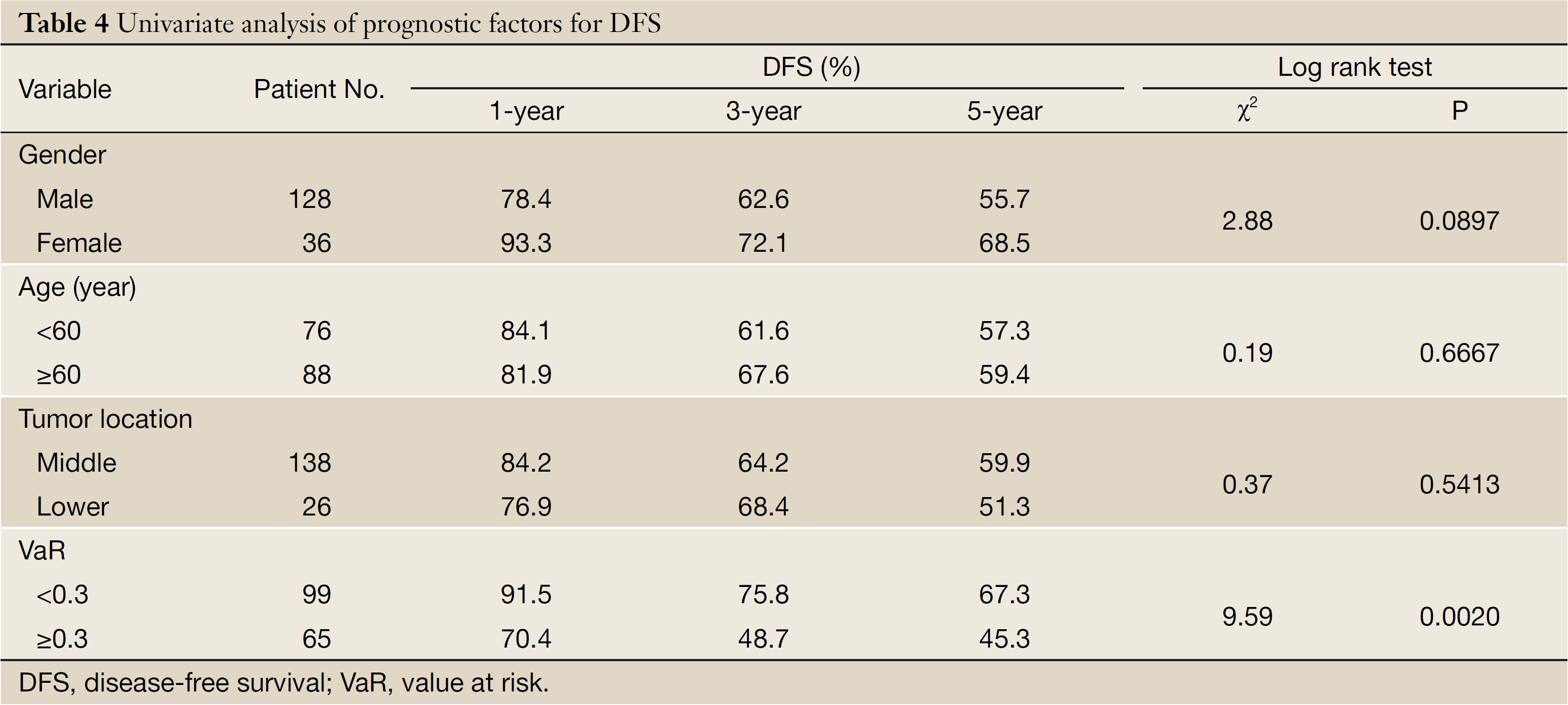
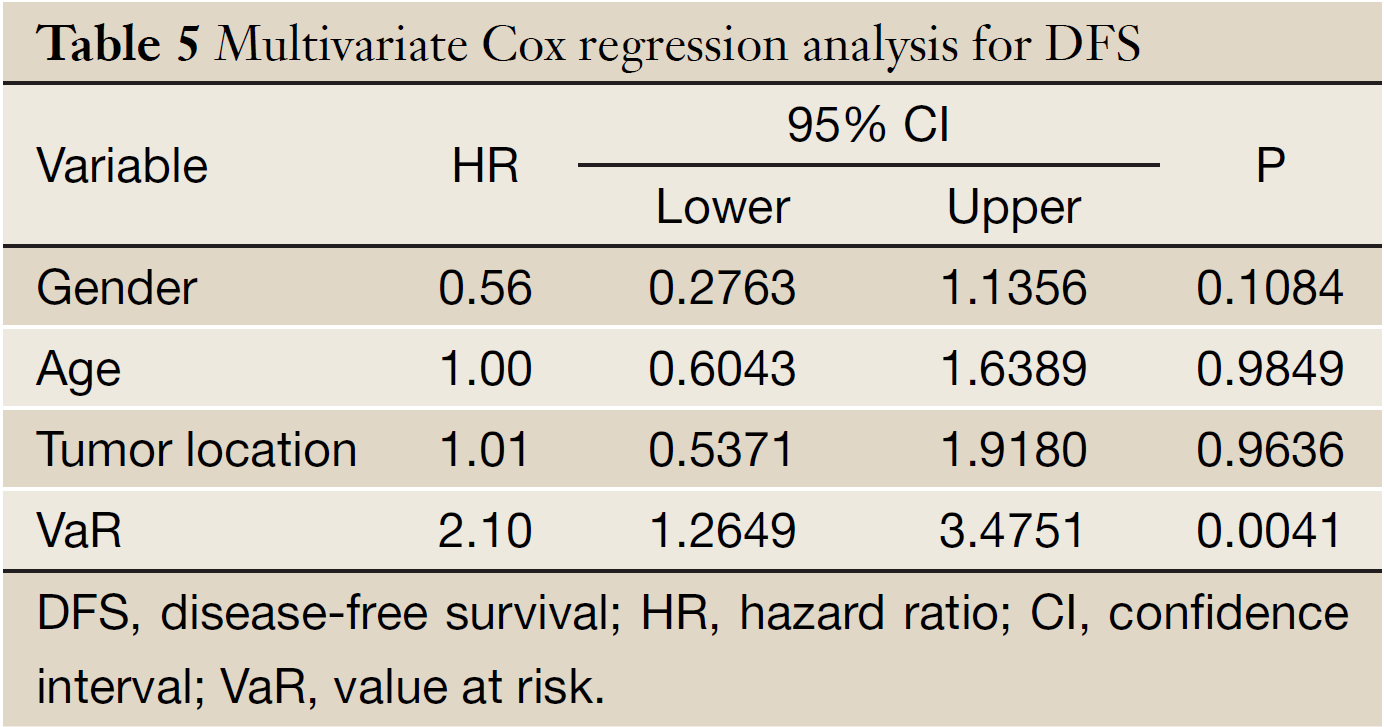
The mean of VaR was 0.39 [95% confidence interval (CI), 0.3016-0.4840] for recurrent patients. The mean of VaR was 0.26 (95% CI, 0.2224-0.2908) for recurrence-free patients. The cutpoint of VaR was 0.3 to infer by stem-and-leaf plot (Figure 1), as well as by independent-samples t-test for recurrence-free time (Table 2). The rate of recurrence was 30.3% (30/99) and 52.3% (34/65), respectively in the VaR <0.3 and VaR ≥0.3 (chi-square test, χ2 =7.984, P=0.005) (Table 3). The 1-, 3-, and 5-year DFS of esophageal cancer after radical surgery was 70.4%, 48.7%, and 45.3%, respectively in the VaR ≥0.3, whereas 91.5%, 75.8%, and 67.3%, respectively in the VaR <0.3 (log-rank test, χ2 =9.59, P=0.0020) (Table 4) (Figure 2). Further confirmed by Cox regression analysis (hazard ratio: 2.10; 95% CI: 1.2649-3.4751; P=0.0041) (Table 5).
Full Table
Full Table
Full Table
Full Table
Comparison of pN and VaR for predicting recurrence
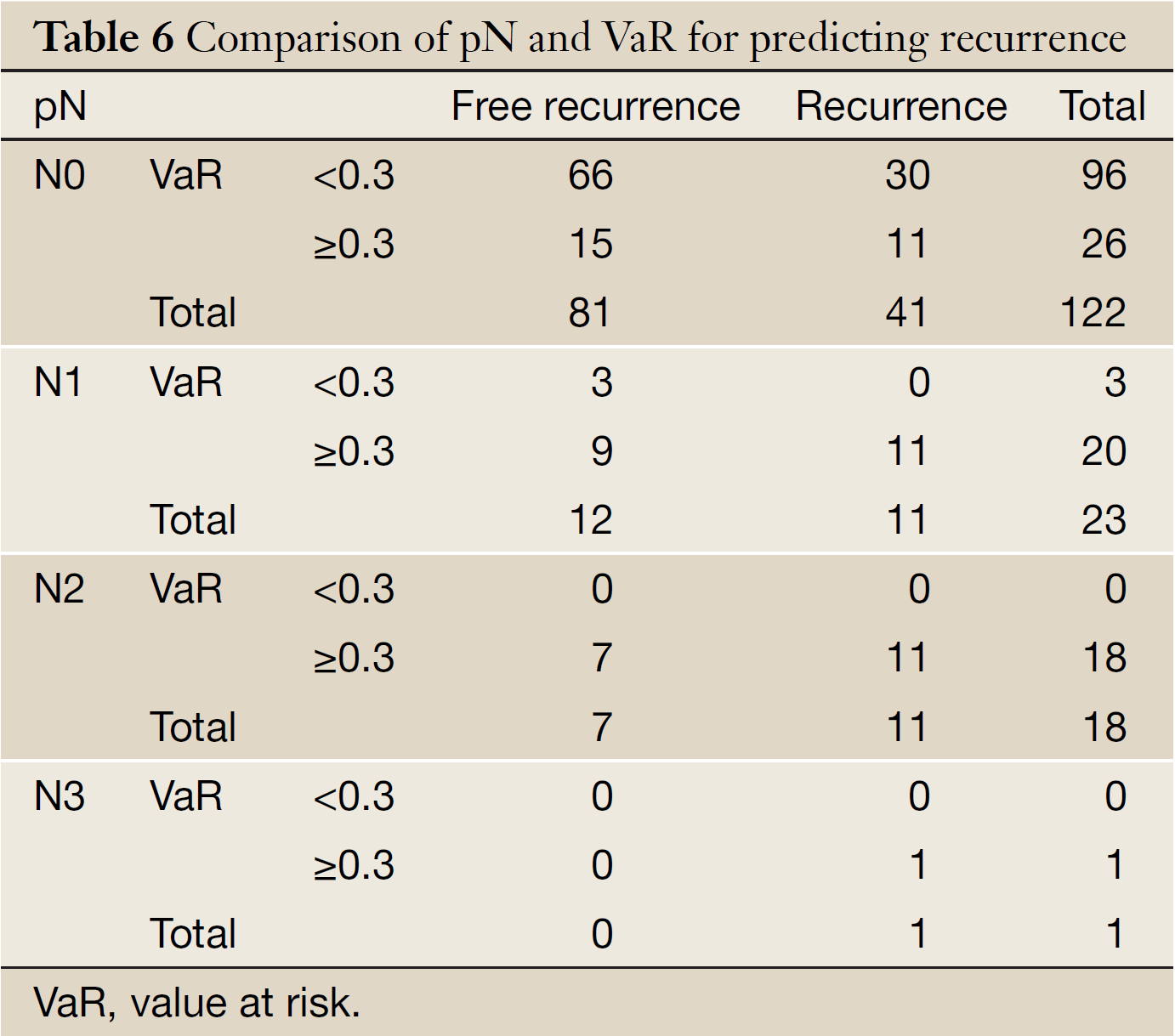
Table 6 shows that 11 were detected relapse in 26 patients (VaR ≥0.3) with the higher recurrent rate of 42.3% than that of 31.3% in those cases (VaR <0.3) among 122 patients with negative pathological lymph node. No recurrence was found in 3 cases (VaR <0.3) among 23 patients of N1. VaR of both N2 and N3 patients was over 0.3.
Full Table
Discussion
Our model refrained the complexity from clinical nomogram (14), and the simplicity from comparison of lymph node positive ratio (15,16). It not only can reflect that variable recurrence risk with the same positive lymph node ratio both at diversity pT stage and diversity total removed lymph node number, which will allow a more accurate comparison of the prognosis for patients with the same lymph node positive ratio (e.g., 1/2 and 6/12), but also can reflect the interplays between the total removed lymph node number and pT stage in pathologically lymph node negative cases. Thus, this model could be applied for integrated assessment of the risk of recurrence after radical surgery for esophageal cancer.
We inferred the cutpoint of VaR was 0.3 by stem-and-leaf plot (Figure 1), as well as independent-samples t-test for recurrence-free time (Table 2), and further confirmed by crosstab chi-square test (Table 3), univariate analysis (Table 4) and Cox regression analysis for the DFS (Table 5). The clinical implication is that the risk of recurrence is higher when VaR ≥0.3, and the potential cases could be recommended for further adjuvant treatment (Figure 2). The result was consistent with previous studies that patients with pathologically positive lymph node, especially in patients with the n more than three, should be performed prophylactic postoperative radiotherapy (17-19). Therefore, this model would be valuable in clinical practice to inform individual patients about their prognosis and be used in direct tailored therapy. Postoperative evaluation of the VaR may help to stratify esophageal carcinoma patients to different risk profiles, which is essential in the area of customized therapy.
Prediction models generally have greater accuracy than reliance on stage or risk groupings, and can be used more rationally to make treatment decisions (20). However, when the VaR reached a fairly low or high level resulted from N, it is not always a prognostic factor for recurrence of esophageal cancer after radical surgery (21). From one point of view, this seems logical that the higher the number of lymph node resected was, the more the possibility of lymph node positive, whereas if the more lymph nodes positive were examined, the more lymph nodes removed were needed to reduce VaR. In fact, inadequate lymph node dissection would be defined as a poor prognostic factor in esophageal cancer, and the number of lymph node dissection can also be embodied in the interference degree of radical surgery which is not only the parameter decided by the tumor natural quality, but excess lymph node dissection often might be meaningless, and lead to more postoperative complications. Therefore, surgeons can only do his best at operation, but in the post-surgery, the prognostic R can help surgeon provide patients with more accurate information regarding to warrant adjuvant therapy.
Currently, most researchers deemed that pathologically positive lymph node should be judgment of whether to be undergone adjuvant therapy or not for postoperative esophageal carcinoma patients (17-19). According to our study of this model, the outcome (Table 6) not only support the view, but also presented that 11 were detected relapse in 26 patients (VaR ≥0.3) with the higher recurrent rate of 42.3% than that of 31.3% in those cases (VaR <0.3) among 122 patients with pathologically negative lymph node. No recurrence was found in 3 cases (VaR <0.3) among 23 patients of N1, which means that our model could recognize more recurrence possible cases from N0 patients and the lower recurrence latent patients.
Some limitations of the present study need to be addressed. First, despite the prospective collection of data, this remains a retrospective study subject to the known or hidden biases inherent in such studies. Second, fewer N in our cohort probably resulted from the application of left thoracotomy via 2-field lymphadenectomy in the mediastinum and upper abdomen, and the absence of a standard protocol for pathological examination could have also produced bias in the studies. Likewise, causes of fewer n in our cohort might be that they had already undergone radiotherapy or chemotherapy after surgery, whereas fewer pT4 might not be indicated to radical surgery or they had already undergone radiotherapy or chemotherapy before surgery. They were removed not only because the addition therapy resulted in decreased frequency of lymph node metastases, but also because nodal localization and pattern were also significantly modified (22). Third, all patients were squamous cell carcinoma in our cohort, while differences in patient characteristics, pathogenesis, and especially survival clearly identify adenocarcinomas of the esophagus and squamous cell carcinoma of the esophagus as two separate tumor entities requiring differentiated therapeutic concepts (23). Therefore, adenocarcinoma needs to be further externally validated. Finally, the recurrence predictions are not perfect when applying this model in clinical practice. The reason for this is that our knowledge of prognostication is still incomplete. Especially, the incorporation of molecular predictors into the model may further increase its performance. Thus far, molecular studies have only included a limited number of patients which could not draw definite conclusions (24). It was our aim, however, to develop a simple model that could be easily applied in routine care. It was respected that validated model predictors could be available in the future.
In conclusion, we constructed a novel model that may be a simple and useful tool for evaluation of the recurrence risk after radical surgery for esophageal cancer. In this way, the patients with high recurrence risk that should be given adjuvant therapy were decided. Future studies are warranted that whether the model could be applied to assess recurrence risk of other tumors, such as breast cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol 2007;25:4110-7. [PubMed]
- Hulscher JB, van Sandick JW, Tijssen JG, et al. The recurrence pattern of esophageal carcinoma after transhiatal resection. J Am Coll Surg 2000;191:143-8. [PubMed]
- Mariette C, Balon JM, Piessen G, et al. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 2003;97:1616-23. [PubMed]
- Chen G, Wang Z, Liu XY, et al. Recurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor-Lewis esophagectomy. World J Surg 2007;31:1107-14. [PubMed]
- Lu JC, Kong C, Tao H. Radiotherapy with or without concurrent chemotherapy for lymph node recurrence after radical surgery of thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2010;78:710-4. [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [PubMed]
- Merkow RP, Bilimoria KY, Chow WB, et al. Variation in lymph node examination after esophagectomy for cancer in the United States. Arch Surg 2012;147:505-11. [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 2008;248:979-85. [PubMed]
- Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763-73.
- Yang HX, Xu Y, Fu JH, et al. An evaluation of the number of lymph nodes examined and survival for node-negative esophageal carcinoma: data from China. Ann Surg Oncol 2010;17:1901-11. [PubMed]
- Jamieson GG, Lamb PJ, Thompson SK. The role of lymphadenectomy in esophageal cancer. Ann Surg 2009;250:206-9. [PubMed]
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248:221-6. [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York: Springer, 2009.
- Gaur P, Sepesi B, Hofstetter WL, et al. A clinical nomogram predicting pathologic lymph node involvement in esophageal cancer patients. Ann Surg 2010;252:611-7. [PubMed]
- Kelty CJ, Kennedy CW, Falk GL. Ratio of metastatic lymph nodes to total number of nodes resected is prognostic for survival in esophageal carcinoma. J Thorac Oncol 2010;5:1467-71. [PubMed]
- Liu YP, Ma L, Wang SJ, et al. Prognostic value of lymph node metastases and lymph node ratio in esophageal squamous cell carcinoma. Eur J Surg Oncol 2010;36:155-9. [PubMed]
- Xiao ZF, Yang ZY, Miao YJ, et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radiat Oncol Biol Phys 2005;62:82-90. [PubMed]
- Schreiber D, Rineer J, Vongtama D, et al. Impact of postoperative radiation after esophagectomy for esophageal cancer. J Thorac Oncol 2010;5:244-50. [PubMed]
- Lee PC, Mirza FM, Port JL, et al. Predictors of recurrence and disease-free survival in patients with completely resected esophageal carcinoma. J Thorac Cardiovasc Surg 2011;141:1196-206. [PubMed]
- Vickers AJ. Prediction models in cancer care. CA Cancer J Clin 2011. [Epub ahead of print]. [PubMed]
- Hsu PK, Wang BY, Chou TY, et al. The total number of resected lymph node is not a prognostic factor for recurrence in esophageal squamous cell carcinoma patients undergone transthoracic esophagectomy. J Surg Oncol 2011;103:416-20. [PubMed]
- Castoro C, Scarpa M, Cagol M, et al. Nodal metastasis from locally advanced esophageal cancer: how neoadjuvant therapy modifies their frequency and distribution. Ann Surg Oncol 2011;18:3743-54. [PubMed]
- Gertler R, Stein HJ, Langer R, et al. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: evaluation of the New Union Internationale Contre le Cancer/American Joint Cancer Committee staging system. Ann Surg 2011;253:689-98. [PubMed]
- Lagarde SM, Reitsma JB, Ten Kate FJ, et al. Predicting individual survival after potentially curative esophagectomy for adenocarcinoma of the esophagus or gastroesophageal junction. Ann Surg 2008;248:1006-13. [PubMed]