Effects of salidroside on glioma formation and growth inhibition together with improvement of tumor microenvironment
Introduction
Glioma is a type of tumor mostly in the brain arising from glial cells. It can cause headaches, nausea, vomiting, seizures, and cranial nerve disorders as a result of increased intracranial pressure (1). Gliomas are rarely curable compared with many other tumors. Glioblastoma multiforme has a worse prognosis with less than a 12-month average survival after diagnosis. Treatment for gliomas is a combined approach of surgery, radiation therapy, and chemotherapy, depending on the tumor location, the cancer cell type and the grade of malignancy (2). Temozolomide is a chemotherapeutic drug that is able to cross the blood-brain barrier effectively and is currently being used in therapy for high-grade tumors, however, the side effects of temozolomide are serious (3). In our previous research, we found out salidroside could inhibit the growth of human lung adenocarcinoma epithelial cells in vitro. Here, we would test the effects of salidroside on the growth of glioma both in vivo and in vitro.
Salidroside [C14H20O7, IUPAC: 2-(4-hydroxyphenyl)ethyl-β-D-glucopyranoside], glucoside of tyrosol from Rhodiola rosea, is thought to be the most effective compounds responsible for the antidepressant and anxiolytic actions of this plant, even more than rosavin, also found in Rhodiola rosea (4). Rhodiola rosea, a traditional Chinese herb, belongs to the Crassulaceae family, growing in cold regions and mountains of China. Rhodiola rosea has been proved to be effective for neural protection, such as improving mood, alleviating depression, and reducing fatigue (5). It has been proved to be effectively prevented stress-induced changes in appetite, physical activity, weight gain and the estrus cycle. Meanwhile, our former research found out salidroside, extracted from Rhodiola rosea, could inhibit the growth of human lung adenocarcinoma epithelial cells. This research mainly focused on the effects of salidroside on the formation and growth of glioma both in vivo and in vitro. The tumor microenvironment would also be studied.
Materials and methods
Cell culture and drug treatments
Salidroside extracted from Rhodiola rosea was purified by high-performance liquid chromatography (HPLC) (ÄKTA, GE Healthcare, USA), then dissolved in 0.9% normal sodium (NS) and stored at 4 °C. Human glioma cells U251 were stored in our lab and propagated at 37 °C with 5% CO2 in IMDM medium (GIBCO, USA) supplemented with L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% (V/V) fetal bovine serum (FBS) (GIBCO, USA). The salidroside concentration of 20 µg/mL will get a good anti-cancer effect on cells according to our former researches.
3-(4,5-dimethylthiazol-2-yl)-2,5-dephenyltetrazolium bromide (MTT) assay for cytotoxicity
Viability of U251 cells was determined by MTT (Invitrogen, USA) assay after treatment of salidroside at the concentration of 20 µg/mL. U251 cells (7×103/well) were seeded into 96-well plates (Corning, USA). Salidroside was diluted in IMDM/10% FBS. The diluted solution was separately incubated with U251 cells for 24-72 h. At least triplicate measurements were made for each group and time point. At the end of the treatment, 20 µL of 2 mg/mL MTT was added into each well, incubating at 37 °C for 2 h. Then, 100 µL acidic isopropanol was added into each well, and the optical density (OD) at 595 nm was measured using a scanning multiwell spectrophotometer plate reader (Shenzhen Procan Electronic Inc, China).
Cell cycle analysis
U251 cells after treatment of salidroside at the concentration of 20 µg/mL for 24-72 h were seeded in 6-well plates (Corning, USA) at the density of 1×105/mL. Tumor cells were trypsinized, centrifuged (1,000 r/min, 10 min), washed with 1× PBS (pH 7.4), and fixed with cold 70% ethanol/30% phosphate-buffered saline at 4 °C overnight. Cells were digested by 1,000 U RNase A (Invitrogen, USA), and then stained with 1% propidium iodide at 37 °C for 30 min. The DNA profiles were determined within 4 h of staining by flow cytometry (FCM) (EPICS® ALTRA™, Beckmann, USA). The results would be divided into G0/G1, S, and G2/M.
Generation of xenotransplantation tumor model and treatment of drug
Human glioma tissue got from clinical operation was cut into 3 mm × 3 mm × 3 mm and then rinsed in DMEM (GIBCO, USA). Male nude mice (2-month old, C57/B6 genetic background) were selected and raised at temperature of 24±2 °C and humidity of (55±15)%. These xenotransplants were subcutaneously injected into the right lower limbs of these mice. After the average tumor size was more than 150 mm3, half of these nude mice were treated with salidroside (intraperitoneal injection) at the concentration of 50 mg/kg.d according to the former research for totally 20 d.
Body weight and tumor size detection
The body weight of these nude mice was weighed every 2 d after the treatment of salidroside. Mice with loss of weight more than 40% would be sacrificed. Tumor size would be determined every 2 d after the treatment of salidroside, together with the body weight detection. Tumor size would be valued as V = π/6 × a × b2 (a: major axis; b: minor axis). Mice with tumor size more than 2,000 mm3 would be sacrificed. For each group, 10 mice would be used and tested.
Detection of oxidative stress
The cerebral cortex was grinded in tris-buffered saline (TBS) containing AEBSF protease inhibitors (1:1,000), centrifuged at 14,000 r/min for 30 min at 4 °C. Supernate was transferred into tubes. The levels of 8-isoprostane, superoxide dismutase (SOD) and malondialdehyde (MDA), special markers for reactive oxygen species (ROS), were determined in the cortex of mice brain in control group and salidroside groups. The isoprostanes were extracted from homogenates of the hemisphere of individual mouse, pulled together and determined using LC/MS/MS method. SOD and MDA extracted from homogenates of the hemisphere were detected using kits (Biobox Company, China). SOD were determined using xanthine oxidase (XO), while thiobarbituric acid (TBA) for MDA.
Immunofluorescence staining detection for astrocytes
Brains of these xenotransplanted nude mice were detected by immunofluorescence staining for the expression of astrocytes. Cryostat mouse brain coronal sections (16 µm) were prepared and stained with glial fibrillary acidic protein (GFAP) antibody (Dako, Denmark, 1:1,000) to assess for proliferation of astrocytes, one of the most important glioma cells following protocol. All slides were stained with 4',6-diamidino-2-phenylindole (DAPI) (Invitrogen, USA, 1:2,000) for the cell nuclei. Images were taken using confocal microscopy (Zeiss, Germany, 50×).
Data analysis
Each assay was carried out for not less than 3 times. Data were presented as x̄±s, and Student’s t-test was used to evaluate the changes between salidroside group and negative control group. All statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA), and P<0.05(*) and P<0.01(**) were considered statistically significant.
Results
Cell viability
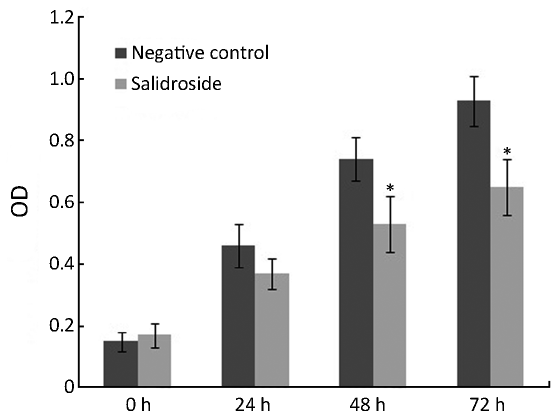
As shown in Figure 1, salidroside showed significant cytotoxic effect and cell growth inhibition on U251 cells. Compared with control group, the growth and viability of U251 cells treated with salidroside for 24-72 h were significantly inhibited at the concentration of 20 µg/mL. These results indicated that salidroside had cytotoxicity on human glioma cells U251 for in vitro study.
Cell cycle analysis
As shown in Table 1, salidroside could inhibit the growth of U251 cells, and induce G0/G1 phase arrest. Compared with control group, the percentage of cells at G0/G1 phase increased, while the percentage in other phases decreased. The results indicated that salidroside could arrest the cell growth at G0/G1 phase.
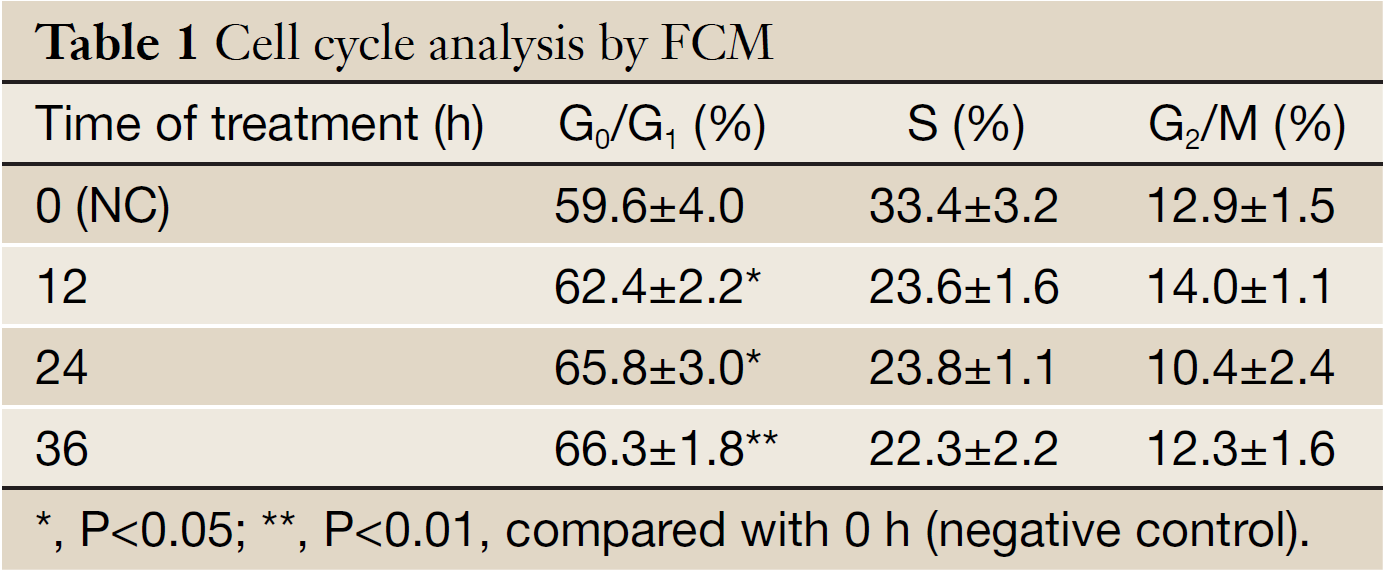
Full Table
Body weight and xenotransplanted tumor size of nude mice
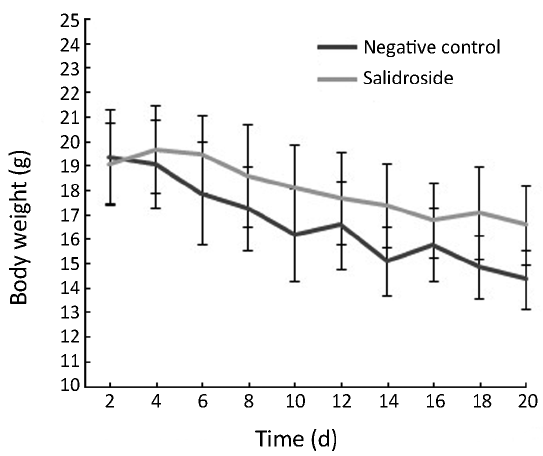
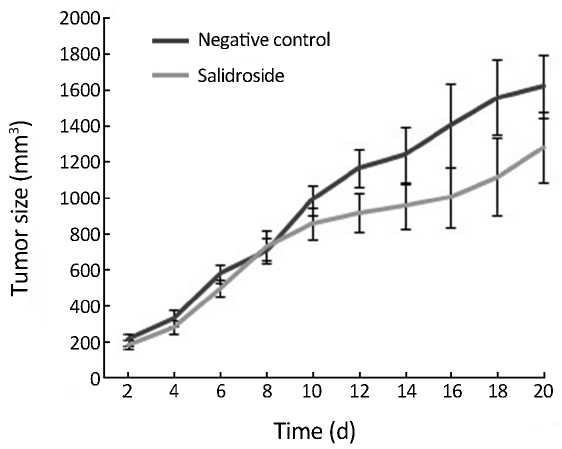
As shown in Figure 2, the body weight of nude mice was decreased in control group due to the quick growth of xenotransplanted tumor. These nude mice seemed to be weak because of lack of nutrition. However, the body weight of nude mice was decreased much slower in salidroside group compared with control group due to the slow growth of tumor. Meanwhile, the physical and mental status in salidroside group seemed to be better than control group. As shown in Figure 3, the xenotransplanted tumor of nude mice in control group grew quickly without any treatment. The uncontrolled growth of tumors would expend the nutrition and make the mice weak. However, the tumors of nude mice in salidroside group grew much slower than those in control group. This indicated that salidroside could inhibit the growth of human glioma in vivo.
Analysis on oxidative stress
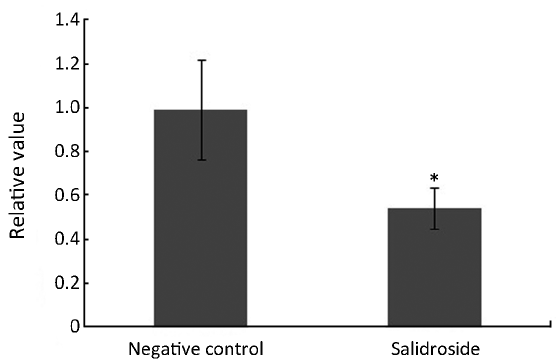
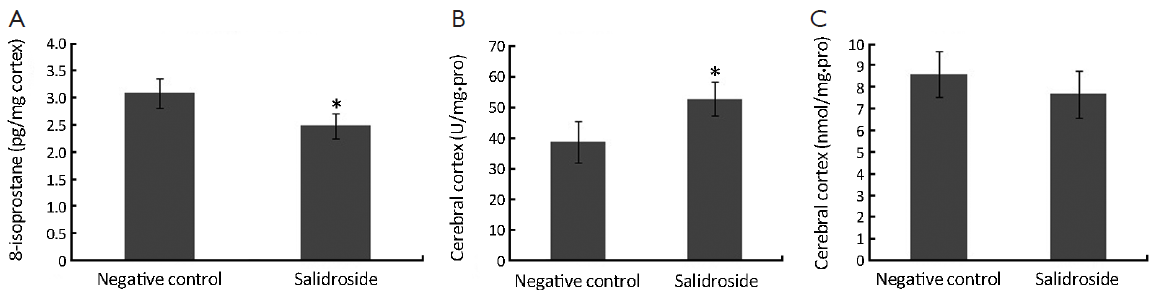
As shown in Figure 4A, increased concentration of 8-isoprostane was detected in control group since the stimulation of xenotransplants. This increased oxidative stress could in turn make the tumor microenvironment even worse. However, the concentration of 8-isoprostane in salidroside group was significantly lower than that in control group. Decreased SOD was found in salidroside group compared with control (Figure 4B) while no obvious change was found for MDA (Figure 4C). These results indicated that salidroside could down-regulate the level of oxidative stress in vivo.
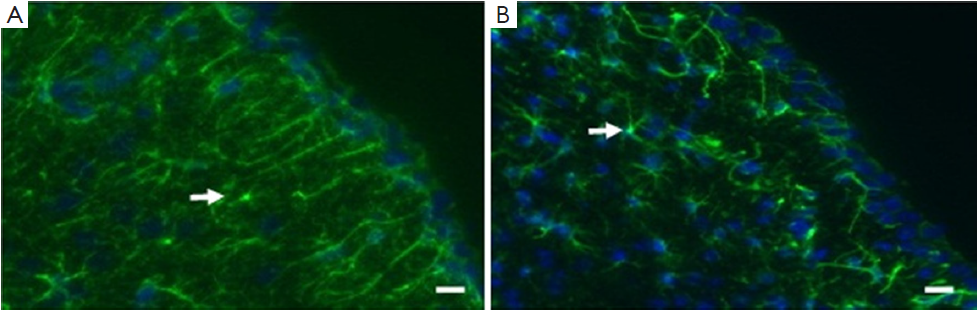
Formation and growth of astrocytes
The formation and growth of astrocytes could be stimulated by many factors such as tumor, oxidative stress, cytokines, and steroid. As shown in Figure 5, increased formation and growth of astrocytes were detected in control group. These increased astrocytes could overgrow and then form into tumor if the microenvironment went even worse, which was called astrocytoma. However, the formation and growth of astrocytes were controlled and decreased significantly in salidroside group compared with control group (Figure 6). This result indicated that salidroside could regulate the formation and growth of astrocytes, and make them grow normally in vivo.
Discussion
Glioma is a type of primary central nervous system (CNS) tumor arising from glial cells, and mostly occurred in the brain. Glioma is the most common and serious form of brain tumors that affect human adults (6). There are several kinds of gliomas: astrocytomas, ependymomas, and oligodendrogliomas. The advanced astrocytoma is called glioblastoma, accounting for 23% of all primary brain tumors (7). Brain tumors are difficult to treat. One treatment is external beam radiation. Another treatment is surgical removal of the tumor, if possible, followed by chemotherapy. All of these treatments are difficult to go through, and pose risks to the patient. Unfortunately, many gliomas recur even after treatment (8), and finding a useful chemical compound for gliomas treatment is urgent. Salidroside, glucoside of tyrosol extracted from Rhodiola rosea, is thought to be the one of the potential compounds for human tumor treatment.
Cytotoxicity analysis is widely used in the pharmaceutical industry for screening compounds. Cytotoxicity can be monitored using MTT assay (9). Salidroside showed cytotoxic effect and cell growth inhibition on U251 cells. The results indicated that the cell cycle of U251 cells would be affected. The cell cycle can be divided in two periods: interphase, during which the cell grows, accumulating nutrients needed for mitosis and duplicating its DNA, and the mitosis phase (M), during which the cell splits itself into two distinct cells (10). Salidroside could arrest the growth of U251 cells at G0/G1 phase.
Xenotransplantation of tumor into nude mice, which lack of immunity, is one of the useful and effective methods for valuing the toxicity of compound on formation and growth of tumor (11). The body weight and the size of xenotransplanted tumor are the most important data for detection (12). In this study, the body weight of nude mice was decreased in control group due to the quick growth of xenotransplanted tumor. These nude mice seemed to be weak because of lack of nutrition. However, the body weight of nude mice decreased much slower in salidroside group due to the slow growth of tumor. Meanwhile, the physical and mental status of mice in salidroside group seemed to be better than those in control group. The xenotransplanted tumor in control group grew quickly without any treatment. The uncontrolled growth of tumors would expend the nutrition and make the mice weak. However, the tumors of nude mice in salidroside group grew much slower than those in control group. This study indicated that salidroside could inhibit the growth of human glioma in vivo.
The formation and growth of tumor depend on microenvironment. The metabolic environment of tumor is directly affected by lack of vasculature and subsequent oxygen starvation (13). Tumor microenvironment affects the status and treatment of cancers (14). While the normal cellular microenvironment can inhibit malignant cell growth, the modifications that occur in the tumor microenvironment synergistically support cell proliferation. Tumors shape their microenvironment and support the development of both tumor cells and non-malignant cells (15).
Oxidative stress is one of the most important factors involved in formation and growth of tumor. Oxidative stress means an imbalance between the systemic manifestation of ROS and ability to readily detoxify the reactive intermediates or to repair the resulting damage (16). Oxidative stress can cause disruptions in normal mechanisms of cellular signaling. In humans, oxidative stress is thought to be involved in the development of many diseases or may exacerbate their symptoms including human tumors (17). High level of ROS is often found in cancer cells or tumor tissues. In this research, we found that salidroside could inhibit the level of ROS by detecting several biomarkers of oxidative stress, 8-isoprostane, SOD and MDA. 8-isoprostane, a prostaglandin (PG)-F2-like compound belonging to the F2 isoprostane class, is produced in vivo by the free radical-catalyzed peroxidation of arachidonic acid. SOD could catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. They are an important antioxidant defense. 8-isoprostane was decreased while SOD increased in salidroside group compared with control group. MDA seems to change little. These results showed salidroside could down-regulate the high and imbalanced condition of oxidative stress.
Astrocytes, star-shaped glial cells in the brain and spinal cord, are the most abundant cells of the human brain (18). They perform many functions, such as biochemical support, provision of nutrients, maintenance of extracellular ion balance, and repairing and scarring of the brain and spinal cord following traumatic injuries, antioxidant and bioenergetic coupling (19,20). Although these important functions, astrocytoma is primary intracranial tumor derived from astrocytes (21). Overgrowth of astrocytes may promote astrocytoma, which is a dangerous glioma in the brain. In this research, we found that salidroside could inhibit the overgrowth of astrocytes partly due to the oxidative stress, making the formation and growth of astrocytes return to normal.
In general, our research showed salidroside could inhibit the formation and growth of glioma both in vivo and in vitro, together with improvement of tumor microenvironment, and making oxidative stress and astrocytes return to normal. The conclusions of in vivo and in vitro study could support each other. Both protection on CNS (22) and inhibition on glioma of salidroside were confirmed. All these suggested that salidroside may be one of the potential compounds responsible for the human tumors, including glioma.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81141080) and Jiangsu Provincial Natural Science Foundation (SBK201340596)).
Disclosure: The authors declare no conflict of interest.
References
- Ricard D, Idbaih A, Ducray F, et al. Primary brain tumours in adults. Lancet 2012;379:1984-96. [PubMed]
- Patil CG, Eboli P, Hu J. Management of multifocal and multicentric gliomas. Neurosurg Clin N Am 2012;23:343-50. [PubMed]
- Chamberlain MC. Temozolomide: therapeutic limitations in the treatment of adult high-grade gliomas. Expert Rev Neurother 2010;10:1537-44. [PubMed]
- Hung SK, Perry R, Ernst E. The effectiveness and efficacy of Rhodiola rosea L.: a systematic review of randomized clinical trials. Phytomedicine 2011;18:235-44. [PubMed]
- Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010;17:481-93. [PubMed]
- Englot DJ, Berger MS, Chang EF, et al. Characteristics and treatment of seizures in patients with high-grade glioma: a review. Neurosurg Clin N Am 2012;23:227-35. [PubMed]
- Kim YH, Kim CY. Current surgical management of insular gliomas. Neurosurg Clin N Am 2012;23:199-206. [PubMed]
- Mattox AK, Lark AL, Adamson DC. Marked response of gliomatosis cerebri to temozolomide and whole brain radiotherapy. Clin Neurol Neurosurg 2012;114:299-306. [PubMed]
- Hasovits C, Clarke S. Pharmacokinetics and pharmacodynamics of intraperitoneal cancer chemotherapeutics. Clin Pharmacokinet 2012;51:203-24. [PubMed]
- Chen T, Stephens PA, Middleton FK, et al. Targeting the S and G2 checkpoint to treat cancer. Drug Discov Today 2012;17:194-202. [PubMed]
- Herreros-Villanueva M, Hijona E, Cosme A, et al. Mouse models of pancreatic cancer. World J Gastroenterol 2012;18:1286-94. [PubMed]
- Meyer LH, Debatin KM. Diversity of human leukemia xenograft mouse models: implications for disease biology. Cancer Res 2011;71:7141-4. [PubMed]
- Dang CV. Links between metabolism and cancer. Genes Dev 2012;26:877-90. [PubMed]
- Jackson C, Ruzevick J, Phallen J, et al. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol 2011;2011:732413.
- Dvorak HF, Weaver VM, Tlsty TD, et al. Tumor microenvironment and progression. J Surg Oncol 2011;103:468-74. [PubMed]
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012;24:981-90. [PubMed]
- Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev 2012;70:257-65. [PubMed]
- Johnson EC, Morrison JC. Friend or foe? Resolving the impact of glial responses in glaucoma. J Glaucoma 2009;18:341-53. [PubMed]
- Fernandez-Fernandez S, Almeida A, Bolaños JP. Antioxidant and bioenergetics coupling between neurons and astrocytes. Biochem J 2012;443:3-11. [PubMed]
- Orellana JA, von Bernhardi R, Giaume C, et al. Glial hemichannels and their involvement in aging and neurodegenerative diseases. Rev Neurosci 2012;23:163-77. [PubMed]
- Molofsky AV, Krencik R, Ullian EM, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 2012;26:891-907. [PubMed]
- Chen SF, Tsai HJ, Hung TH, et al. Salidroside improves behavioral and histological outcomes and reduces apoptosis via PI3K/Akt signaling after experimental traumatic brain injury. PLoS One 2012;7:e45763. [PubMed]