Effects of tyrosine kinase inhibitor E7080 and eNOS inhibitor L-NIO on colorectal cancer alone and in combination
Introduction
Cancer is a leading cause of death worldwide, accounting for 7.6 million deaths (around 13% of all deaths) in 2008 (1). In the Western world, colorectal cancer is the third most commonly diagnosed malignancy in men after prostate and lung cancer and in women after breast and lung cancer. It is also the second leading cause of cancer-related death for both sexes combined. In the United States, an estimated 142,570 new cases will be diagnosed and 51,370 deaths will occur in 2010 (2). Twenty percent of patients have clinical evidence of hepatic metastases at diagnosis and about 50% of patients will develop liver metastatic disease later (3).
5-Fluorouracil (5-FU) has been the only treatment for disseminated disease for many decades used either alone or combined with leucovorin (LV) (4). Recently, irinotecan and oxaliplatin have been shown to enhance efficacy of 5-FU/LV combinations both in terms of response and survival, and capecitabine has been demonstrated to be equivalent to continuous infusion 5-FU/LV. Two drug regimens containing infusional 5-FU/LV or capecitabine together with irinotecan or oxaliplatin are now considered as the standard treatment of metastatic colorectal cancer patients (5,6).
Even with the significant improvement in traditional chemotherapy, there are remaining limitations with this treatment. These drugs carry substantial toxicity, and the complete response to these agents is rare. Therefore, several novel targets are being investigated both as single agents and in combination with chemotherapy to assess the potential for increased efficacy. Some of the most promising targets include vascular endothelial growth factor (VEGF) (7).
VEGF plays a pivotal role in cancer neoangiogenesis and its importance in colorectal cancer growth and development has been extensively documented. It is secreted by both tumor and normal cells during cancer processes. VEGF originating from normal myeloid cells in response to tumor stimulation may be essential in tumorigenesis and may reduce susceptibility of tumors to standard chemotherapy (8).
More recently, a new class of agents targeted to VEGF has provided further benefit. Currently available targeted agents fall into two groups: monoclonal antibodies that bind to the ligand or the extracellular domain of a receptor, and small-molecule tyrosine kinase (TK) inhibitors that target the intracellular part (the TK domain) of a receptor. Both groups of targeted agents inhibit the signal transduction pathways through TK receptors that are necessary for cancer cell growth (9).
E7080 (lenvatinib) is an orally active inhibitor of multiple tyrosine kinases including receptors for growth factors with pro-angiogenic properties, such as VEGF, fibroblast growth factor (FGF), stem cell factor (SCF) and platelet-derived growth factor (PDGF) (10). E7080 has effects on both VEGF and tyrosine kinase and it’s in vivo antitumor activities in small cell lung cancer (SCLC) and breast cancer based on angiogenesis inhibition have been demonstrated previously (11). Despite the promising results in different types of cancers, evaluating the effects of E7080 on colorectal cancer remains to be investigated.
Nitric oxide (NO) is synthesized from L-arginine and oxygen by four major isoforms of nitric oxide synthase (NOS): neuronal NOS, endothelial NOS (eNOS), inducible NOS (iNOS), and mitochondrial NOS. Although iNOS is considered as a viable candidate for purposes of cancer prevention and treatment, it has been suggested that targeting eNOS may also be a viable strategy or at least deserves consideration (12). Also genetic comparison studies on healthy people and cancer patients have shown that gene polymorphisms in eNOS are associated with the development of multiple cancers (13). In cell culture models, eNOS plays an essential role in endothelial cell proliferation and is a central mediator of several endothelium growth stimulators, such as VEGF and prostaglandin E2 (PGE2). Both molecules activate the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and the former (VEGF) increases eNOS activity by enhancing eNOS phosphorylation. As reviewed by Duda et al., VEGF can also activate eNOS by the induction of calcium flux and the recruitment of heat shock protein 90 (14).
Although it is well known that N5-(1-iminoethyl)-L-ornithine dihydrochloride (L-NIO) is a selective eNOS inhibitor and it has been shown that L-NIO inhibits the proliferative effect of pancreastatin on hepatoma cell via eNOS pathway (15), there is few studies demonstrating the effects of L-NIO on colorectal cancer.
We hypothesized that modulation of tumor oncogenes with multi-directed therapy, in the form of combinatory treatment with E7080 and L-NIO, would have improved efficacy in angiogenesis and antitumoral effect. Therefore we aimed to investigate the effects of these agents on colorectal cancer alone and in combination. Our goal is to develop more effective treatment strategies for patients with colon cancer.
Materials and methods
Reagents
E7080 was purchased from Selleck Chemicals LLC. (Boston, USA), and L-NIO was purchased from Sigma-Aldrich. All medium, solution and enzymes for cell culture were purchased from Sigma-Aldrich.
Cell culture
Human colon adenocarcinoma cells HT29 were purchased from the Sap Institute. Cells were multiplied in three passages, frozen in aliquots and stored in liquid nitrogen. The cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) with phenol red and NaHCO3. The culture medium was supplemented with 10% heat inactivated fetal bovine serum (FBS), 1% penicilin and streptomicin. Cells were grown in T75 cm2 culture flasks in a humidifed atmosphere containing 5% CO2 at 37 °C.
Instrumentation
xCELLigence system
The xCELLigence system was used according to the instructions of the supplier (Roche Applied Science and ACEA Biosciences) (16). The core of the xCELLigence system is the E-plate 16: this is a single use, disposable device used for performing cell-based assays on the RTCA DP instrument, which has similar application like commonly used 96-well microtiter plate. However the E-plate 16 differs from standard 96-well microtiter plates vastly with its incorporated gold cell sensor arrays in the bottom, which contributes cells in-side each well to be monitored and assayed. The E-plate 16 has a low evaporation lid design: the bottom diameter of each well is 5.0±0.05 mm; with a total volume of 210±5 µL, approximately 80% of the bottom areas of each well is covered by the circle-on-line-electrodes, which is designed to be used in an environment of 15 to 40 °C, relative humidity 98% maximum without condensation. The electronic impedance of sensor electrodes is measured to allow monitoring and detection of physiological changes of the cells on the electrodes. The voltage applied to the electrodes during RTCA measurement is about 20 mV (RMS). The impedance measured between electrodes in an individual well depends on electrode geometry, ion concentration in the well and whether cells are attached to the electrodes or not. In the absence of cells, electrode impedance is mainly determined by the ion environment both at the electrode/solution interface and in the bulk solution. In the presence of cells, cells attached to the electrode sensor surfaces will act as insulators and thereby alter the local ion environment at the electrode/solution interface, leading to an increase in impedance. Thus, the more cells that are growing on the electrodes, the larger the value of electrode impedance. The RTCA associated software allows users to obtain parameters such as: average value, maximum and minimum values, standard deviation, half maximum effect of concentration (EC50), half maximum inhibition of concentration (IC50), cell index (CI), and in addition graphics. The data expressed in CI unit scan is exported to Excel for any type of mathematical analysis.
Cell growth and proliferation assay using xCELLigence system
HT29 cells were grown and expanded in tissue-culture flasks. After reaching about 75% confluence, the cells (passage 6) were washed with PBS, afterwards detached from the flasks by a brief treatment with trypsin/EDTA. Subsequently, 100 µL of cell culture media at room temperature was added into each well of E-plate 16. After this, the E-plate 16 was connected to the system and checked in the cell culture incubator for proper electrical contacts and the background impedance was measured. Meanwhile, the cells were resuspended in cell culture medium and adjusted to 400,000 cells/mL. Cell suspension 100 µL was added to the 100 µL medium containing wells on E-plate 16. After 30 min incubation at room temperature, E-plate 16 was placed into the cell culture incubator. Finally, proliferation of the cells was monitored every hour for a period of up to 72 h via the incorporated sensor electrode arrays of the E-plate 16. The electrical impedance was measured by the RTCA-integrated software of the xCELLigence system as a dimensionless parameter termed CI.
Cytotoxicity assay using xCELLigence system
First, the optimal seeding concentration for proliferation experiments of HT29 was determined. After seeding the respective number of cells in 100 µL medium to each well of the E-plate 16, the proliferation of the cells was monitored every 30 min by the xCELLigence system. Approximately 18 h after seeding, when the cells were in the log growth phase, the cells were exposed to 10 µL of medium containing E7080 (100, 50, 25, 12.5 and 6.25 nmol/L) and L-NIO (100, 50, 25, 12.5 and 6.25 nmol/L) both alone and in combination. Controls received either medium only, or medium + E7080 or medium + L-NIO. All experiments were run for 72 h.
Angiogenesis assay using chorioallantoic membrane (CAM) assay
Preparation of pellets
In this trial, the effects of E7080 and L-NIO alone and in combination on angiogenesis were studied. E7080 and L-NIO were prepared and mixed with agarose in order to form pellets. The agarose (Merck, Damstadt, Germany) is added to distilled water to obtain a 2.5% (W/V) solution. This solution is put into the autoclave in 121 °C and under 1 atmospheric pressure to provide dissolution and sterilization. Subsequently, it was cooled in a sterile container up to 37 °C. The drug is added at this stage. Appropriate volumes of solutions were used to achieve three different concentrations of E7080 and L-NIO (100, 10 and 1 nmol/L per 10 µL pellet). Approximately one hundred pellets for each study set are used. Therefore, approximately 1 mL of combined agar and drug solution (10 µL ×100=1 mL) was prepared initially for E7080 and L-NIO. The drug solutions of 10 and 1 nmol/L concentrations were prepared by diluting these initial mixtures ten folds with the agarose solution again. Using a micropipette, 10 µL drops of this mixed solution were placed on previously sterilized, vertical, cylindrical stainless steel rods which were 5 mm in diameter to obtain circular pellets with the same diameter. The pellets were then solidified at room temperature in a sterile setting.
CAM assay
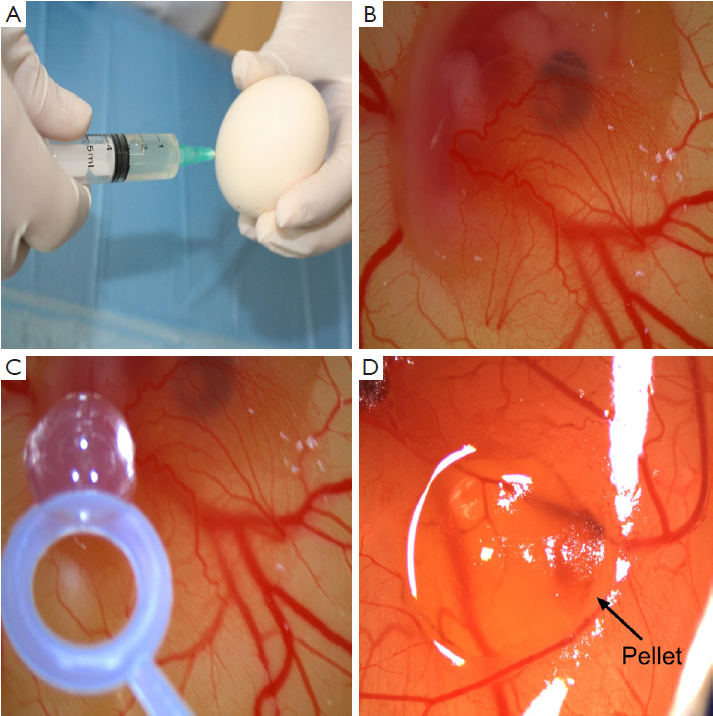
Ross 308 strain fertilized hen’s eggs were obtained from Yemsel Poultry Company (Kayseri, Turkey). The study protocol was approved by the Cumhuriyet University Animal Ethics Committee. The fertilized hen’s eggs were incubated in horizontal position with environmental conditions of 37.5 °C and 80% relative humidity. On the fifth day of the incubation period, 5 mL of albumen was taken through the eggshell with a syringe (Figure 1A) and a shell piece of 2-3 cm in diameter was removed from the contrary side of the eggs. Normal development of the CAM was verified (Figure 1B) and malformed or dead embryos were excluded. The windows on the egg shells were sealed with gelatin, and thereafter, the eggs were incubated for more than 72 h to have CAM reaching 2 cm in diameter. Subsequently (on day 8), the seal was removed and the pellets were placed on the CAM of each egg (Figure 1C). The seal was placed again and the eggs were then incubated for 24 h. The angiogenesis level was evaluated after that period. For each concentration of sorafenib, 20 eggs were used. As the negative control group, pellets containing just agar were utilized. As the positive control group, pellets containing bevacizumab, FDA approved antiangiogenic agent, were used. All the tests were duplicated. The eggs in which the pellets caused inflammation and embryo toxicity were excluded.
Angiogenesis scoring
The inhibitory effects of the drugs on angiogenesis in CAM were evaluated under a stereoscopic microscope and assessed according to the scoring system used previously in several studies (17,18). In this scoring system, the change in the density of the capillaries around the pellet and the extent of the effect were evaluated (Figure 1D). In the initial scoring of each subject, score 0 indicated the absence of any demonstrable antiangiogenic effect (normal embryo and no difference in surrounding capillaries); score 0.5 represented a very weak effect (no capillary-free area but an area with reduced density of capillaries, which is not larger than the pellet area); score 1, a weak-moderate effect (a small capillary-free area or a small area with significantly decreased density of capillaries; less than double size of the pellet is involved); and score 2, a strong antiangiogenic effect (a capillary free area around the pellet, which is equal to or more than double size of the pellet itself). The equation used for the determination of the average score was as follows: average score = [number of eggs (score 2) ×2 + egg number (score 1) ×1]/[total number of eggs (score 0,1,2)].
According to this scoring system, a score of <0.5 meant that there was no antiangiogenic effect; a score of 0.5 to 1 indicated a weak antiangiogenic effect, and a score of >1 implied a strong antiangiogenic effect.
Detection of apoptosis by Annexin V method
Apoptotic cell death was measured using a flouresin isothiocynate (FITC)-conjugated Annexin V/propidium iodide (PI) assay kit by flow cytometry. Briefly, 5×105 cells were washed with icecold PBS, resuspended in 100 mL binding buffer, and stained with 5 mL of FITC-conjugated Annexin V (10 mg/mL) and 10 mL of PI (50 mg/mL). The cells were incubated for 15 min at room temperature in the dark, 400 mL of binding buffer was added, and the cells were analyzed (FACScan, Becton-Dickinson, USA). HT29 cells were gated separately according to their granularity and size on forward scatter (FSC) vs. side scatter (SSC) plots. Early and late apoptosis was evaluated on fluorescence 2 (FL2 for PI) vs. fluorescence 1 (FL1 for Annexin) plots. Cells stained with only Annexin V were evaluated as being in early apoptosis; cells stained with both Annexin V and PI were evaluated as being in late apoptosis or in a necrotic stage.
Statistical analysis
All data are expressed as x̄±s. Groups were compared statistically using general linear models of analysis of variance (ANOVA) followed by Tukey test and t-test when appropriate. Also Kruskal-Wallis and Mann-Whitney U tests have been used when the parametric test assumptions have been violated. P<0.05 was considered statistically significant.
Results
Monitoring dynamic cell proliferation and attachment in real-time using CELLigence system
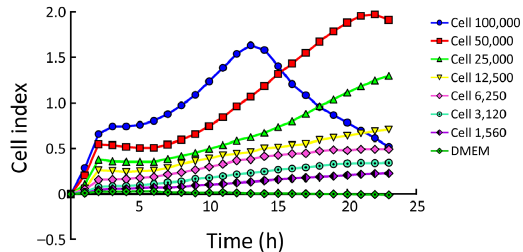
First, we determined the optimal concentration for cell proliferation and viability measurements. To this end, 100,000, 50,000, 25,000, 12,500, 6,250, 3,125 and 1,562 cells/well were seeded in the E-plate 16 and the impedance determined. The impedance CI of 100,000, 50,000, 25,000, 12,000, 6,250, 3,125 and 1,562 cells/well increased proportionally to cell number (Figure 2). As shown in Figure 2, the CI of each cell concentration sharply increased after seeding up to reach its maximum at 2.5 h. Thereafter, the CI of 50,000 cells/well slowly decreased to reach the minimum at 7 h to increase again to the maximum at 22 h. In all, we conclude that the response seen in the 12,500-50,000 cells/well experiments reflects cell cycle effects, while the concentration of 100,000 cells/well was not suited for further experimentation, possibly because of a too high cell density and the resulting contact inhibition.
Monitoring of cytotoxicity in real-time using xCELLigence system
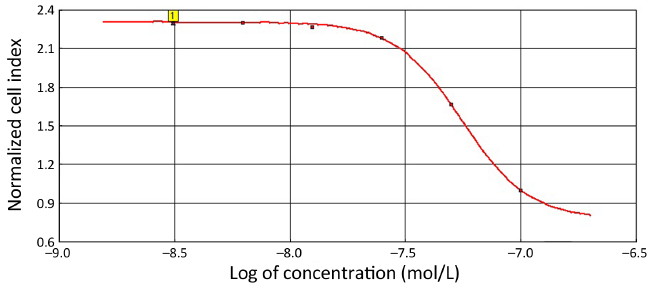
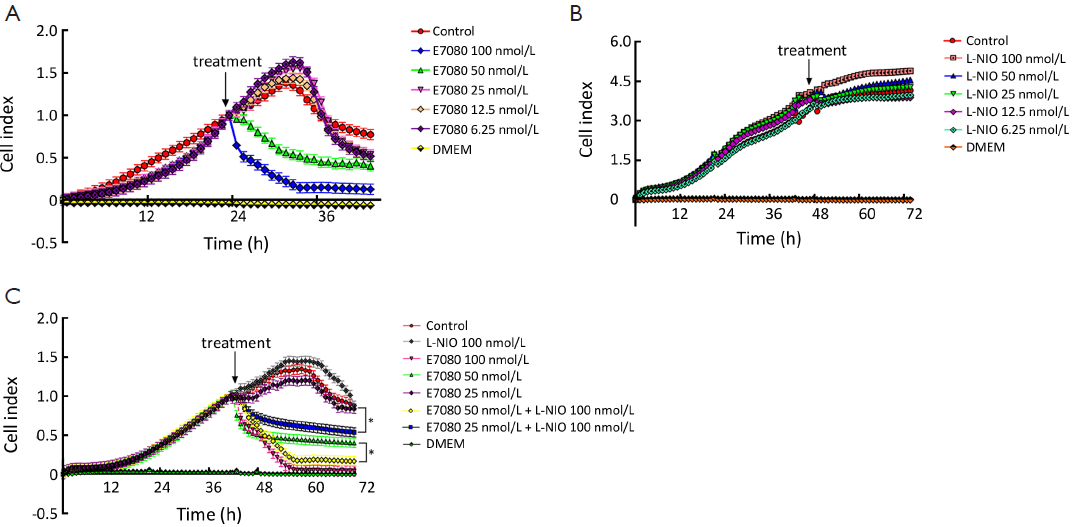
Next, we used the 40,000 cells/well concentration in the xCELLigence assay to examine the antiproliferative effects elicited by E7080 and L-NIO, as the 40,000 cells/well concentration has an optimal treatment window during 16-24 h (Figure 2). E7080 treated HT29 cells exhibited decreasing CI values in a concentration-dependent manner. While 100 nmol/L has shown complete cytotoxic effect and decreased CI to 0.1, 50 nmol/L decreased CI to 0.4. Both showed statistically significant cytotoxic effect when compared with the control (P<0.05) (Figure 3A). On the other hand, none of the concentrations of L-NIO caused any cytotoxic effect (Figure 3B). After L-NIO administration, CIs were the same as the CI of control. In order to determine interaction between E7080 and L-NIO, 50 and 25 nmol/L E7080 were combined with 100 nmol/L L-NIO which has no cytotoxic effect on HT29 cells. E7080 50 nmol/L + L-NIO 100 nmol/L caused higher cytotoxic effect on HT29 cells when compared with E7080 50 nmol/L alone (P<0.05) (Figure 3C). The CI of E7080 50 nmol/L + L-NIO 100 nmol/L was significantly low when compared the E7080 50 nmol/L alone (P<0.05). Also for the combination of L-NIO with 25 nmol/L E7080, the concentration which has no cytotoxic effect on HT29, with the ineffective concentration of L-NIO caused statistically significant cytotoxic effect on HT29 cells when compared with the control group (P<0.05) (Figure 3C). Twenty-four hours after treatment with E7080, the IC50 of 5.60×10–8 mol/L was achieved (Figure 4).
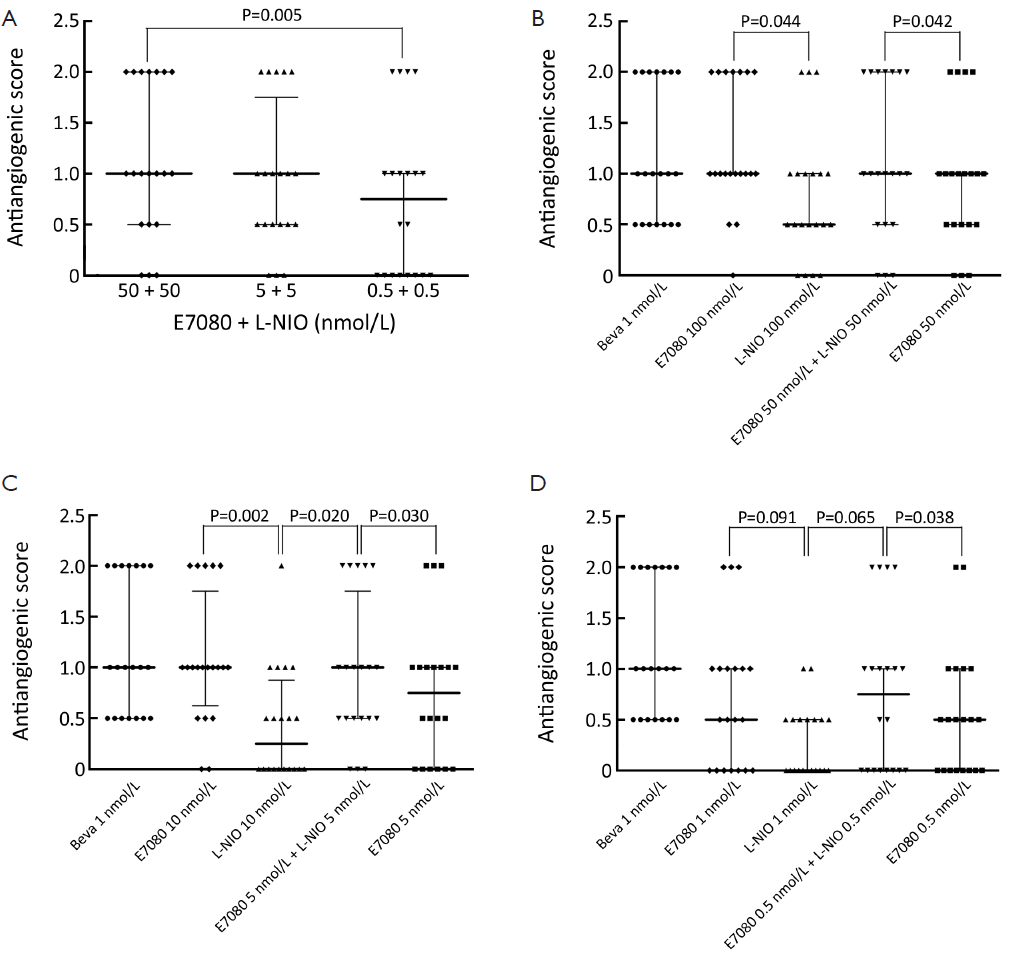
Determining antiangiogenic effects of E7080 and L-NIO alone and in combination
The eggs on which a 10 µL-agarose pellet with no drug was installed demonstrated no significant antiangiogenic effect (average antiangiogenic score =0.2). All the study drugs demonstrated some antiangiogenic effect compared with the negative control (P<0.05). Each tested drug is evaluated separately and the results with different solutions were compared.
Figure 5 shows the antiangiogenic scores of E7080, L-NIO and E7080 + L-NIO in 100, 10 and 1 nmol/L concentrations. Antiangiogenic scores of E7080 100, 10 and 1 nmol/L were 1.2, 1.0 and 0.6, respectively. These scores show that E7080 caused concentration-dependent antiangiogenic effect on CAM. On the other hand, administration of L-NIO in 100, 10 and 1 nmol/L did not cause antiangiogenic effect. The antiangiogenic scores of L-NIO were 0.6, 0.3 and 0.1, respectively. In order to determine the interaction between E7080 and L-NIO, these agents were combined in 50+50, 5+5 and 0.5+0.5 nmol/L. The antiangiogenic scores were 1.1, 0.8 and 0.7, respectively. Also antiangiogenic scores of E7080 50, 5, and 0.5 nmol/L alone were 0.8, 0.65 and 0.4, respectively. The antiangiogenic scores of E7080 + L-NIO 50+50 nmol/L were significantly high when compared with the scores of E7080 50 nmol/L alone and there was no significant difference when compared with E7080 100 nmol/L alone (P<0.05). Similar results were observed when lower concentrations of E7080 and L-NIO were combined. A combination of 5+5 nmol/L of both drugs had better antiangiogenic effect than E7080 10 nmol/L alone and a combination of 0.5+0.5 nmol/L both drugs had better antiangiogenic effect than E7080 1 nmol/L alone.
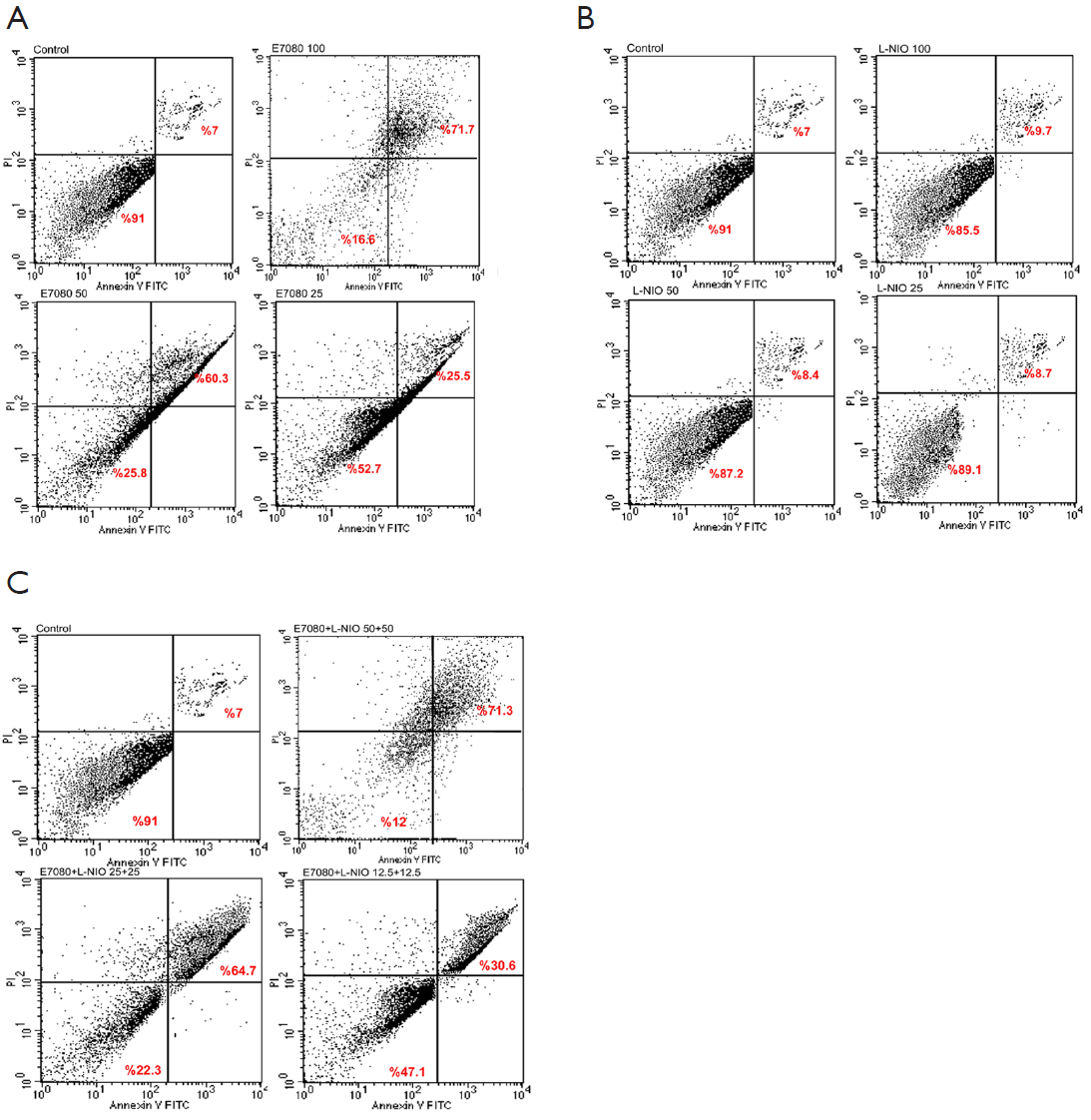
Determining apoptotic effects of E7080 and L-NIO alone and in combination
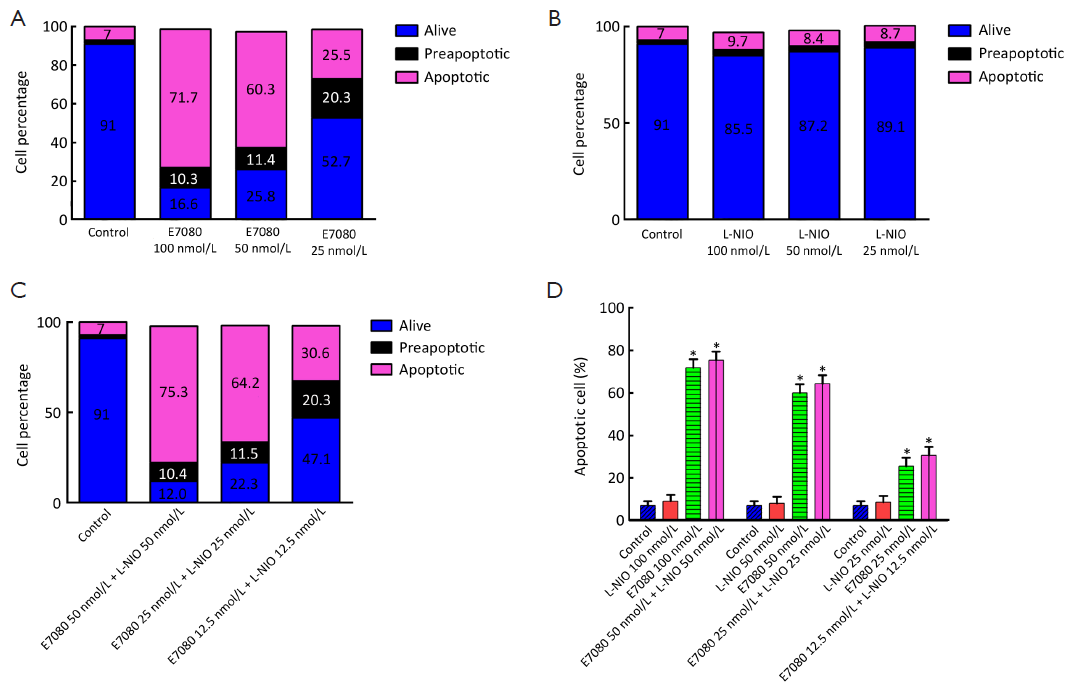
The apoptosis induction was assessed by Annexin V-FITC assay. In the dot plot of flow cytometric analysis (Figure 6), the lower-right (LR) area was the Annexin V positive/PI negative portion which represented the preapoptotic fraction, while the upper-right (UR) area was the Annexin V positive/PI positive portion which represented the apoptotic fraction. The percentage of UR (apoptosis portion) area increased gradually according to the concentration of E7080 from 7% in control group to 71% in 100 nmol/L E7080, which means E7080 caused concentration-dependent apoptosis in HT29 cells. On the other side, none of the concentrations of L-NIO caused any apoptotic effect. But the combination of E7080 with L-NIO of the concentrations which did not cause any apoptotic affect, significantly increased the apoptotic effect of E7080 on HT29 cells (P<0.05) (Figure 7).
Discussion
Colorectal cancer is one of the common human malignant diseases and a leading cause of cancer-related deaths worldwide. Despite recent advances in chemotherapy, the 5-year survival rate for metastatic colorectal cancer remains below 10% (19).
Tumor angiogenesis is defined as the process of the formation of blood vessels within a tumor, and it plays a key role in tumor growth. Enhanced expression of VEGF is correlated with Dukes’ grade, distant metastasis, and poor prognosis. VEGF is a potent, specific mitogen for endothelial cells; it activates the angiogenic switch in vivo and enhances vascular permeability. VEGF binds to three tyrosine kinase receptors, two of which, flt-1 [VEGF receptor 1 (VEGFR-1)] and flk-1/KDR (VEGFR-2), are present on endothelial cells, and one, flt-4 (VEGFR-3), is present on lymphatic endothelial cells (20-22). VEGFR-2 is considered to be the dominant signaling receptor for endothelial cell permeability, proliferation, and differentiation. It has been reported that increased tyrosine kinase activity of VEGFR is capable of enhancing the expression of malignant phenotypes. Therefore, the VEGF tyrosine kinase receptor may be a useful target for cancer therapy (23). Also it is well known that in tumor cells, VEGFR2 is strongly auto-phosphorylated by increased expression of VEGFs and mediates a series of mitogenic and survival downstream responses including mitogen activated protein kinase (MAPK) pathway PI3K/Akt pathway survival signaling pathway (24). The PI3K/Akt pathway is one of the most popular areas for targeted cancer therapy. Both preclinical and clinical data indicate that alterations of this pathway lead to oncogenesis (25).
There are different kinds of tyrosine kinase inhibitors (TKIs) including C-kit, EGFR/Her1 and Her2, VEGF and PDGF TKIs. These different kinds of TKIs are found to be effective in treatment of various types of cancers including chronic myelogenous leukemia (CML) (26), non-small-cell lung cancer (NSCLC) (27), renal cell carcinoma (28) and prostate carcinoma (29).
E7080 is a potent inhibitor of VEGFR-2 and VEGFR-3 with IC50 of 4 and 5.2 nmol/L respectively, but also has activity against VEGFR-1, FGFR-1, and PDGFRa/b tyrosine kinases although the IC50 is around 10 fold higher (12). E7080 shows antitumor activity in human cancer cell xenografts, which has been attributed to its ability to inhibit angiogenesis predominantly through effects on VEGFR-2 inhibition but also through inhibition of KIT and FGFR-1 (30,31).
It has been shown that E7080 inhibited proliferation of human SCLC, H526 cells, which expressed KIT, at the concentrations required for the inhibition of KIT kinase (11). In a recently published study, Bruheim S et al. found that E7080 had broad antitumor activity against a panel of xenografts derived from various histotypes of human sarcomas, accompanied by a substantial reduction in microvessel densities (30). Also Glen H et al. showed that E7080 treatment (both at 1 and 10 µmol/L) resulted in a significant inhibition of cell migration of both DX3 melanoma and U2OS osteosarcoma cell lines. E7080 also inhibited the ability of DX3 melanoma cells to invade into a Matrigel plug. In the same study, it has been shown that E7080 inhibited the proliferation of cell lines including E375 melanoma and DUI145 prostate cancer cells (32). All these studies are consistent with our study. In our study, we found that E7080 inhibited the proliferation of HT29 colorectal cancer cells in a concentration-dependent manner with 56 nmol/L IC50. In the studies mentioned above, it has been pointed out that the antiproliferative effect of E7080 has been seen at higher concentrations and the antiangiogenic effect mediated by VEGFR2 was the main mechanism of antitumoral effect. In our study, E7080 showed its antiproliferative effect at lower concentrations and it seems the antiproliferative effect was as important as the antiangiogenic effect. The difference may be related to that E7080 shows potent antitumor effects in xenograft models of various types of tumors by inhibiting angiogenesis, especially through VEGFR suppression (10). Moreover, E7080 can cause regression of tumors induced by human lung cancer H146 cells, which produce SCF, due to its antiangiogenic activity mediated by inhibition of both C-kit and VEGFR signaling (11). These findings suggest that E7080 may inhibit VEGF-dependent and VEGF-independent angiogenesis and have antitumor activity against a broad spectrum of human solid tumors by suppressing not only VEGFR2, but also other receptor tyrosine kinases (31).
The studies about E7080 are mainly focused on angiogenesis. Ikuta K et al. (31) showed E7080 inhibited VEGF-induced HMVEC proliferation in a dose-dependent manner, and showed complete blockade at 10 nmol/L. In a study in which tube formation of HUVEC was evaluated, it has been determined that E7080 inhibited either VEGF or SCF-induced tube formation of HUVEC (11). In addition to these in vitro studies, Matsui J et al. showed that E7080 has inhibited the metastasis to either regional lymph nodes or distant lung in the MDA-MB-231, human breast adenocarcinoma cells, xenograft model (10). Consistently, in our study, we demonstrated that E7080 caused inhibition of cell proliferation of HUVECs in vitro (data not shown) and the microvessels developing on CAM in vivo CAM model. It is obvious that E7080 has a strong antiangiogenic effect possibly mediated by VEGF2R.
Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt/mTOR pathways are often regulated by tumor suppressor P53. Furthermore, P53 activity is likewise regulated by the Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt/mTOR pathways. p53 is a critical tumor suppressor gene which encodes a transcription factor that is frequently mutated in human cancer (33). Akt can phosphorylate MDM-2 which leads to its proteasomal degradation and prevents it ability to interact with and destabilize P53 (34). The p53 and MDM families of genes are critically involved in the response to apoptosis (35). Although it is expected that using TKIs which are mainly target PI3K/Akt/mTOR pathway results in apoptosis, there is no conclusive data about the effects of E7080 on apoptosis. In our study, we demonstrated that E7080 has a strong apoptotic effect on HT29 colorectal cancer cells. This apoptotic effect possibly takes part in strong antitumor effect of E7080 along with antiangiogenic properties.
Treatments with TKIs, in some cases, have given promising results. However, most tumors treated with TKIs became resistant to treatment in a short time (36). Clinical experience has shown that only a percentage of patients respond to targeted therapies, even if their tumor expresses the altered target. This primary resistance to treatment is often due to constitutive activation of downstream signal transducers (37). It seems resistance is the major threat for the tyrosine kinase treatment. When it is thought major mechanism for resistance is related to the downstream signal transducers, it seems wise to combine TKIs with an agent which does not share the same mechanism with kinase inhibitors.
A number of activities mediated by NO may contribute to its tumor enhancing effects, including induction of DNA damage (38), increased angiogenesis (39), and prevention of apoptosis (40). Multiple clinical observations indicate that eNOS is not only expressed in normal tissues, but also extensively expressed in tumor tissues. eNOS is relevant in tumor progression, as it has been shown to modulate cancer-related events like angiogenesis, apoptosis, cell cycle, invasion and metastasis (41).
In our study, we determined the effects of L-NIO, eNOS inhibitor, on HT29 cell proliferation, angiogenesis and apoptosis. While L-NIO did not show any antiproliferative and apoptotic effect, it showed slightly low antiangiogenic effect. It may be an expected result. Although eNOS is important in carcinogenesis, the main source of NO in certain circumstances including inflammation and cancer is inducible NO synthase. During the last two decades, iNOS has been reported to be associated with several human malignant tumors including breast, brain, lung, prostate, colorectal and pancreatic carcinomas, Kaposi’s sarcoma (35) and melanoma (42). Ineffective effects of L-NIO may also be the results of concentrations used in this study.
Surprisingly, when we combined E7080 with ineffective concentrations of L-NIO, the antiproliferative, antiangiogenic and apoptotic effects of E7080 were increased by L-NIO. This result suggests that there should be a link between MI3K/Akt/mTOR pathway and eNOS. Tanimoto T et al. mentioned in 2002 that VEGF stimulates PI3K and Akt-dependent phosphorylation of eNOS, resulting in activation of eNOS and increased NO production (43). Furthermore, Wang Y et al. showed that phosphorylation of eNOS and NO production can be upregulated by the PI3K/AKT pathway in the angiogenesis of endothelial cells, and osteopontin, an integrin-binding protein, increases AKT and eNOS phosphorylation and NO production thereby stimulates angiogenesis. The inhibition of AKT phosphorylation by treatment with the PI3K inhibitor LY294002 reduced eNOS phosphorylation and NO production, leading to the suppression of angiogenesis in vitro (44). These studies may indicate that PI3K/Akt/mTOR and eNOS/NO pathways are connected to each other, and simultaneous inhibition of both pathways may increase downstream effects.
In conclusion, in this study we found that E7080, multityrosine kinase and VEGF2-VEGF3 inhibitor, has a strong antiproliferative, antiangiogenic and apoptotic effects on HT29 colorectal cancer cells. While administration the eNOS inhibitor L-NIO alone did not show any effect, the combination of E7080 with L-NIO showed stronger antiproliferative, antiangiogenic and apoptotic effects than E7080 alone. This study shows that the combination of TKIs with eNOS inhibitors may enable the use of TKI in lower concentrations which may prevent resistance to TKIs. Further studies are needed to enlighten the mechanism of action and understand the nature of these two drug’s combination.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Nasti G, Ottaiano A, Berretta M, et al. Pre-operative chemotherapy for colorectal cancer liver metastases: an update of recent clinical trials. Cancer Chemother Pharmacol 2010;66:209-18. [PubMed]
- Köhne CH, Wils J, Lorenz M, et al. Randomized phase III study of high-dose fluorouracil given as a weekly 24-hour infusion with or without leucovorin versus bolus fluorouracil plus leucovorin in advanced colorectal cancer: European organization of Research and Treatment of Cancer Gastrointestinal Group Study 40952. J Clin Oncol 2003;21:3721-8. [PubMed]
- Comella P, Casaretti R, Sandomenico C, et al. Capecitabine, alone or in combination, in the management of patients with colorectal cancer: a review of the evidence. Drugs 2008;68:949-61. [PubMed]
- Köhne CH, van Cutsem E, Wils J, et al. Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J Clin Oncol 2005;23:4856-65. [PubMed]
- Iqbal S, Lenz HJ. Integration of novel agents in the treatment of colorectal cancer. Cancer Chemother Pharmacol 2004;54 Suppl 1:S32-9. [PubMed]
- Stockmann C, Doedens A, Weidemann A, et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 2008;456:814-8. [PubMed]
- Cohen SJ, Cohen RB, Meropol NJ. Targeting signal transduction pathways in colorectal cancer more than skin deep. J Clin Oncol 2005;23:5374-85. [PubMed]
- Matsui J, Funahashi Y, Uenaka T, et al. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res 2008;14:5459-65. [PubMed]
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 2008;122:664-71. [PubMed]
- Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res 2007;67:1407-10. [PubMed]
- Lu J, Wei Q, Bondy ML, et al. Promoter polymorphism (–786t>C) in the endothelial nitric oxide synthase gene is associated with risk of sporadic breast cancer in non-Hispanic white women age younger than 55 years. Cancer 2006;107:2245-53. [PubMed]
- Duda DG, Fukumura D, Jain RK. Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends Mol Med 2004;10:143-5. [PubMed]
- Díaz-Troya S, Najib S, Sánchez-Margalet V. eNOS, nNOS, cGMP and protein kinase G mediate the inhibitory effect of pancreastatin, a chromogranin A-derived peptide, on growth and proliferation of hepatoma cells. Regul Pept 2005;125:41-6. [PubMed]
- Roche Diagnostics GmbH. Introduction of the RTCA SP Instrument Operator’s Manual, A. Acea Biosciences, Inc. 2008;14-6.
- Bürgermeister J, Paper DH, Vogl H, et al. LaPSvS1, a (1-->3)-beta-galactan sulfate and its effect on angiogenesis in vivo and in vitro. Carbohydr Res 2002;337:1459-66. [PubMed]
- Demirci B, Dadandi MY, Paper DH, et al. Chemical composition of the essential oil of Phlomis linearis Boiss. & Bal., and biological effects on the CAM assay: a safety evaluation. Z Naturforsch C 2003;58:826-9. [PubMed]
- Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2005;23:8664-70. [PubMed]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249-57. [PubMed]
- Fan F, Wey JS, McCarty MF, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene 2005;24:2647-53. [PubMed]
- Pradeep CR, Sunila ES, Kuttan G. Expression of vascular endothelial growth factor (VEGF) and VEGF receptors in tumor angiogenesis and malignancies. Integr Cancer Ther 2005;4:315-21. [PubMed]
- Lu Y, Zhang J, Qian J. The effect of emodin on VEGF receptors in human colon cancer cells. Cancer Biother Radiopharm 2008;23:222-8. [PubMed]
- Wu LW, Mayo LD, Dunbar JD, et al. Utilization of distinct signaling pathways by receptors for vascular endothelial cell growth factor and other mitogens in the induction of endothelial cell proliferation. J Biol Chem 2000;275:5096-103. [PubMed]
- Witzig TE, Reeder CB, LaPlant BR, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia 2011;25:341-7. [PubMed]
- Druker BJ. Imatinib and chronic myeloid leukemia: validating the promise of molecularly targeted therapy. Eur J Cancer 2002;38 Suppl 5:S70-6. [PubMed]
- Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003;290:2149-58. [PubMed]
- Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006;295:2516-24. [PubMed]
- Ko YJ, Small EJ, Kabbinavar F, et al. A multi-institutional phase II study of SU101, a platelet-derived growth factor receptor inhibitor, for patients with hormone-refractory prostate cancer. Clin Cancer Res 2001;7:800-5. [PubMed]
- Bruheim S, Kristian A, Uenaka T, et al. Antitumour activity of oral E7080, a novel inhibitor of multiple tyrosine kinases, in human sarcoma xenografts. Int J Cancer 2011;129:742-50. [PubMed]
- Ikuta K, Yano S, Trung VT, et al. E7080, a multi-tyrosine kinase inhibitor, suppresses the progression of malignant pleural mesothelioma with different proangiogenic cytokine production profiles. Clin Cancer Res 2009;15:7229-37. [PubMed]
- Glen H, Mason S, Patel H, et al. E7080, a multi-targeted tyrosine kinase inhibitor suppresses tumor cell migration and invasion. BMC Cancer 2011;11:309. [PubMed]
- Demidenko ZN, Korotchkina LG, Gudkov AV, et al. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci USA 2010;107:9660-4. [PubMed]
- Martelli AM, Evangelisti C, Chiarini F, et al. The emerging role of the phosphatiylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in normal myelopoiesis and leukemogensis. Biochim Biophys Acta 2010;1803:991-1002.
- O’Prey J, Crighton D, Martin AG, et al. p53-mediated induction of Noxa and p53AIP1 requires NFkappaB. Cell Cycle 2010;9:947-52. [PubMed]
- Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev 2008;18:73-9. [PubMed]
- Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res 2009;69:1851-7. [PubMed]
- Liu RH, Hotchkiss JH. Potential genotoxicity of chronically elevated nitric oxide: a review. Mutat Res 1995;339:73-89. [PubMed]
- Fukumura D, Jain RK. Role of nitric oxide in angiogenesis and microcirculation in tumors. Cancer Metastasis Rev 1998;17:77-89. [PubMed]
- Kolb JP. Mechanisms involved in the pro- and anti-apoptotic role of NO in human Leukemia 2000;14:1685-94. [PubMed]
- Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res 2007;75:247-60. [PubMed]
- Singh S, Gupta AK. Nitric oxide: role in tumour biology and iNOS/NO-based anticancer therapies. Cancer Chemother Pharmacol 2011;67:1211-24. [PubMed]
- Tanimoto T, Jin ZG, Berk BC. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS). J Biol Chem 2002;277:42997-3001. [PubMed]
- Wang Y, Yan W, Lu X, et al. Overexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the avβ3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells. Eur J Cell Biol 2011;90:642-8. [PubMed]