Apoptosis of bladder transitional cell carcinoma T24 cells induced by adenovirus-mediated inducible nitric oxide synthase gene transfection
Bladder cancer is one of the most common urinary tract tumors in China with an increase of incidence year by year, more than 90% of which are transitional cell carcinoma. Recently, NO has been demonstrated to be one of regulatory signals responsible for tumor cell apoptosis and it plays bilateral roles in tumor genesis and development, which are intracellular NO concentration- and time- dependent (1). High NO concentration could suppress tumor genesis and promote cell apoptosis, which might be a novel approach of anti-tumor therapy (2). Inducible nitric oxide synthase (iNOS) gene is located on chromosome 17 with a length of 37 kb. It has 26 exons and 25 introns, encoding a homologous protein dimer of 260 kD. Its product is NO. iNOS exerts its effect through a NO acting mechanism. Hubert et al. (3) and Hayashi et al. (4) observed the over-expression of iNOS mRNA and protein in bladder cancer tissues. iNOS is also widely found in many tumor cell lines and solid tumor tissues, such as head and neck squamous cell carcinoma, prostate cancer, gynecological cancer, breast cancer and lung cancer. Recent studies suggested iNOS found in solid tumors might vital roles in tumor genesis and development. However, the evidences of correlation between iNOS vs. tumor cell proliferation and apoptosis were still inconsistent. Kagoura et al. (5) and Aaltoma et al. (6) reported iNOS correlates to tumor cell proliferation in case of skin cancer and prostate cancer. But Kong et al. (7) and Ropponen et al. (8) found iNOS is related to tumor apoptosis in case of pancreatic cancer.
In this study, exogenous iNOS was transfected into T24 human bladder carcinoma cells by the adenovirus-mediated method, which induced intracellular iNOS expression and high NO concentration. The effect of iNOS over-expression was examined, which provided an experimental basis for the iNOS gene therapy against bladder transitional cell carcinoma.
Materials and methods
Experimental materials
Human bladder cancer cell line T24 was supplied by the cell center, Xiangya Hospital of Central South University. E. coli DH5α was supplied by the Central Laboratory, the Third Xiangya Hospital of Central South University. The eukaryotic expression vector of pReceive-M29-iNOS is constructed by our hospital laboratory. Adenovirus shuttle vector pYr-adshuttle-1, the control adenovirus rAd-NC and rAd-EGFP was purchased from Changsha Yinrun Biotechnology Co., Ltd. Adenoviral backbone vector pAd/BL-DEST was purchased from Invitrogen Corporation. Adenovirus packaging cell line HEK293 was purchased from ATCC (USA).
Methods
Construction and identification of recombinant adenovirus rAd-iNOS
In order to ensure the correctness of template plasmid pReceive-M29-iNOS sequence, the template plasmid was sequenced. After sequencing, the iNOS segment strips of double enzyme digestion pReceive-M29-iNOS and adenovirus shuttle vector pYr-adshuttle-1 and the linear strip of pYr-adshuttle-1 vector were recovered during agarose gel electrophoresis. After ligation, transformation and shaking amplification of competent E. coli DH5α, recombinant plasmid pYr-adshuttle-1-iNOS was extracted for the identification of restriction enzyme digestion and DNA sequencing. With the correctly identified recombinant plasmid, the expression cassette of iNOS was subcloned into adenoviral expression vector pAd/BL-DEST using LR in vitro homologous recombination approaches. The adenovirus vector pAd-iNOS was digested by Pac enzyme. Packaging cell HEK 293 was transfected by the linearized recombinant adenovirus vector to afford adenovirus rAd-iNOS which was used for PCR identification and viral titer measurement.
Apoptosis and related gene detection of T24 cells with rAd-iNOS transfection
After T24 cell culture, the optimal transfection replicates of T24 cells were determined by recombinant adenovirus with green fluorescent protein expression. T24 cells were selected in their exponentially growth phase in 24 hours before infection. There were three experimental groups designed in this study: (I) recombinant adenovirus rAd-iNOS infection group; (II) control adenovirus infection group; (III) blank control group. When a density of 60-70% was reached, 100 MOI recombinant adenovirus or control adenovirus were added and the culture was placed in a 37 °C, 5% CO2 incubator for 4 hours, and fresh complete culture medium was replaced, cells of each well was collected after culture for 48 hours and trypsin digestion. The mRNA profiles of iNOS and P53 were examined by RT-PCR method; the protein expression profiles of iNOS and P53 were examined by Western approach; the NO secretion profiles of cells in each group were examined by NO detection kit. Apoptosis and necrosis of each group were examined under fluorescence microscope; cell apoptosis of each group were examined by flow cytometric analysis.
Statistics
SPSS10.0 statistical software was employed for data processing. Data were expressed as mean ± standard deviation. Comparison of each set of data was conducted by using t-test and P<0.05 was considered to be statistically significant.
Results
Identification of recombinant adenovirus vector rAd-Inos
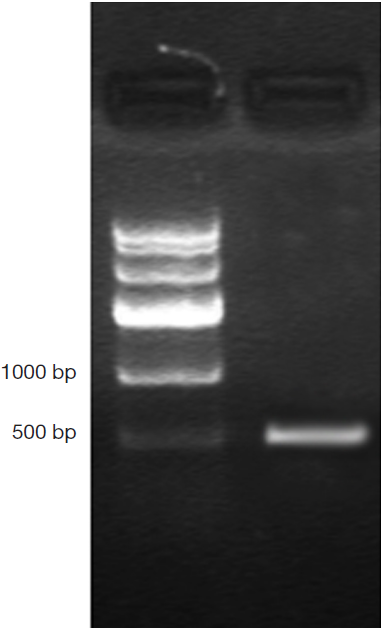
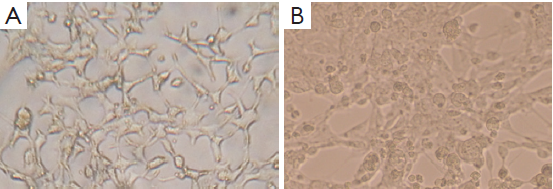
DNA genome of RAd-iNOS was extracted and iNOS gene fragments were amplified with the designed iNOS primers. The PCR products of iNOS fragments were analyzed by agarose gel electrophoresis and examined by GIS gel system. iNOS straps (approx. 500 bp) were developed, see Figure 1. The size of amplified PCR fragments were consistent with the theoretical value of iNOS fragments and the packaging of recombinant adenovirus rAd-iNOS packaging was identified successfully. HEK293 cell line was used in rAd-iNOS adenovirus amplification. These cells are in a disease state after virus infection, see Figure 2. The viral titer of rAd-iNOS was 5.8×108 PFU/mL, as determined by double dilution method.
rAd-iNOS transfection promoted T24 cell apoptosis
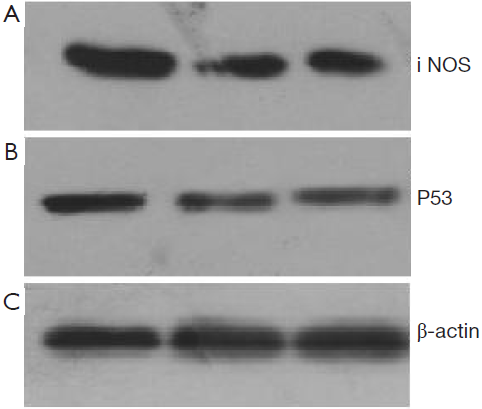
mRNA and protein expression of iNOS and P53 examined by RT-PCR and Western blot approaches
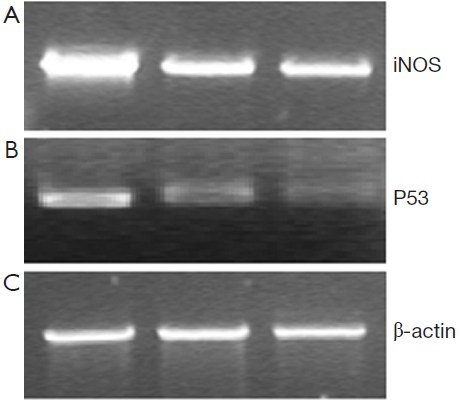
48 hours after rAd-iNOS infection, the mRNA expression profiles of iNOS and mutant p53 gene were examined by RT-PCR with untransfected T24 cells as the blank control group and control adenovirus rAd-NC T24 cells as a negative control group. The results suggested the expression profiles of iNOS and mutant p53 gene of recombinant adenovirus rAd-iNOS infection group was higher significantly than negative and blank control groups. See Figure 3. The protein expression profiles and iNOS and P53 were examined by Western blot method. The protein expression profiles of iNOS and mutant P53 of recombinant adenovirus rAd-iNOS infection group was higher significantly than negative and blank control groups. See Figure 4. P<0.05.
Concentration of NO in the cell culture medium
Forty-eight hours after adenovirus infection for each group, the culture supernatant NO concentrations of recombinant adenovirus rAd-iNOS infected group, control adenovirus rAd-NC infection group and blank control group were determined to be 311.42±21.34, 135.85±16.74 and 133.39±9.51 µmol/L, respectively. After the infection of iNOS recombinant adenovirus, the secretion of NO level was higher than pre-transfection level (P<0.05). There was no significant difference between the blank control group and the control adenovirus group (P>0.05).
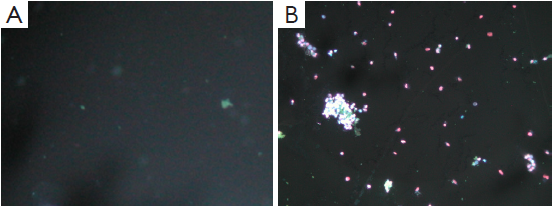
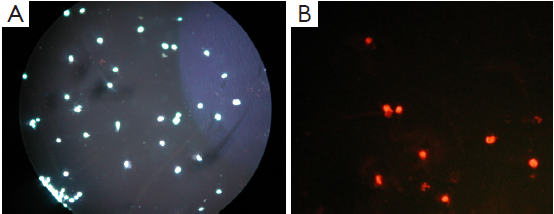
Apoptosis and necrosis under fluorescence microscope
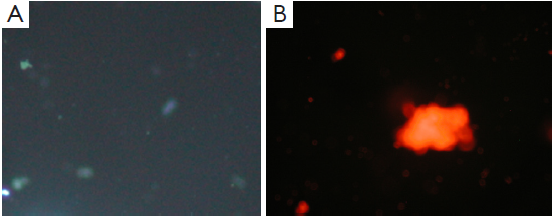
Apoptosis and necrosis were examined under fluorescence microscope after the double staining of Hoechst 33342 and PI. Fluorescence images of T24 cells were shown in Figures 5-7 for the blank control group, adenovirus infection control group and recombinant adenovirus group. Under fluorescence microscopy, findings of the vector group and the control group were substantially the same. However, the cell apoptosis of rAd-iNOS transfected group was significantly increased.
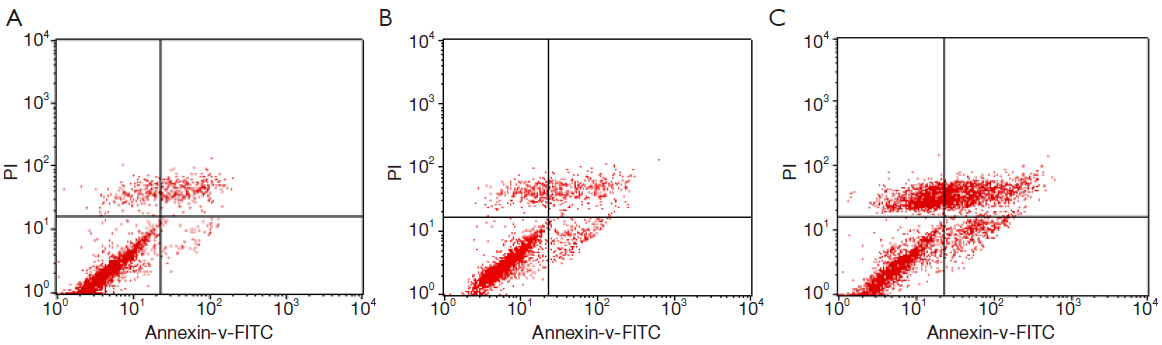
Apoptosis and necrosis analyzed by flow cytometry
The apoptosis rates of blank control group, adenovirus infection control group and recombinant adenovirus group were 15.87%±1.24%, 18.49%±2.01% and 41.32±3.03%, respectively. The apoptosis rates of T24 cells with recombinant adenovirus rAd-iNOS infection were higher than those of adenovirus infection control group and blank control group. The differences were statistically significant (P<0.05). See Figure 8.
Discussion
Bladder cancer is one of the most common malignancies of urogenital system. Most of Bladder tumors are transitional cell carcinoma, with characteristics of multi-center origin and easy relapse. Tumor genesis and development is a multi-stage, multi-step process. Malignant cells are associated with genetic changes and abnormalities for each step, which might become potential targets of gene therapy. The effectiveness of gene therapy is related to the selection of validated target gene. Meanwhile, it is also important to choose appropriate vector to carry target genes to target cells and to maintain highly efficient and long-term expression. Adenovirus vectors are vectors commonly used for current gene therapies. Adenovirus has the characteristics of tropism to bladder (9) and bladder is a semi-open organ, which made it possible to treat bladder cancer by the gene therapy of adenoviral vectors. Currently, the relationships between tumors and inducible nitric oxide synthase (iNOS), as well as the product of nitric oxide (NO), have attracted growing concerns. In this study, in order to investigate the effect of iNOS on the bladder transitional cancer cell growth, a recombinant adenovirus carrying the iNOS gene rAd-iNOS was constructed and transfected into bladder transitional cell cancer T24 cells by adenoviral transfection system to promote apoptosis of bladder cancer cells. This study provided a novel insight to the gene therapy of bladder cancer.
NO is a kind of free radical in vivo. Its structure is simple and is generated by NOS catalysis. It is involved in the pathological and physiological processes of many diseases (10), with complicated and diverse biological activities. The basal activities of inducible nitric oxide synthase (iNOS) is generally very low, and high concentration of NO will be synthesized in pathological conditions (inflammation, cancer, etc.) and produce cytotoxicity activities through direct and monocyte-macrophage-mediated cytotoxic activities to suppress tumor cell growth or tumor cell death. It has been demonstrated that NO is one of the critical signals for tumor apoptosis (11-13), but endogenous NO can also stimulate tumor growth and metastasis through promoting blood vessel growth and blood flow regulation. Therefore, NO has a bidirectional effect on tumor genesis and development. At low concentration, NO could promote tumor development. But at high concentration, NO could suppress tumor genesis and stimulate apoptosis (14). The specific mechanism of the cytotoxic effect of NO is still unknown. Most studies suggested NO could bind to a FeS site of the critical enzyme responsible for tumor cell metabolism and form iron-nitrosyl complexes, which in term cause iron loss, energy metabolism blockage and DNA replication, tumor cell suppression or apoptosis. NO could stimulate the expression of multiple tumor suppressor genes including p53 (15), suppress anti-apoptotic factor NF-κB (16) and induce apoptosis. It can also react with superoxide anion O2- to form ONOO-, which could be protonated and split into more toxic species of hydroxyl radical (OH.) and stable NO2. The highly active OH. species could induce cell injury from many aspects, e.g., lipid peroxidation, protein and amino acid cross-linkage, as well as covalent linkage between RNA and DNA. All these activities contribute to the anti-tumor activities of NO (1,17).
It has been demonstrated that regulation of iNOS gene activation of tumors in vivo could produce anti-tumor effects (18-20). Soler et al. (21) injected cDNA containing iNOS into medullary thyroid cancer directly and observed rat tumor necrosis and slowed tumor growth. Khare et al. (22) used engineering recombinant retroviral vector to selectively identify cells expressing carcinoembryonic antigen which induces targeted apoptosis of cancer cells and suppresses tumor growth. By cloning the cDNA of human iNOS transfected into human embryonic kidney cells and packaging into microcapsules, high NO concentration could be achieved. After the administration of these microcapsules into nude mice, the growth of colon and ovarian cancer could be suppressed significantly (23). In this study, we applied iNOS transgenic technology in the gene therapy of bladder cancer. Recombinant adenovirus carrying iNOS gene has been constructed successfully and it was transfected into transfected bladder carcinoma T24 cells successfully to achieve high iNOS expression. Determination of NO content showed the NO concentration was significantly higher than the baseline before viral infection (P<0.05), and was significantly higher than control adenovirus infection group. As shown by flow cytometry analysis, the apoptosis rate of cells infected by recombinant adenovirus rAd-iNOS was significantly higher than blank cell group and control adenovirus infection group. All these results suggest iNOS over-expression could produce high NO concentration and induce apoptosis of bladder carcinoma T24 cells. p53 is one of the genes closely correlated to human tumors. NO has been demonstrated to induce p53 gene expression and P53 protein accumulation (15). It was reported that iNOS mRNA is highly expressed in head and neck cancer, which correlated to P53 expression positively. The process of NO induced apoptosis is associated with p53 gene expression and P53 protein synthesis (24). Wang BS et al. (25) reported the high expression profiles of iNOS mRNA and proteins could result in P53 protein overexpression, most of which occur at the transcriptional level. As shown by our study, the iNOS protein expression of rAd-iNOS infected cell group was significantly higher than blank and control adenovirus groups. Concomitantly, this was associated with P53 protein over-expression, which shows some correlation to iNOS expression. Meanwhile, the apoptosis rate of adenovirus infection group was increased significantly, which suggested iNOS over-expression contributes to the up-regulation of p53 gene expression of cancer cells, P53 protein accumulation and then apoptosis.
As shown in our study, iNOS over-expression could produce high concentration of NO, up-regulate P53 expression of T24 cells and promote T24 apoptosis. As demonstrated by relevant studies, NO could enhance the sensitivity of tumor cells to drugs and radiation (26,27), which could be utilized to reduce chemotherapy and radiotherapy dosages, and then to reduce damages to normal tissue cells. These evidences supported the broad application prospects of iNOS gene studies in the treatments of bladder transitional cell carcinoma.
Acknowledgements
Funding: No. B2010033 Scientific Research Foundation, Health Department, Hunan Province.
Disclosure: The authors declare no conflict of interest.
References
- Muntané J, De la Rosa AJ, Marín LM, et al. Nitric oxide and cell death in liver Cancer cells. Mitochondrion 2013;13:257-62. [PubMed]
- Hussain SP, Trivers GE, Hofseth LJ, et al. Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res 2004;64:6849-53. [PubMed]
- Swana HS, Smith SD, Perrotta PL, et al. Inducible nitric oxide synthase with transitional cell carcinoma of the bladder. J Urol 1999;161:630-4. [PubMed]
- Hayashi H, Kuwahara M, Fujisaki N, et al. Immunohistochemical findings of nitric oxide synthase expression in urothelial transitional cell carcinoma including dysplasia. Oncol Rep 2001;8:1275-9. [PubMed]
- Kagoura M, Matsui C, Toyoda M, et al. Immunohistochemical study of inducible nitric oxide synthase in skin cancers. J Cutan Pathol 2001;28:476-81. [PubMed]
- Aaltoma SH, Lipponen PK, Kosma VM. Inducible nitric oxide synthase (iNOS) expression and its prognostic value in prostate Cancer. Anticancer Res 2001;21:3101-6. [PubMed]
- Kong G, Kim EK, Kim WS, et al. Inducible nitric oxide synthase (iNOS) immunoreactivity and its relationship to cell proliferation, apoptosis, angiogenesis, clinicopathologic characteristics, and patient survival in pancreatic Cancer. Int J Pancreatol 2001;29:133-40. [PubMed]
- Ropponen KM, Kellokoski JK, Lipponen PK, et al. Expression of inducible nitric oxide synthase in colorectal Cancer and its association with prognosis. Scand J Gastroenterol 2000;35:1204-11. [PubMed]
- Mufson MA, Belshe RB. A review of adenoviruses in the etiology of acute hemorrhagic cystitis. J Urol 1976;115:191-4. [PubMed]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993;329:2002-12. [PubMed]
- Edwards P, Cendan JC, Topping DB, et al. Tumor cell nitric oxide inhibits cell growth in vitro, but stimulates tumorigenesis and experimental lung metastasis in vivo. J Surg Res 1996;63:49-52. [PubMed]
- Ziche M, Morbidelli L, Choudhuri R, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 1997;99:2625-34. [PubMed]
- Leon-Bollotte L, Subramaniam S, Cauvard O, et al. S-nitrosylation of the death receptor fas promotes fas ligand-mediated apoptosis in cancer cells. Gastroenterology 2011;140:2009-18, 2018.e1-4.
- Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 2005;310:1966-70. [PubMed]
- Hussain SP, Amstad P, Raja K, et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res 2000;60:3333-7. [PubMed]
- Reynaert NL, Ckless K, Korn SH, et al. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A 2004;101:8945-50. [PubMed]
- Trachootham D, Alexandre J, Huang P. Targeting Cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 2009;8:579-91. [PubMed]
- Chakraborty JB, Mahato SK, Joshi K, et al. Hydroxychavicol, a piper betle leaf component, induces apoptosis of CML cells through mitochondrial reactive Oxygen species-dependent JNK and endothelial nitric oxide synthase activation and overrides imatinib resistance. Cancer Sci 2012;103:88-99. [PubMed]
- El-Najjar N, Chatila M, Moukadem H, et al. Reactive Oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis 2010;15:183-95. [PubMed]
- Kleinert H, Pautz A, Linker K, et al. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol 2004;500:255-66. [PubMed]
- Soler MN, Bobé P, Benihoud K, et al. Gene therapy of rat medullary thyroid cancer by naked nitric oxide synthase II DNA injection. J Gene Med 2000;2:344-52. [PubMed]
- Khare PD, Shao-Xi L, Kuroki M, et al. Specifically targeted killing of carcinoembryonic antigen (CEA)-expressing cells by a retroviral vector displaying single-chain variable fragmented antibody to CEA and carrying the gene for inducible nitric oxide synthase. Cancer Res 2001;61:370-5. [PubMed]
- Xu W, Liu L, Charles IG. Microencapsulated iNOS-expressing cells cause tumor suppression in mice. FASEB J 2002;16:213-5. [PubMed]
- Shu C, Du XD, Wang F, et al. The expression and clinical Impl ication of inducible nitric oxide synthase(iNOS)mRNA and p53 in head and neck carcinoma. Acta Academiae Medicinae Suzhou 2003;23:431-46.
- Wang BS, Song DM, Zhang JH, et al. Apoptosis of transplanted tumor ce lls induced by gene transfection of inos in nude mice and the corre lation with p53 expression. Journal of Hebei Medical University 2008;29:804-7.
- Hirakawa M, Oike M, Masuda K, et al. Tumor cell apoptosis by irradiation-induced nitric oxide production in vascular endothelium. Cancer Res 2002;62:1450-7. [PubMed]
- Adams DJ, Levesque MC, Weinberg JB, et al. Nitric oxide enhancement of fludarabine cytotoxicity for B-CLL lymphocytes. Leukemia 2001;15:1852-9. [PubMed]