Skull metastasis revealing a papillary thyroid carcinoma
Introduction
Thyroid carcinomas account for approximately 1.1% of all carcinomas, and 0.2% of all carcinoma deaths (1). The incidence of thyroid carcinoma has increased in recent years. The National Cancer Institute estimates that approximately 22,500 new cases of thyroid cancer are diagnosed each year (2). Among these, differentiated thyroid carcinomas (DTC) comprising papillary and follicular thyroid carcinoma subtypes, represent more than 90% of all thyroid carcinomas.
Papillary thyroid carcinoma (PTC) is the most common type of DTC. It is classically described as having an indolent nature, as it is typically low-grade and slowly progressive. Consequently, the prognosis is usually favorable with high survival rate. The most frequently occurring metastasis affects the regional lymph nodes, especially the cervical and mediastinal nodes. The most common sites of distant metastasis are lung and bone. Bone metastases are most likely to occur in the scapula, sternum and ilium. Skull metastases are uncommon, being found in only 2.5% to 5.8% of cases of thyroid carcinoma (3).
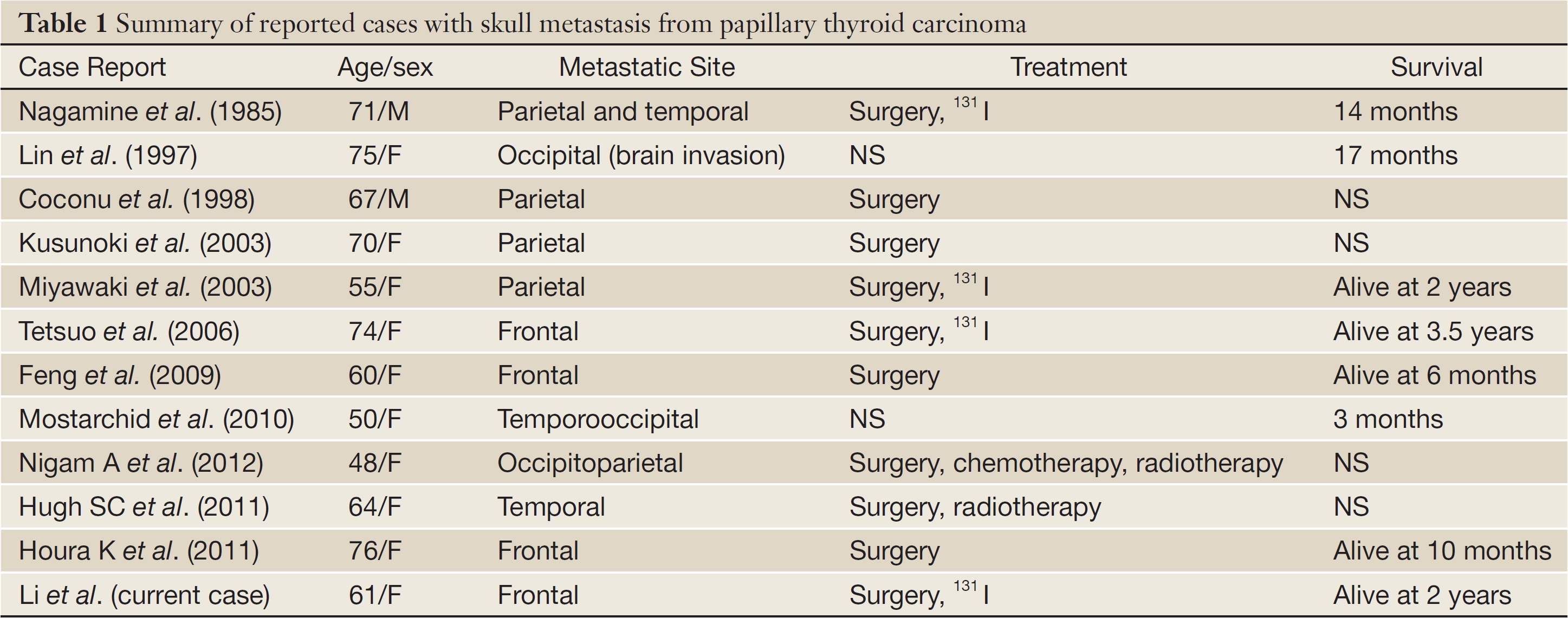
To date, details of only ten cases of skull metastasis, including three cases of frontal skull metastasis from PTC have been published. In this article, we describe a case of frontal skull metastasis of PTC occurring 13 years after near total thyroidectomy. We also discuss and review the differential diagnosis of skull metastases arising from thyroid carcinoma together with its treatment management and prognosis.
Case report
A 61-year-old Chinese woman was admitted in our Hospital with a one year history of a growing well circumscribed mass on the center of the frontal and parietal part of the skull. Detailed medical history revealed that she had undergone a near total thyroidectomy due to thyroid adenoma 13 years ago. Physical examination showed a 1.5 cm × 1.5 cm, firm, and immobile mass of the frontal and parietal area. Neurological function and visual acuity were both normal, and physical examination was unremarkable. Laboratory studies, including tumor markers, showed no abnormalities except for increased serum thyroglobulin (138.77 IU/mL) and anti-thyroglobulin antibody levels (18.51 IU/mL). All vital signs were normal.
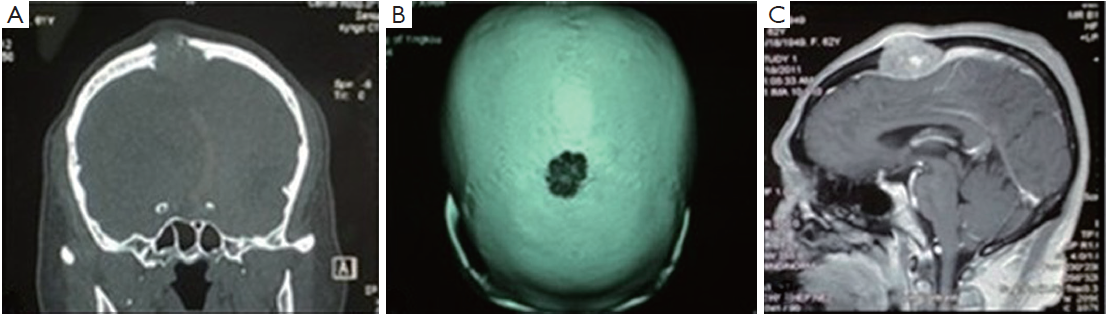
Coronal magnetic resonance imaging (MRI) showed an expansive, osteolytic solid tumor extending from the dura mater into the subcutis, which had begun to destroy part of the frontal and parietal bone (Figure 1A,B). Sagittal contrast-enhanced MRI images confirmed the presence of a destructive tumor on the frontal and parietal bone which was compressing the adjacent cortical sulci (Figure 1C).
The tumor originated from the epidural space, and was attached to the skull. It had begun to spread on both sides and was infiltrated by brown multilobulated, firm, vascular tumor tissue. The tumor tissue was resected at the bone level.
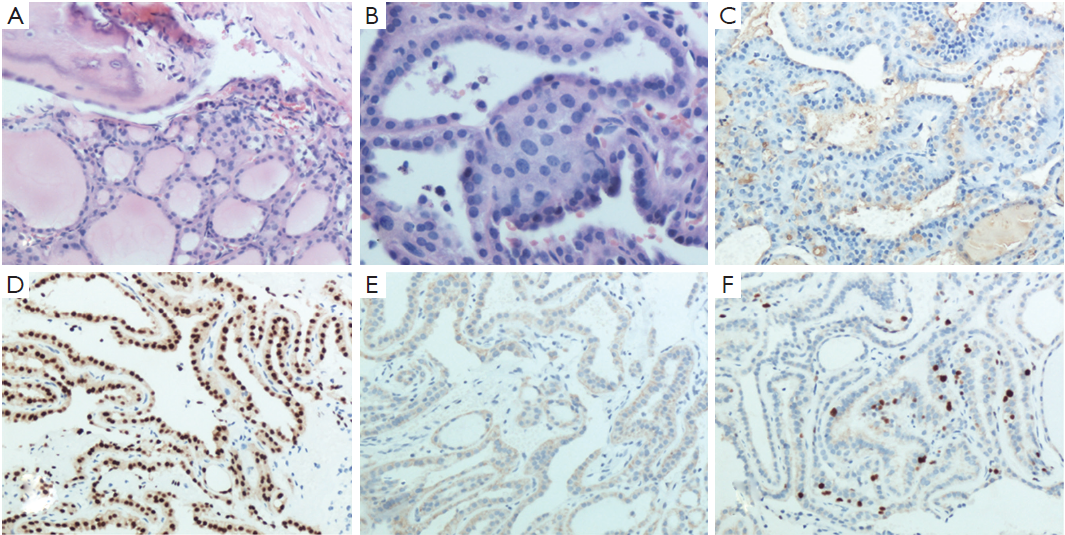
The histopathological report of the surgical biopsy revealed differentiated papillary thyroid carcinoma mixed with small fragments of skull bone. The nuclei of the tumor cells were enlarged, oval in shaped and overlapped each other. They had a typical ground glass appearance (Figure 2A,B). On immunohistochemical analysis, the tissue stained positively for thyroglobulin (Tg), thyroid transcription factor-1 (TTF-1), cytokeratin 19 (CK19) and Ki67 (Figure 2C,D,E,F). Thus, both morphology and immunohistochemistry supported the pathological diagnosis of skull metastasis from papillary thyroid carcinoma. Based on these findings, the tumor was definitively diagnosed as a metastasis from the papillary carcinoma of the thyroid.
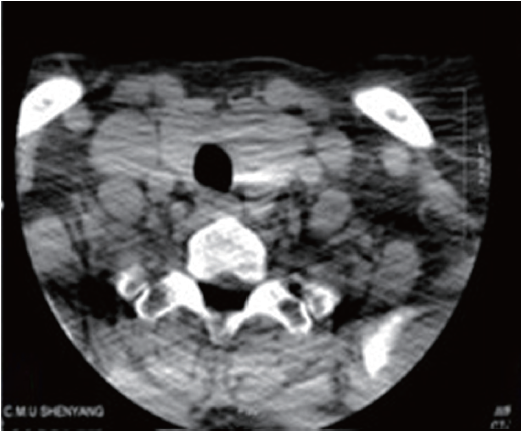
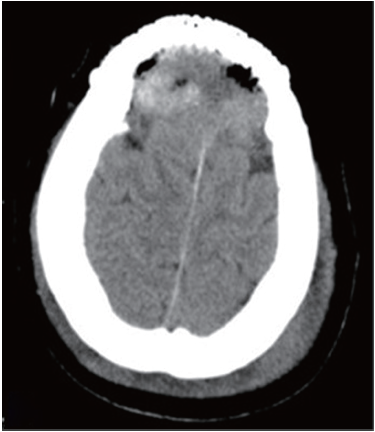
Thyroid ultrasound revealed a heteroechoic lesion with increased vascularity and foci of calcification in both the left and right lobes. CT of the neck showed that the left lobe of the thyroid was enlarged with a 1.4 cm diameter, round low density lesion that had caused tracheal compression and deviation. Both investigations showed that cervical lymph nodes findings were unremarkable (Figure 3). Based on these imaging findings the patient underwent a total thyroidectomy one month later. Histopathological examination revealed a small PTC affecting part of the left side of the thyroid. Follow up CT confirmed total postoperative removal of the tumor and no complications (Figure 4).
The patient received suppressive doses of L-thyroxin for three months postoperatively. Adjuvant radioiodine treatment using 100 mCi of 131I, given orally to treat the skull base metastasis, caused no discomfort or clinical symptoms. At present, the patient is alive two years after surgery, without evidence of recurrence or metastasis during.
Discussion
PTC is the most common type of thyroid carcinoma, accounting for 80% of all thyroid cancers in the United States. This form of cancer is two to three-times more common among women than among male subjects. Papillary thyroid carcinoma is usually associated with slow progression, with loco regional spread to the lymph nodes in the neck. Distant metastases occur in about 10% of patients. Most studies, indicate that papillary carcinoma is the least likely subtype to show bone metastases (1.4-7%) (4).
Papillary thyroid carcinoma metastases have been reported to arise in the following areas of the skull base: sella turcica and pituitary gland (5), cavernous sinus (6), and sphenoid sinus (7). Only three case of frontal skull metastasis from PTC have been previously published (Table 1).
As a result of their rarity, PTC metastasis in the skull can easily be mistaken for other skull base tumors, including meningioma, schwannoma, chondrosarcoma and paraganglioma, as was the case in our subject.
The mean period from the initial diagnosis of PTC to the detection of skull metastasis has been estimated at 23.3 years (8). However, such a long silent period between initial diagnosis is, probably atypical. Indeed, the patient described here had undergone the near total thyroidectomy due to thyroid adenoma 13 years ago.
Both clinical and radiologic evidence are important for obtaining an accurate differential diagnosis. Non-invasive diagnostic techniques such as ultrasonography (9), transillumination, CT and/or MRI-scanning are essential for detecting, localizing and assessing the extension of the lesions. An incorrect diagnosis is also likely to occur if it is based on clinical presentation and radiological findings, without a tumor biopsy.
Biopsies should be performed at the time of diagnosis and repeated postoperatively. The presence of intranuclear inclusion bodies and/or ground-glass nuclei are typical pathological findings that help to confirm the diagnosis.
Accurate diagnosis has major implications for tumor management, as different tumor types respond differently. The preferred treatment algorithm for most primary thyroid carcinomas is near-total thyroidectomy followed by postoperative radiotherapy, or 131I ablation and thyroid-stimulating hormone (TSH) suppression with levothyroxine. Thyroxine withdrawal causes prolonged TSH stimulation and may accelerate metastatic progression, with important clinical consequences, especially for patients with skull metastases. Therefore, administration of 131I therapy or radiotherapy after L-thyroxine stimulation should be considered to control residual disease and metastases (10).
Bisphosphonates are effective inhibitors of osteoclastic activity. They have been shown to reduce complications and pain, and represent an alternative approach for managing bone metastases (11). The optimal timing and duration of bisphosphonate therapy and their efficacy in preventing of bone metastases is currently under investigation in clinical trials for solid tumors. Other new approaches to prevent bone metastases are also being developed, including bone resorption inhibitors, anti-angiogenic factors and gene therapy (12).
The ability of differentiated thyroid carcinomas to metastasize to the skull base cannot be accurately determined because of the lack of standardization of treatment. Primary, well-differentiated thyroid carcinomas are slow-growing and are associated with a good overall prognosis. However, this is not the case with cranial metastases. According to previous reports (Table 1) overall survival among patients undergoing definitive treatment ranged from 14 months to 3.5 years from the discovery of the metastasis. The survival of untreated patients was even shorter. Overall 5- and 10-year survival probabilities in patients with bone metastases have been estimated a 29% and 13%, respectively (13).
Full Table
In conclusion, metastatic thyroid tumors infringing on the skull are rare but potentially hazardous. Prompt diagnosis and appropriate treatment are essential keys to successful management. Diagnosis of a skull metastasis from thyroid carcinoma is usually based on clinical judgment, in conjunction with results of ultrasonography, transillumination, CT and/or MRI-scanning and biopsy findings. Although unexpected, the diagnosis of these tumors has important implications on the choice of clinical management and patient outcomes. There are currently no established treatment protocols in patients with skull metastasis from PTC. A review of previous literature indicates that effective treatment options rely on a multimodal approach of surgery followed by 131I, or external beam radiation, and chronic TSH suppression. This form of combined management can contribute to prolonged and asymptomatic survival.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mehta MP, Goetowski PG, Kinsella TJ. Radiation induced thyroid neoplasms 1920 to 1987: a vanishing problem? Int J Radiat Oncol Biol Phys 1989;16:1471-5. [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review 1975-2010. Bethesda: National Cancer Institute, 2007.
- Mydlarz WK, Wu J, Aygun N, et al. Management considerations for differentiated thyroid carcinoma presenting as a metastasis to the skull base. Laryngoscope 2007;117:1146-52. [PubMed]
- Proye CA, Dromer DH, Carnaille BM, et al. Is it still worthwhile to treat bone metastases from differentiated thyroid carcinoma with radioactive iodine? World J Surg 1992;16:640-5; discussion 645-6. [PubMed]
- Masiukiewicz US, Nakchbandi IA, Stewart AF, et al. Papillary thyroid carcinoma metastatic to the pituitary gland. Thyroid 1999;9:1023-7. [PubMed]
- Takami T, Ohata K, Tsuyuguchi N, et al. Cavernous sinus metastasis from thyroid papillary adenocarcinoma. J Clin Neurosci 2002;9:598-600. [PubMed]
- Freeman JL, Gershon A, Liavaag PG, et al. Papillary thyroid carcinoma metastasizing to the sphenoid-ethmoid sinuses and skull base. Thyroid 1996;6:59-61. [PubMed]
- Rosenbaum MA, Mchenry CR. Contemporary management of papillary carcinoma of the thyroid gland. Expert Rev Anticancer Ther 2009;9:317-29. [PubMed]
- Brugge WR. Aspiring to new levels of achievement: EUS in the therapeutic endoscopy Olympics. Endoscopic Ultrasound 2012;1:59-60.
- Rosahl SK, Erpenbeck V, Vorkapic P, et al. Solitary follicular thyroid carcinoma of the skull base and its differentiation from ectopic adenoma--review, use of galectin-3 and report of a new case. Clin Neurol Neurosurg 2000;102:149-55. [PubMed]
- Cascinu S, Graziano F, Alessandroni P, et al. Different doses of pamidronate in patients with painful osteolytic bone metastases. Support Care Cancer 1998;6:139-43. [PubMed]
- Muresan MM, Olivier P, Leclere J, et al. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer 2008;15:37-49. [PubMed]
- Tickoo SK, Pittas AG, Adler M, et al. Bone metastases from thyroid carcinoma: a histopathologic study with clinical correlates. Arch Pathol Lab Med 2000;124:1440-7. [PubMed]
