Selective killing effect of oxytetracycline, propafenone and metamizole on A549 or Hela cells
Introduction
In the course of drug discovery, it is a very time-consuming process (it may take more than 15 years before a drug receives approval), and some promising compounds were failed because of the failure of clinical trials, although more than millions of dollars had been spent (1,2). While, when a new function of a clinical drug is found, it could have much more chance to be used clinically. It is commonly called “old drugs for new applications”.
Aspirin is a typical nonsteroidal anti-inflammatory drug (NSAID) used to relieve pain and reduce inflammation (3). Then it was found by clinical practice that it has the function to prevent generation of cerebral thrombosis. It was also used to prevent next attack of myocardial infarction (4). Later, its anticancer effect was also found and studied (5,6).
Berberine (BR), the main alkaloid component in Huang Lian and other medicinal herbs, was used mainly as an antibacterial drug. Then, by clinical practice, it was found that it has the function of treating type II diabetes (7). The most promising actions of BR are its inhibition on cell growth and induction of apoptosis in several human cancer cells (8,9).
Vitamin C takes part in amino acid metabolism and synthesis of collagen. It can also decrease permeability of capillary and accelerate blood coagulation. Later on, it was found that it has the function of antitumor. According to experiments in vitro, vitamin C can only display antitumor effect at high concentrations (10-12). It was reported that vitamin C can enhance the generation speed and effect of some induced pluripotent stem cells. The results will make vitamin C be used in a new pathway (13,14).
Many drugs have multiple functions. So, we had screened more than 60 kinds of clinical drugs, which had no obvious anticancer function reported, to test whether they could inhibit proliferation of tumor cells, such as A549 and Hela cells. Finally, we found three kinds of clinical drugs could inhibit proliferation of A549 or Hela cells greatly. They were oxytetracycline, propafenone, and metamizol (dipyrone).
Oxytetracycline is a broad-spectrum antibiotic produced by Streptomyces rimosus (15). About 30 years ago, it was reported that the proliferation of Zajdela tumor cells, grown in vivo in Wistar rats, was arrested by low amounts of oxytetracycline because of the inhibition of mitochondrial protein synthesis (16). Propafenone is a Vaughan-Williams class Ic antiarrhythmic drug frequently used in the management of atrial fibrillation and other supraventricular arrhythmias (17). Metamizol (dipyrone), a potent drug also centrally acting as a non-opioid analgesic and antipyretic agent with an additional spasmolytic effect, is widely used in humans to relieve postoperative pain alone and in combination with opioids (18). Although it is not available in all countries (e.g., USA and Sweden), there is a wide experience in the use of metamizol, particularly in the management of postoperative pain in animals (19).
Materials and methods
Materials
All reagents were of analytical grade or chemically pure. DMEM and RPMI 1640 were obtained from Gibco invitrogen Co. Bovine calf serum was provided by Ding Guo Biotechnology (Beijing, China). Sulforhodamine B (SRB) (Cat. No. S-9012) was provided by Sigma-Aldrich (USA). Tablets of oxytetracycline, propafenone and metamizole were purchased from Shandong Jinyang Drug Company, Shandong Renhetang Drug Company, and Peking Shuguang Drug Company, respectively.
Cell culture
A549 lung cancer cells were cultured in RPMI 1640 medium at 37 °C with 5% CO2 and 95% air, supplemented with 10% (V/V) bovine calf serum and 80 U/mL gentamicin. Hela cells were cultured in DMEM (high glucose) medium under the same condition as above. The cells were seeded onto 96-well plates or other appropriate dishes containing the medium at the density of about 6,000/cm2 (20).
Antiproliferative activity
Screening of synthesized compounds was carried out using Hela cell line. Proliferation percentage was determined by the SRB assay. Cells were incubated with the drugs at the concentrations of 0.005-0.2 g/L for 48 h, and the cell proliferation/viability was determined using the survival percentage with the cells treated only with dimethyl sulfoxide (DMSO) at 0.1% as a reference (20-22). The results were expressed as the average of triplicate assays.
Lactate dehydrogenase (LDH) assay
Cell culture medium was collected after 48 h treatment with 0.1% DMSO (as control) or the drugs. LDH assay was performed using LDH kit (Nanjing Jiancheng Co., China) according to the manufacturer’s instructions (20).
Hoechst 33258 staining to detect apoptosis
Hela and A549 cells were fixed with 4% formaldehyde in phosphate buffered saline (PBS) for 10 min before staining with 2 µg/mL of Hoechst 33258 at 37 °C for 30 min. Subsequently, the cells were gently washed once with PBS, and then observed under a fluorescence microscope (Nikon). The condensed DNA of apoptotic cells was identified by intense local staining in the nucleus, in contrast to diffused staining of DNA in normal cells. A minimum of 500 cells was counted, and each experiment was performed in triplicate (23-25).
Flow cytometry analysis of cell cycle distribution
Cells were harvested, then fixed with 70% ethanol, and stained with 50 µg/mL propidium iodide (PI) containing 10 µg/mL RNase A at 4 °C for 1 h. The stained cells were analyzed using FACSCalibur flow cytometer (BD Bioscience, USA). Cell cycle distribution was analyzed with ModiFit software (BD Bioscience, USA) (25-27).
Western blot analysis
Cells were washed twice with ice cold PBS, and then lysed in protein lysis buffer [1% sodium dodecyl sulfate (SDS) in 25 mmol/L Tris-HCl, pH 7.5, 4 mmol/L ethylene diamine tetraacetic acid (EDTA), 100 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 10 µg/mL leupeptin and 10 µg/mL soybean trypsin inhibitor]. The protein concentration of the cells was determined by Bradford method (28). Equal amount of protein was loaded onto 12% SDS-polyacrylamide gel. After electrophoresis, the resolved protein was electrophoretically transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, MA, USA). The membrane was incubated in TBST (10 mmol/L Tris-HCl, pH 7.6, 150 mmol/L NaCl, 0.1% Tween-20) containing 2% (W/V) bovine serum albumin (BSA) and 2% (W/V) skim milk powder for 12 h at 4 °C. Subsequently, the membrane was probed with the primary antibody of P53 (sc-126, Santa Cruz, 1:200 diluted in TBST), LC3-II (sc-271625, Santa Cruz, 1:500 diluted in TBST), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (2118S, CST, 1:500 diluted in TBST) overnight at 4 °C, and was then washed twice with PBS-Tween 20 (PBST), each time for 5 min. The membranes were respectively incubated with peroxidase-conjugated anti-mouse IgG for P53 and LC3-II, and anti-rabbit IgG for GAPDH for 1 h at room temperature (secondary antibodies 1:5,000 diluted in TBST, Santa Cruz). After washed with PBST, the membrane was incubated with horseradish peroxidase (HRP) substrate until the protein strip could be visualized, and the luminous intensity was detected with X-ray films. Intensity of the protein bands was quantified using ImageJ software (National Institutes of Health) (29).
Statistical analysis
Data were presented as 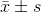 from at least three independent experiments and analyzed by Student’s t-test. P<0.05 was considered statistically significant.
from at least three independent experiments and analyzed by Student’s t-test. P<0.05 was considered statistically significant.
Results
Inhibitory effects of oxytetracycline, propafenone and metamizole on proliferation of A549 and Hela cells
All the drugs selected were evaluated for their cytotoxicity against A549 and Hela cells. Firstly, the assay incubated with drugs at the concentrations from 0.005 to 0.2 g/L for 48 h was carried out, and the cells were treated with SRB to measure their growth/viability (% of the untreated control) using a spectrophotometer as described previously. Oxytetracycline, propafenone and metamizole all displayed a most potent inhibitory effect on the growth of A549 cells in a dose-dependent manner (Figure 1A). Propafenone displayed an evident inhibitory effect on the growth of Hela cells in a dose-dependent manner (Figure 1B).
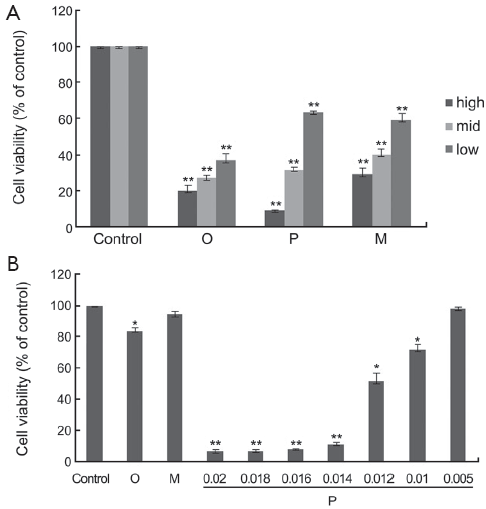
The half maximal inhibitory concentrations (IC50) (g/L) of the drugs at 48 h in A549 and Hela cells are shown in Table 1.
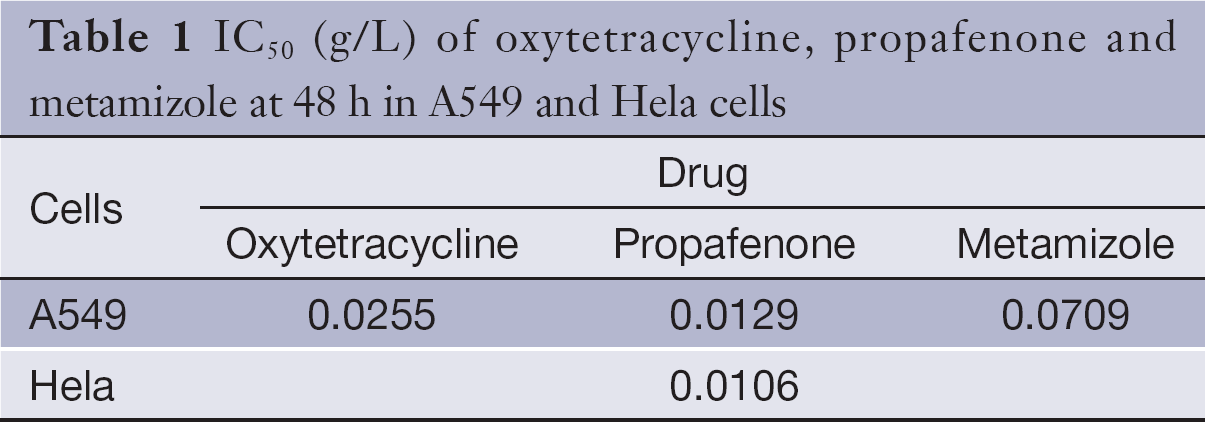
Full table
Effects of drugs on morphology of A549 and Hela cells
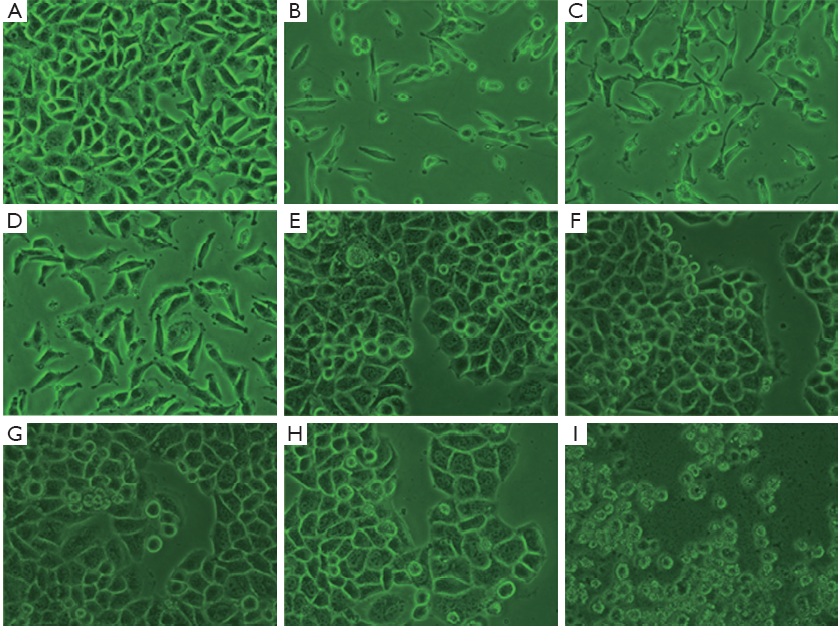
The morphological image of A549 cells treated with oxytetracycline, propafenone and metamizole at the concentration of 0.1, 0.02, or 0.1 g/L for 48 h was observed under an inverted phase contrast microscope (Nikon). It was found that the quantities of the cells treated with these drugs were decreased greatly. In addition, the shape of some A549 cells changed to globular. In the case of Hela cells, there were some morphological changes such as volume shrinkage, fragment and changing to globular, when the concentration of propafenone is more than 0.014 g/L. Such morphological changes were not apparent in the control cells (Figure 2).
Oxytetracycline, propafenone and metamizole do not cause necrosis in A549 and Hela cells
In order to determine whether the growth inhibitory effects were due to necrosis that is believed to be an unwanted side effect of cancer-fighting agents, LDH assay was carried out on cells treated with oxytetracycline, propafenone, or metamizole at the concentration of 0.1, 0.02 or 0.1 g/L or 0.1% DMSO (as control) for 48 h. As shown in Figure 3, for A549 and Hela cells, there were no significant differences in LDH release between the cells in the control group and the drug treatment group. The results demonstrated that the drugs at the test range of concentration did not cause necrosis in the cells.
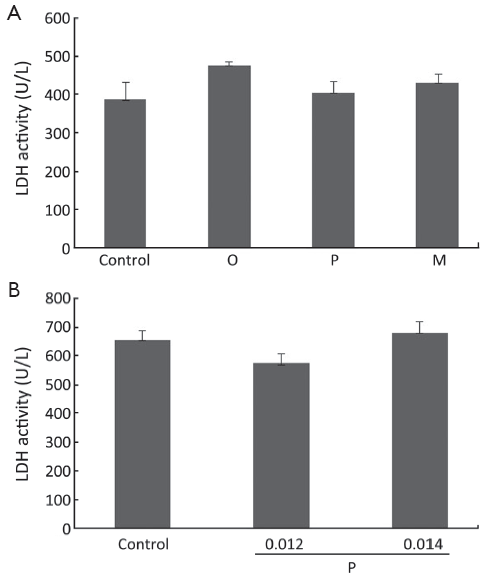
 ; n=3. O, oxytetracycline; P, propafenone; M, metamizole.
; n=3. O, oxytetracycline; P, propafenone; M, metamizole.Compounds induced apoptosis in A549 and Hela cells
To detect whether oxytetracycline, propafenone and metamizole resulted in apoptosis of the cells, Hoechst 33258 staining assay was performed. It is well known that DNA fragmentation, chromatin condensation, cell shrinkage, and membrane blebbing are the characteristics of apoptotic cells. The chromatin condensation and DNA fragmentation in the cells were determined by Hoechst 33258 staining under a fluorescence microscope. Treatment with oxytetracycline, propafenone and metamizole at the concentration of 0.1, 0.02 or 0.1 g/L for 48 h could not induce apoptosis in A549 cells greatly (Figure 4A). It was interestingly found that propafenone at the concentration of 0.014 g/L or higher for 48 h could induce apoptosis in Hela cells greatly (P<0.05 vs. control). While, treatment with oxytetracycline and metamizole at the concentration of 0.20 g/L for 48 h could not induce apoptosis in Hela cells significantly (Figure 4A,B).
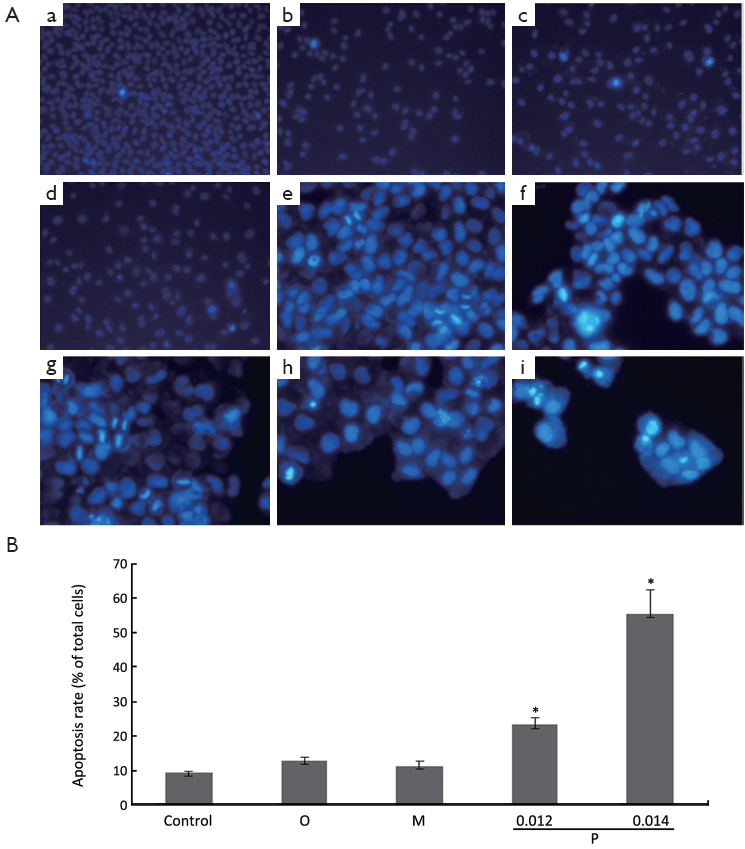
 from three independent experiments. *, P<0.05 vs. control.
from three independent experiments. *, P<0.05 vs. control.Cell cycle distribution
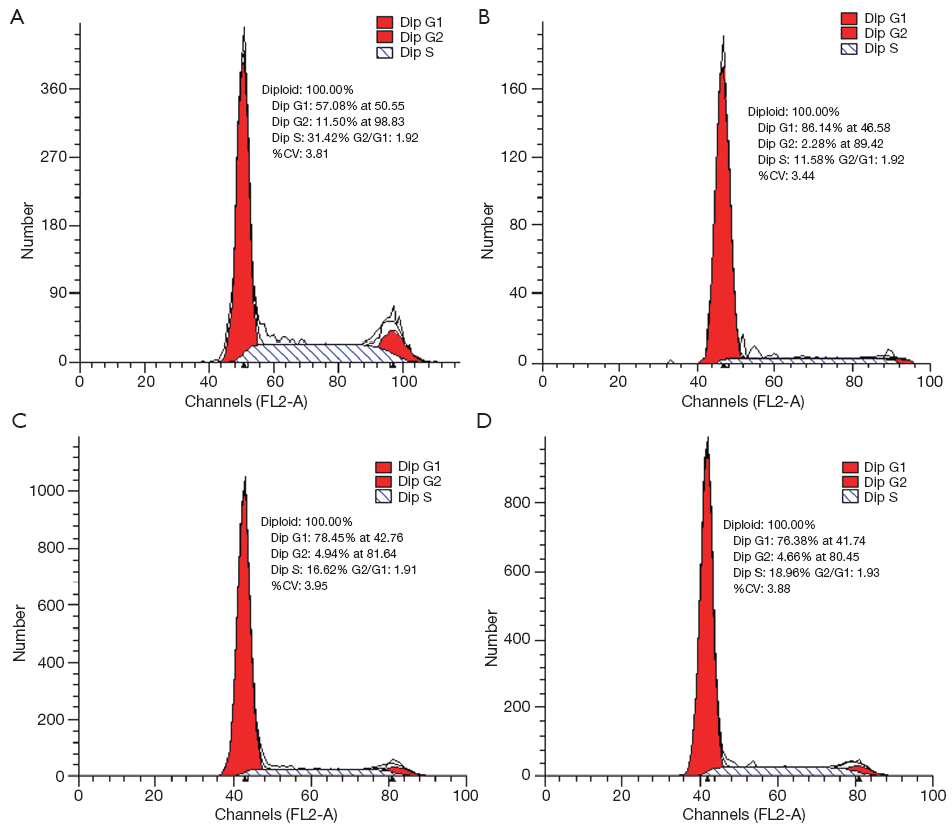
In order to get a more precise insight of growth inhibition, flow cytometry analysis was performed to detect whether oxytetracycline, propafenone and metamizole could induce A549 cell cycle arrest. The results showed that treatment with 0.1 g/L, 0.02 g/L or 0.1 g/L of oxytetracycline, propafenone and metamizole for 48 h induced G1-phase arrest effectively (Figure 5). After 48 h treatment with 0.1 g/L, 0.02 g/L or 0.1 g/L, the G1-phase cell population was noticeably enhanced by about 29%, 21% or 19%, respectively, compared with control. The increase in the G1-phase cell population was accompanied by a decrease in S and G2-phase cell populations. The fact that oxytetracycline, propafenone and metamizole induced strong G1-phase arrest of A549 cells is concomitant with the growth inhibitory effect.
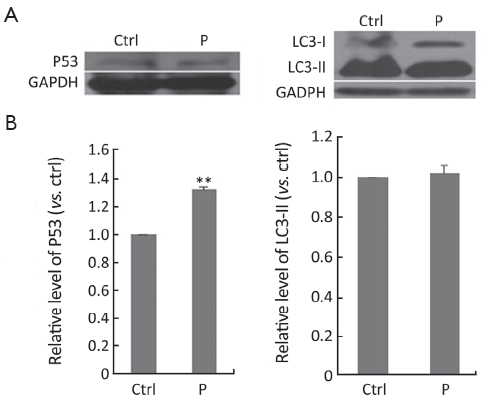
Western blot
To find out the inhibitory mechanism of propafenone on Hela cells, the expressions of P53 and LC3-II were detected, after Hela cells were treated with propafenone for 48 h. By way of western blot analysis, it could be seen that propafenone can elevate the protein level of P53 effectively (P<0.01). Protein level, treated with propafenone, of LC3-II was not greatly altered (Figure 6).
Discussion
According to the application instruction, oxytetracycline tablets may be taken 0.25-0.5 g once and every 6 h for an adult. So, one adult can take at most 2.0 g every day. It was used at the concentration of 0.1 g/L in the experiment. We also found that when the tablet of oxytetracycline was dissolved with DMSO for more than 10 d at 4 °C, the solution became dark gradually and its antitumor effect also became stronger and stronger. In the experiment, we used the solution made within one week.
According to the application instruction, propafenone tablets may be taken 100-200 mg once and every 6 h for an adult. So, one adult can take at most 0.8 g every day. It was used at the concentration of 0.02 g/L in the experiment. According to the application instruction, metamizole tablets may be taken 0.75-1.25 g for an adult every day. It was used at the concentration of 0.1 g/L in the experiment.
Preliminary biological evaluation showed that oxytetracycline, propafenone and metamizole could inhibit the growth of A549 cells in a dose-dependent manner. Propafenone could also inhibit the growth of Hela cells in a dose-dependent manner.
Globally, cervical cancer is second only to breast cancer as the leading cause of cancer death in women. Despite advances in the study of cervical cancer over the past 20 years, its pathogenesis is still not fully understood. Hela cell line, which was established by Gey et al., was derived from cervical adenocarcinoma of a black woman (30). The cell line is one of the most representative cervical squamous carcinoma cell lines, and is used widely (31). Lung cancer is one of the leading causes of death worldwide (32). A549 cells, as a kind of lung cancer cell line, were often used to examine antitumor effects of some compounds (33,34).
P53 protein suppresses the accumulation of mutations in somatic cells and substantially decreases the probability of malignant diseases (35). Propafenone can elevate the protein level of P53 effectively (P<0.01), indicating that the antitumor effect of propafenone is induced by way of protein P53.
Microtubule-associated protein 1 light chain 3-β (MAP1LC3-II) is one kind of markers of autophagy. When the expression level of LC3-II is elevated, it usually indicates that autophagy is induced (36). After treatment with propafenone, the protein level of LC3-II was not greatly altered. So, the antitumor effect of propafenone is not induced by way of autophagy.
This study showed that oxytetracycline, propafenone and metamizol (dipyrone) all displayed evident inhibitory effects on the proliferation of A549 cells. Propafenone also displayed evident inhibitory effects on the proliferation of Hela cells. At the same time, we found the new function of these old drugs by screen experiments, while, new functions of other old drugs were found usually by clinical practice. In addition, as the drug has already undergone clinical trials and tested to be bioactive, this strategy will greatly reduce the cost and increase the chance of drug discovery.
Acute arrest of bone marrow will be produced after treatment of some anticancer drugs, while oxytetracycline, propafenone and metamizol do not. This is an advantage of them. The three kinds of drugs may be used in treatment of lung cancer or cervical cancer along with normal anticancer drugs.
Acknowledgements
I thank the technical platform in Shandong University and Doctor research fund of Hubei University of Art and Science (2013B009).
Disclosure: The authors declare no conflict of interest.
References
- Dickson M, Gagnon JP. Key factors in the rising cost of new drug discovery and development. Nat Rev Drug Discov 2004;3:417-29. [PubMed]
- Pan X, Cheng W, Wang J, et al. ‘Old drugs for new applications’: can orthopedic research benefit from this strategy? Ther Adv Musculoskel Dis 2011;3:201-5.
- Yasuda O, Takemura Y, Kawamoto H, et al. Aspirin: recent developments. Cell Mol Life Sci 2008;65:354-8. [PubMed]
- Howard PA, Delafontaine P. Non-steroidal anti-inflammatory drugs and cardiovascular risk. J Am Coll Cardiol 2004;43:519-25. [PubMed]
- Thakkar A, Sutaria D, Grandhi BK, et al. The molecular mechanism of action of aspirin, curcumin and sulforaphane combinations in the chemoprevention of pancreatic cancer. Oncol Rep 2013;29:1671-7. [PubMed]
- Chen RH, Tian YJ. Enhanced Anti-tumor Efficacy of Aspirin Combined with Triptolide in Cervical Cancer Cells. Asian Pac J Cancer Prev 2013;14:3041-4. [PubMed]
- Zhang H, Wei J, Xue R, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism 2010;59:285-92. [PubMed]
- Marverti G, Ligabue A, Lombardi P, et al. Modulation of the expression of folate cycle enzymes and polyamine metabolism by berberine in cisplatin-sensitive and -resistant human ovarian cancer cells. Int J Oncol 2013;43:1269-80. [PubMed]
- Liu Q, Jiang H, Liu Z, et al. Berberine Radiosensitizes Human Esophageal Cancer Cells by Downregulating Homologous Recombination Repair Protein RAD51. PLoS One 2011;6:e23427. [PubMed]
- Chen P, Yu J, Chalmers B, et al. Pharmacological ascorbate induces cytotoxicity in prostate cancer cells through ATP depletion and induction of autophagy. Anticancer Drugs 2012;23:437-44. [PubMed]
- Mandl J, Szarka A, Bánhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol 2009;157:1097-110. [PubMed]
- Park S. The effects of high concentrations of vitamin C on cancer cells. Nutrients 2013;5:3496-505. [PubMed]
- Esteban MA, Wang T, Qin B, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 2010;6:71-9. [PubMed]
- Wang T, Chen K, Zeng X, et al. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell 2011;9:575-87. [PubMed]
- Chopra I, Hawkey PM, Hinton M. Tetracyclines, molecular and clinical aspects. J Antimicrob Chemother 1992;29:245-77. [PubMed]
- van den Bogert C, Dontje BH, Wybenga JJ, et al. Arrest of in vivo proliferation of Zajdela tumor cells by inhibition of mitochondrial protein synthesis. Cancer Res 1981;41:1943-7. [PubMed]
- Yeung A, Shanks D, Parwana H, et al. Acute propafenone toxicity after two exposures at standard dosing. Can J Cardiol 2010;26:209-10. [PubMed]
- Schug SA, Manopas A. Update on the role of non-opioids for postoperative pain treatment. Best Pract Res Clin Anaesthesiol 2007;21:15-30. [PubMed]
- Baumgartner C, Koenighaus H, Ebner J, et al. Comparison of dipyrone/propofol versus fentanyl/propofol anaesthesia during surgery in rabbits. Lab Anim 2011;45:38-44. [PubMed]
- Shen SL, Shao JH, Luo JZ, et al. Novel chiral ferrocenylpyrazolo[1,5-a][1,4]diazepin-4-one derivatives-synthesis, characterization and inhibition against lung cancer cells. Eur J Med Chem 2013;63:256-68. [PubMed]
- Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990;82:1107-12. [PubMed]
- Rubinstein LV, Shoemaker RH, Paull KD, et al. Comparison of in vitro anticancer -drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst 1990;82:1113-8. [PubMed]
- Shi J, Zheng D, Liu Y, et al. Overexpression of soluble TRAIL induces apoptosis in human lung adenocarcinoma and inhibits growth of tumor xenografts in nude mice. Cancer Res 2005;65:1687-92. [PubMed]
- Xu N, Wang YS, Pan WB, et al. Human alpha-defensin-1 inhibits growth of human lung adenocarcinoma xenograft in nude mice. Mol Cancer Ther 2008;7:1588-97. [PubMed]
- Liu N, Zhang JH, Zhao BX, et al. Microwave-assisted synthesis, crystal structure of pyrazolo[1,5-a]pyrazin-4(5H)-ones and their selective effects on lung cancer cells. Eur J Med Chem 2011;46:2359-67. [PubMed]
- Lee PJ, Alam J, Wiegand GW, et al. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci U S A 1996;93:10393-8. [PubMed]
- Lee JY, Leonhardt LG, Obeid LM. Cell-cycle-dependent changes in ceramide levels preceding retinoblastoma protein dephosphorylation in G2/M. Biochem J 1998;334:457-61. [PubMed]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54. [PubMed]
- Lian S, Su H, Zhao BX, et al. Synthesis and discovery of pyrazole-5 -carbohydrazide N-glycosides as inducer of autophagy in A549 lung cancer cells. Bioorg Med Chem 2009;17:7085-92. [PubMed]
- Herz F, Miller OJ, Miller DA, et al. Chromosome analysis and alkaline phosphatase of C41, a cell line of human cervical origin distinct from HeLa. Cancer Res 1977;37:3209-13. [PubMed]
- Jiang L, Zeng X, Wang Z, et al. Oral cancer overexpressed 1 (ORAOV1) regulates cell cycle and apoptosis in cervical cancer HeLa cells. Mol Cancer 2010;9:20-8. [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [PubMed]
- Zheng LW, Shao JH, Zhao BX, et al. Synthesis of novel pyrazolo[1,5-a]pyrazin-4(5H)-one derivatives and their inhibition against growth of A549 and H322 lung cancer cells. Bioorg Med Chem Lett 2011;21:3909-13. [PubMed]
- Lee ER, Kang YJ, Choi HY, et al. Induction of apoptotic cell death by synthetic naringenin derivatives in human lung epithelial carcinoma A549 cells. Biol Pharm Bull 2007;30:2394-8. [PubMed]
- Gu B, Zhu WG. Surf the Post-translational Modification Network of p53 Regulation. Int J Biol Sci 2012;8:672-84. [PubMed]
- Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008;4:151-75. [PubMed]
