Clinical significance of serum miR-21 in breast cancer compared with CA153 and CEA
Introduction
Breast cancer (BC) is the most common cancer among women. Stage at diagnosis is an important predictor of BC survival and quality of life, so there is an urgent need for diagnostic indicators. With simpleness, rapidness and noninvasiveness, tumor markers are playing an increasingly important role in BC diagnosis and treatment.
The most commonly used markers for BC are cancer antigen 153 (CA153) and carcinoembryonic antigen (CEA). However, the specificity and sensitivity of them are low. MicroRNAs (miRNAs) are a class of noncoding RNAs of 19-25 nucleotides that have been implicated in regulating diverse cellular processes. They have a close relationship with tumor formation and development. miR-21 is significantly elevated in the majority of human tumors. It plays an important role in tumor formation and development, and it has been shown to be a key regulator of oncogenic processes. Iorio et al. (1) had reported that miR-21 is overexpressed in BC tissue, and it may be a useful marker of BC. But it is invasive to get tissue. The serum sample has the advantages of simple collection, less invasiveness, and easy monitoring. We have evaluated the expression of miR-21 by stem-loop real-time reverse transcription-polymerase chain reaction (RT-PCR) based on SYBR-Green using miR-16 as reference in 89 BC patients and 55 healthy controls. Levels of miR-21 expression by disease stage and hormone receptor status were compared. Then the sensitivity was compared among miR-21, CA153 and CEA in diagnosing BC.
Materials and methods
Subjects
During the study, all tumor serum samples were collected consecutively from patients who underwent therapy for BC in Beijing Cancer Hospital between March 2011 and May 2011. All patients included in the study had a histologically confirmed diagnosis of primary BC, aged from 29 to 80 years, and the median age was 56 years. All normal human serum samples were collected from healthy women who underwent a medical examination in Beijing Cancer Hospital between March 2011 and April 2011, aged from 28 to 65 years, and the median age was 50 years. This study was approved by the Ethic Committee of Beijing Cancer Hospital, and written informed consent of all the patients was obtained.
Detection of miR-21
Serum samples and preparation of total RNA
Fresh serum samples were stored at –80 °C until processing. Total RNAs were extracted from serum using TRIzol reagent (Invitrogen life technologies) according to the manufacturer’s instructions.
Reverse transcription
Each RNA sample of 10 µL was mixed with 3 µL stem-loop RT primers of miR-21 and miR-16, and 4 µL of 5× RT Buffer, 2 µL of 1 mol/L dithiothreitol [DTT; Tiangen Biotech (Beijing) Co., Ltd.], 0.2 µL of RNasin [Tiangen Biotech (Beijing) Co., Ltd.], 0.5 µL of dNTPs [Tiangen Biotech (Beijing) Co., Ltd]., and 1 µL of Moloney murine leukemia virus (M-MLV) reverse transcriptase [Promega (Beijing) Biotech Co., Ltd.] were added (Table 1). miR-16 was used as an endogenous control. The mixture in a final volume of 20.7 µL was incubated at 16 °C for 30 min, 37 °C for 30 min and 70 °C for 10 min, and then held at 4 °C.
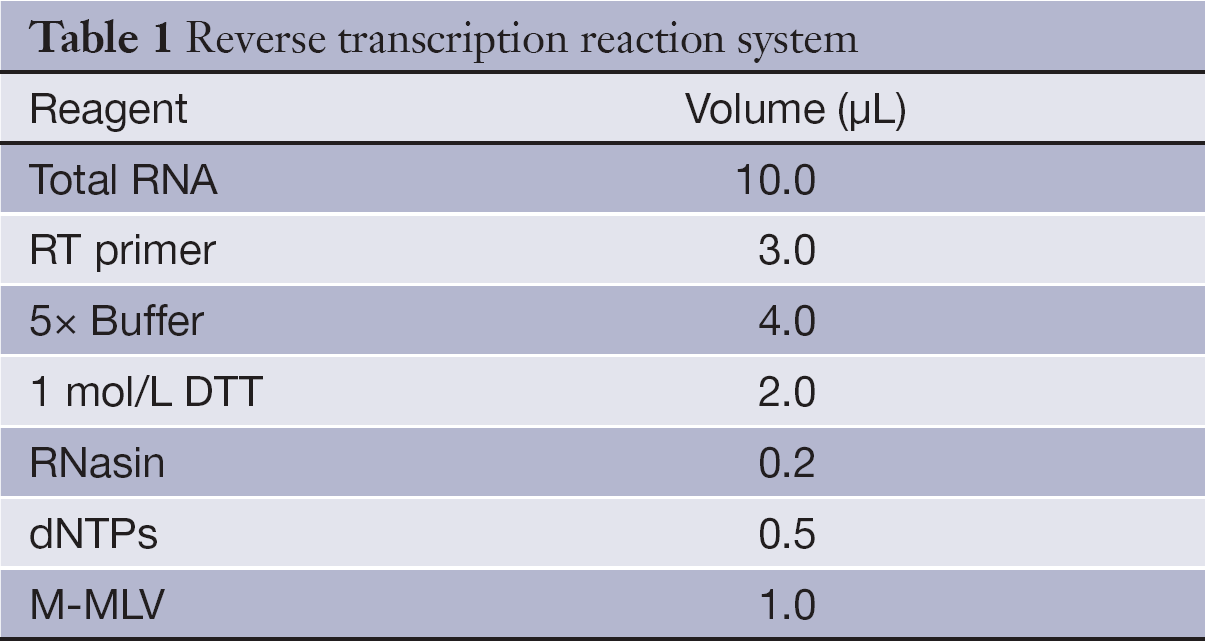
Full table
Real-time PCR
After reverse transcription, 1.6 µL cDNA product along with 1 µL PCR primers and 10 µL SYBR Green Master (Roche Co., Ltd.) were mixed with other PCR reagents (Table 2), The PCR conditions included denaturing at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The reaction was carried out in the ABI 7500 Fast PCR system.
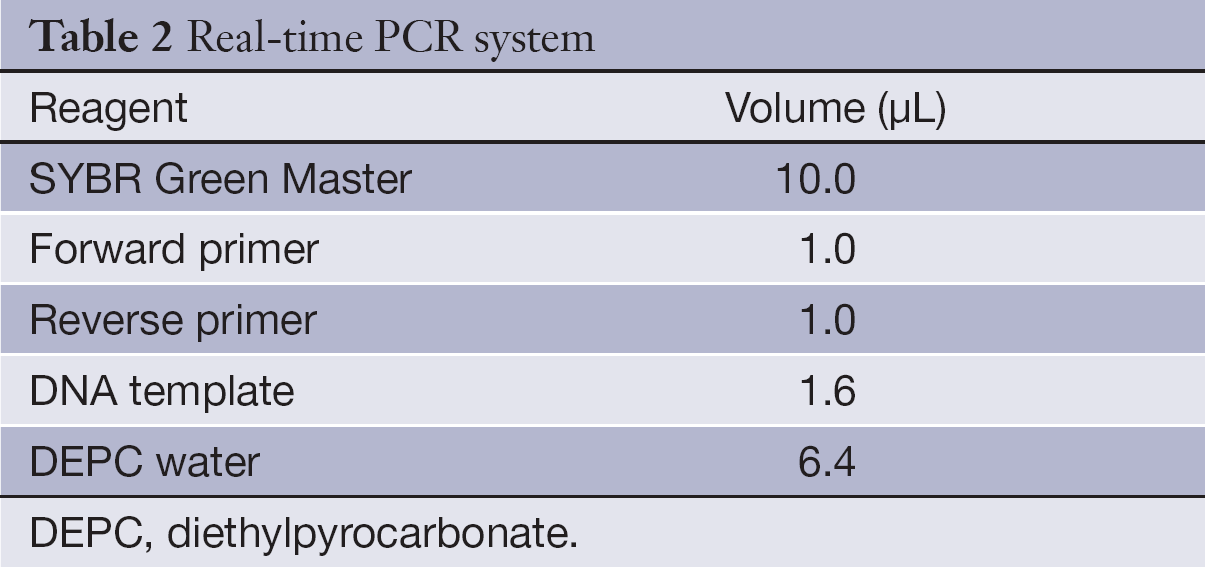
Full table
Detection of CA153 and CEA
The levels of CA153 and CEA were measured through electrochemiluminescence assays. The reaction was carried out in a Roche E170 MODULAR Immunoassay Analyzer
Statistical analysis
The expression of circulating miR-21 was normalized through relative change folds. The relative expression levels of miR-21 were characterized by their median and ranges from the 25th to the 75th percentile. The associations of miR-21 with patients’ clinical stage were analyzed with the Kruskal-Wallis test, and the associations of miR-21 with hormone receptor status were analyzed with the Mann-Whitney test. The expression of miR-21 in BC and healthy control was also calculated using the Mann-Whitney test. The cut-off value was determined via receiver operating characteristic (ROC) curve, and then the sensitivity and specificity of diagnosis were obtained according to the cut-off value. P<0.05 was considered statistically significant, and all statistical analyses were performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
Amplification of target gene
The melting curves of miR-21 and miR-16 were sharply defined curves with a narrow peak, indicating that pure, homogeneous PCR products were obtained (Figure 1). The amplification curves are shown in Figure 2.
miR-21 expression in BC
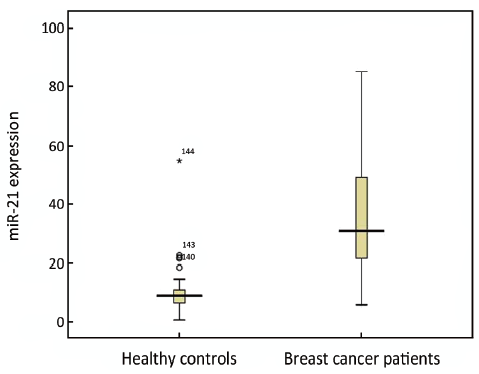
We have evaluated the expression of miR-21 in 89 BC patients and 55 healthy controls. The relative expression of miR-21 in BC (30.82) was significantly higher than that in healthy controls (9.1) (P<0.001) with the ratio of 3.39 (Figure 3).
ROC curve for miR-21
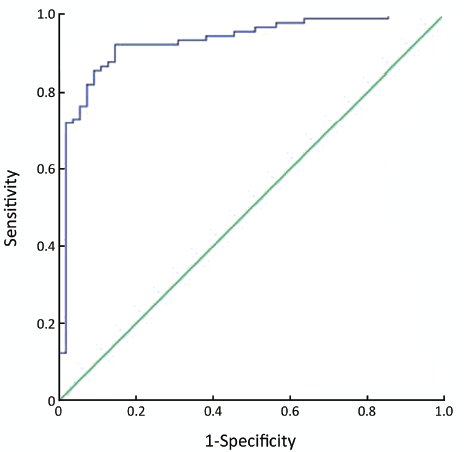
The best cut-off value was 13.22, the area under ROC curve (ROC-AUC) was 92.9% (95% confidence interval: 88.3%, 97.4%), and the sensitivity and specificity were 87.6% and 87.3%, respectively (Figure 4).
Association of miR-21 expression with clinical and pathological features
Table 3 shows the median expression of miR-21 in various groups of patients classified by clinical stage and hormone receptors status. Correlations of miR-21 with the clinical and pathological features were not observed, including clinical stage and hormone receptors status (P>0.05).
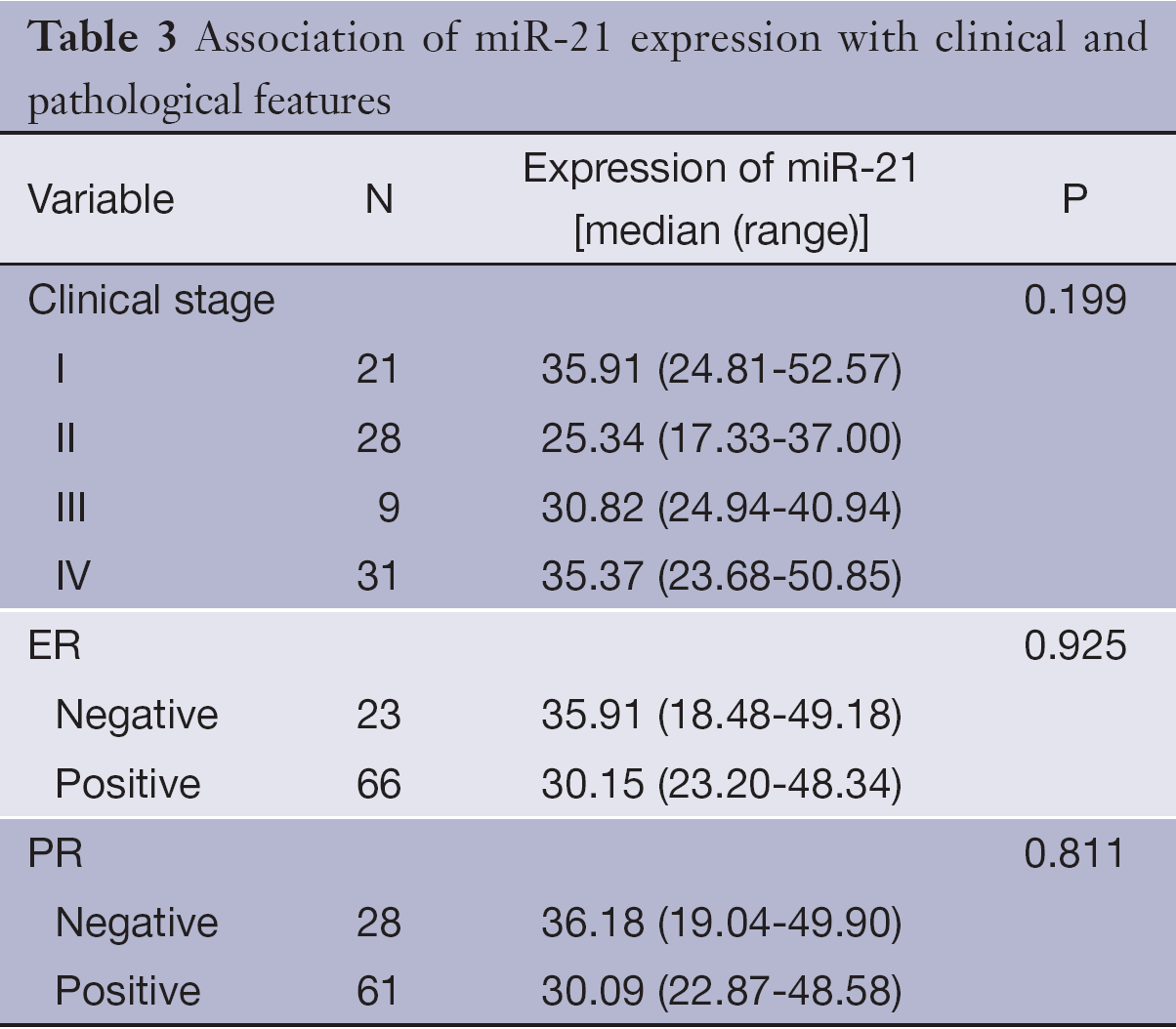
Full table
Comparison of miR-21 with conventional tumor markers CA153 and CEA
Table 4 shows the median expression of miR-21, CA153 and CEA in various groups of patients classified by different clinical stage. The overall sensitivity of miR-21 was 87.64%, while the overall sensitivity of CA153 and CEA was only 22.47% and 15.73%, respectively. Besides, serum miR-21 also has higher sensitivity (95.24%) in the diagnosis of early-stage (stage I) BC compared with CA153 and CEA (4.76%) (Table 4).
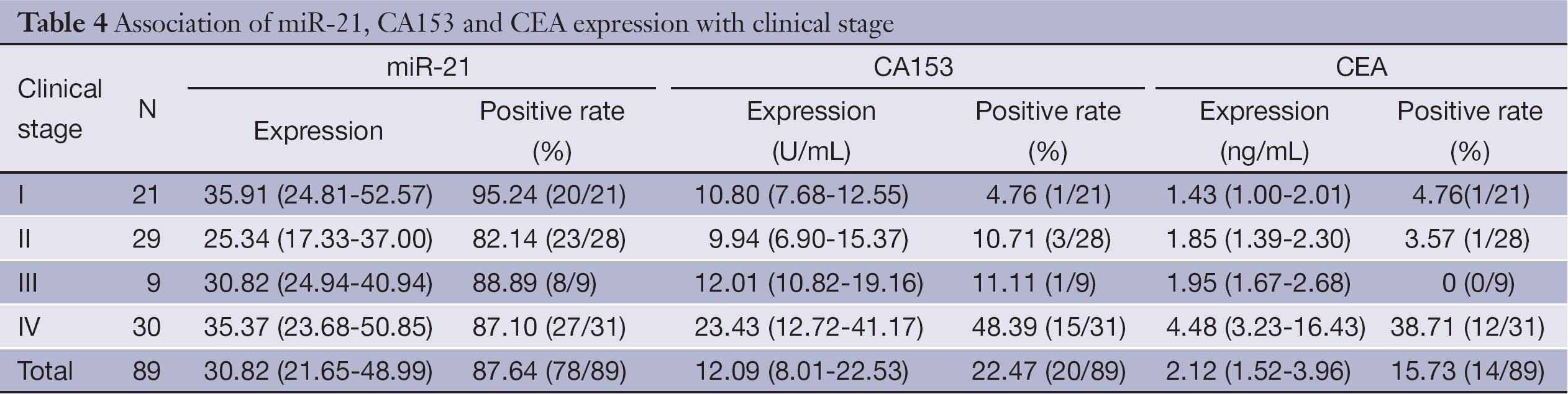
Full table
Discussion
miRNAs are small, noncoding RNA molecules of 19-25 nucleotides in length which participated in the regulation of a variety of biological signaling pathways (2). Many studies have found that miRNA closely related to the development, invasion, metastasis and other characteristics of tumors, which showed the possibility to be the foundation of a new strategy for cancer therapy (3). The abnormal expression of miRNA in tissue or serum was closely related to a variety of malignant tumors (4,5). It is getting more and more attention as a new marker for tumor diagnosis and prognosis.
BC is the most common cancer in women, and early diagnosis and treatment of BC has important prognostic significance. Serum markers have become increasingly important to diagnosis of BC for its simple collection and less invasiveness. CA153 and CEA are the most widely used markers in monitoring patients with BC, and elevated serum levels are associated with BC relapse, but both lack of sensitivity for early-stage disease and specificity (6). What is more, CEA is a non-specific tumor marker and has a lower positive rate in early diagnosis of BC. It cannot serve as an indicator of early diagnosis of BC (7).
Currently, some miRNAs have been found differentially expressed in BC and normal tissues, such as miR-145 (1), miR-21 and let-7a (8). They play different roles in cell proliferation and apoptosis, and regulate biological processes of BC cells. Recently, it have been reported miRNA could be a tumor maker for the diagnosis and treatment of BC (9).
miR-21 located in 17q23.2, and has independent transcriptional units (10). It participated in regulating the expression of multiple tumor suppressor genes, and played an important role in the development of colon cancer (11,12), lung cancer (13,14), stomach cancer (15), BC (1,16,17) et al. Several studies have proved miR-21 was up-regulated in BC both in vivo and in vitro by different kinds of methods including Northern blotting (1), microarray (1,18), bead-based flow cytometric miRNA expression profiling method (19), in situ hybridization (ISH) (16), and real-time RT-PCR (20-23), and a preliminary study has been taken about the overexpression of miR-21 in BC tissue (1,21,24-26). The results showed that the expression level of miR-21 in tumor samples was higher than that in the normal breast samples, and the expression of miR-21 was associated with some clinical and pathological variables (27). But, there was no evaluation and in-depth discussion of its application in BC diagnosis. Furthermore, all previous studies were about BC tissue, and there were few studies about serum miR-21. We have evaluated the expression of miR-21 by stem-loop real-time RT-PCR based on SYBR-Green in serum of BC patients, and evaluated the value of miR-21 as a diagnostic indicator to discuss its role as a serum marker in BC diagnosis and treatment monitoring. The results showed that the level of serum miR-21 was significantly higher (about 3.39 times) in BC patients than in controls (P<0.001). The sensitivity and specificity of miR-21 in the diagnosis of BC were 87.6% and 87.3%, respectively, whereas the sensitivities of CA153 and CEA were only 22.47% and 15.73%. Besides, no significant association was found between expression of miR-21 in serum and clinical stages, and there was also no significant association between expression of miR-21 in serum and the status of estrogen receptor (ER) and progesterone receptor (PR). This result was consistent with other reports (21,24).
In conclusion, miR-21 is a more sensitive marker for BC compared with CA153 and CEA. It can improve the early diagnosis of BC, thereby improving the prognosis of BC. miR-21 is a potential tumor marker in the diagnosis of early-stage BC. The preliminary study was carried out. In the follow-up study, we will expand the sample size, and take more in-depth analysis. It will lay a good foundation for serum miR-21 detection in diagnosis of BC.
Acknowledgements
This study was supported by National High-Tech Research and Development (863) Program of China (No. 2012AA02A504).
Disclosure: The authors declare no conflict of interest.
References
- Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res 2005;65:7065-70. [PubMed]
- O'Hara SP, Mott JL, Splinter PL, et al. MicroRNAs: key modulators of post-transcriptional gene expression. Gastroenterology 2009;136:17-25. [PubMed]
- Chou J, Shahi P, Werb Z. microRNA-mediated regulation of the tumor microenvironment. Cell Cycle 2013;12:3262-71. [PubMed]
- Zhang B, Pan X, Cobb GP, et al. microRNAs as oncogenes and tumor suppressors. Dev Biol 2007;302:1-12. [PubMed]
- Zheng D, Haddadin S, Wang Y, et al. Plasma microRNAs as novel biomarkers for early detection of lung cancer. Int J Clin Exp Pathol 2011;4:575-86. [PubMed]
- Samy N, Ragab HM, El Maksoud NA, et al. Prognostic significance of serum Her2/neu, BCL2, CA15-3 and CEA in breast cancer patients: A short follow-up. Cancer Biomark 2010;6:63-72. [PubMed]
- Wu ZC, Zhai HS, Yan CL, et al. The value of serum CA15-3, CEA in the early diagnosis of breast cancer. Qiqihar Yi Xue Yuan Xue Bao 2010;31:1849-50.
- Song EW. Small molecule RNA for basic research and clinical applications. Beijing: Science and Technology Press, 2007:115-20.
- Si H, Sun X, Chen Y, et al. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J Cancer Res Clin Oncol 2013;139:223-9. [PubMed]
- Fujita S, Ito T, Mizutani T, et al. miR-21 Gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J Mol Biol 2008;378:492-504. [PubMed]
- Slaby O, Svoboda M, Fabian P, et al. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology 2007;72:397-402. [PubMed]
- Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA 2008;299:425-36. [PubMed]
- Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006;9:189-98. [PubMed]
- Markou A, Tsaroucha EG, Kaklamanis L, et al. Prognostic value of mature microRNA-21 and microRNA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR. Clin Chem 2008;54:1696-704. [PubMed]
- Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 2006;103:2257-61. [PubMed]
- Sempere LF, Christensen M, Silahtaroglu A, et al. Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res 2007;67:11612-20. [PubMed]
- Li J, Zhang Y, Zhang W, et al. Genetic heterogeneity of breast cancer metastasis may be related to miR-21 regulation of TIMP-3 in translation. Int J Surg Oncol 2013;2013:875078.
- Mattie MD, Benz CC, Bowers J, et al. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer 2006;5:24. [PubMed]
- Blenkiron C, Goldstein LD, Thorne NP, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol 2007;8:R214. [PubMed]
- Si ML, Zhu S, Wu H, et al. miR-21-mediated tumor growth. Oncogene 2007;26:2799-803. [PubMed]
- Qian B, Katsaros D, Lu L, et al. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-β1. Breast Cancer Res Treat 2009;117:131-40. [PubMed]
- Wang B, Zhang QY. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J Cancer Res Clin Oncol 2012;138:1659-66. [PubMed]
- Kumar S, Keerthana R, Pazhanimuthu A, et al. Overexpression of circulating miRNA-21 and miRNA-146a in plasma samples of breast cancer patients. Indian J Biochem Biophys. 2013;50:210-4. [PubMed]
- Yan LX, Huang XF, Shao Q, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA 2008;14:2348-60. [PubMed]
- Huang GL, Zhang XH, Guo GL, et al. Clinical significance of miR-21 expression in breast cancer: SYBR-Green I-based real-time RT-PCR study of invasive ductal carcinoma. Oncol Rep 2009;21:673-9. [PubMed]
- Walter BA, Gómez-Macias G, Valera VA, et al. miR-21 expression in pregnancy-associated breast cancer: a possible marker of poor prognosis. J Cancer 2011;2:67-75. [PubMed]
- Ozgün A, Karagoz B, Bilgi O, et al. MicroRNA-21 as an indicator of aggressive phenotype in breast cancer. Onkologie 2013;36:115-8. [PubMed]