Clinical antiangiogenic effect of recombinant adenovirus-p53 combined with hyperthermia for advanced cancer
Introduction
Tumor suppressor gene p53, well known as the genome guardian of cells, plays a key role in cell cycle control, apoptosis, and inhibition of tumor cell proliferation. p53 gene also acts as a transcription factor and mediates cellular response to DNA damage induced by irradiation, hyperthermia, and cytotoxic agents (1,2).
Introduction of normal p53 gene using viral vectors results in suppression and reversal of the malignant phenotype of tumors and induces thermosensitization or radiosensitization, which is a new strategy to convert a thermo- or radio-resistant phenotype into a thermo- or radio-sensitive one (3-5). Thus, recombinant adenovirus-p53 (rAd-p53) could act as a strong thermosensitizer or radiosensitizer for tumor therapy. These results support the combination use of p53 gene therapy and hyperthermia or radiotherapy in antitumor treatment. Up to now, hyperthermia is not considered as a unitary clinical treatment method for cancer, because hyperthermia alone is negligible for late-stage cancer. Current hyperthermia alone has been an assistant method for cancer treatment. rAd-p53 acts as a thermosensitizer for hyperthermia, and upgrades hyperthermia to radical cure for patients with cancer.
rAd-p53 (trademarked as Gendicine) is an E1-substituted replication-incompetent recombinant adenovirus encoding human p53 gene. Gendicine is a p53 gene therapy drug approved to market by China SFDA at October of 2003.
Vascular epithelial growth factor (VEGF) has been considered to induce angiogenesis, which is indispensable to tumorigenesis and progression. Introduction of wild-type p53 gene into cancer cells with mutant p53 gene markedly inhibited the expression of an angiogenic factor, VEGF, and increased the expression of a novel antiangiogenic factor, brain-specific angiogenesis inhibitor 1 (BAI 1), resulting in decrease in neovascularization in vivo. Microvessel density and microvessel counts were lower in xenografts from tumor cells containing wild-type p53. Overall data suggest that rAd-p53 is antiangiogenic, which may explain, in part, the mechanism of clinical antiangiogenic effect of p53 gene combined with hyperthermia plus or not plus radiotherapy for advanced cancer, shown as tumor regression and obvious necrosis (6-8).
Continuously, a combination of rAd-p53 and hyperthermia was adopted in this study. This study aimed to further confirm that the effects and mechanism of combination of rAd-p53 and hyperthermia in 44 patients with advanced cancer.
Materials and methods
Immunohistochemistry (IHC) imaging
After intratumoral injection of rAd-p53, the adenoviral particle infects targeted tumor cells and delivers the adenoviral genome carrying the therapeutic p53 gene to the cell nucleus for transcription. Biopsies before injection and 48 h after the first intratumoral injection of rAd-p53 were assessed for P53 protein and P53-targeted genes p21, Bax, and VEGF, the downstream P53-transactivated genes, by IHC staining in 50 couples of available samples of 25 patients. IHC staining of paraffin-embedded tissues was performed according to standard methods. Sections in which more than 75% of cells had definitive nuclear reactivity were scored 4; 50% to 75%, 3; 25% to 50%, 2; 5% to 25%, 1; and fewer than 5%, 0.
Patients’ characteristics
This is a clinical observation protocol only, because the enrolled patients were in advanced stage including recurrent cases failing in surgery, radiotherapy or chemotherapy, and all enrolled tumors were refractory and uncurable. Antitumor effects of rAd-p53 alone was not confirmed in clinical study before, so there were no patients receiving rAd-p53 alone used as control in this study. Our previous preclinical in vitro study demonstrated that after wild-type p53 mediated by adenovirus was transferred into four kinds of human gastric carcinoma cell lines with different p53 status, the expression of P53 protein in cell nucleus, increased radiation-inducing G2/M arrest and apoptosis, and increased radiosensitivity were found. So in clinic, it does not need to detect patient’s p53 gene status beforehand (1-4).
Details about patient approval to participate
Inclusion criteria
Patients should be 18 to 80 years old, and had a histological diagnosis of malignant tumor in advanced stage with measurable disease and no distant metastasis. The patients were clinically staged according to the fifth edition of the International Union against Cancer (UICC) TNM staging systems [1997]. Patients must have a projected life expectancy of at least three months and a Karnofsky performance score of at least 70%. Patients were required to have adequate bone marrow function (white blood cell count ≥4.0×109/L, hemoglobin ≥7 g/L, platelet count ≥70×109/L) and adequate liver and renal function [aspartate transaminase (AST), alanine transaminase (ALT), blood urea nitrogen (BUN) and creatinine (Cr) <1.5 times of the upper limit).
Exclusion criteria
Pregnant or nursing women, patients with uncontrolled serious infection, or patients with serious heart and lung failure were excluded.
Study protocol
This is a non-randomized controlled clinical study, and was approved by the Ethics Committee of Beijing Cancer Hospital at Sep 12, 2001.
In this study, we used intratumoral injection of rAd-p53 combined with hyperthermia plus or not plus radiotherapy for treatment of advanced cancer. Between October 2001 and December 2009, 44 patients, including 33 males and 11 females with a median age of 54.3 years old (range of 23-80 years old), were enrolled. All these patients were diagnosed as advanced cancer by pathohistological examination. Among the 44 patients, 21 patients had squamous cell carcinoma (9 in nasopharynx, 3 in larynx, et al.), 6 patients had adenocarcinoma (1 adenoid cystic carcinom, 1 thyroid adenocarcinoma, et al.), and 17 patients had soft tissue sarcoma (4 liposarcoma, 2 malignant neurinoma, et al.). There were 38 patients with recurrent tumors and 6 patients with primary tumor receiving no treatment before they were enrolled. Of these 38 patients with recurrent tumors, 34 person-times failed in surgery, 30 person-times failed in radiotherapy, and 21 person-times failed in chemotherapy. Most of the 38 patients failed in combination of three types of treatment, 12 patients failed in two types of treatment, and 5 patients failed in one type of treatment.
Treatment options
All patients were intratumorally injected with rAd-p53 at multiple points, at a dose of up to 1×1012 viral particles (vp) once a week, with a total of 4-54 (mean 7.7) times. Intratumoral injection was done directly, or guided by ultrasound for neck node, abdominal, pelvic deep-seated tumor, or guided by computed tomography (CT) for lung tumor. Hyperthermia was given weekly 2 d after rAd-p53 injection at 43-44 °C using a 915 MHz microwave machine for 1 h for superficial tumor, and at 42-43 °C using a 41 MHz radiofrequency machine for 1 h for deep-seated tumor. Total of 4-29 (mean 8.5) times of hyperthermia was given to all patients.
Among the 44 patients, 30 patients were concurrently added with radiotherapy using the 6 or 8 MV linear accelerator X-ray with the conventional fractionation 2 Gy once a day from Monday to Friday to a total each week at a total dose of 30-76 Gy/15-38 f/3-8 w (mean 58 Gy).
Response assessment and adverse events
One longest diameter of each tumor lesion was measured according to CT or magnetic resonance imaging (MRI) findings of tumor. Tumor shrinkage rate of rAd-p53-injected tumor after 2-month treatment was counted by comparison to the pre-treatment tumor size. The tumor shrinkage rate was graded using Response Evaluation Criteria in Solid Tumor (RECIST) Guidelines (one-dimension): complete response (CR), disappearance of lesions; partial response (PR), more than a 30% decrease; stable disease (SD), neither PR nor progressive disease (PD); and PD, more than a 20% increase (9). The tumor density measurement (Hounsfield unit, HU) on CT images is a good indicator and provides a reliable quantitative means of monitoring the tumor. After treatment, low-density area [low density area (LDA), CT value <25 HU] on CT images appeared and expanded within the tumor that did not regress completely. Histopathologic examinations showed the LDA to be massive coagulation necrosis. A greater extent of LDA indicated the improvement of local tumor control and survival of patients (10-12).
The patients were monitored for adverse events. Toxic and adverse events were assessed as light (grade 1), mild (grade 2), serious (grade 3), and life-threatening (grade 4) according to the WHO’s evaluation standard for adverse events. Particular attention was paid to body temperature.
Statistical analysis
Overall survival (OS) was calculated according to the Kaplan-Meier method from the first date of treatment to the date of death. If a patient was not dead, then survival was censored at the time of the last visit. Version 11.0 of the SPSS statistical program (SPSS Inc., Chicago, IL, USA) was used for analysis. P<0.05 was considered statistically significant.
Results
Treatment and efficacy
The research showed that rAd-p53-specific p53 mRNA was detected by reverse transcription-polymerase chain reaction (RT-PCR) analysis in the 16 tissue samples out of 17 (94.1%) assessable samples taken at 48 h after intratumoral injection of rAd-p53. The expression of p53 gene and p53 downstream genes in the tumor samples taken at 48 h after intratumoral injection of rAd-p53 were assayed by IHC. Up-regulation of cell cycle relative gene p21 and apoptosis relative gene Bax, and down-regulation of VEGF were observed in tumor tissues after injection of rAd-p53. Immunostaining was scored semiquantitatively. The positive cell scores before and after injection were 1.44 and 2.48 for P53 (P=0.050), 0.32 and 0.88 for P21 (P=0.015), 0.92 and 1.63 for Bax (P=0.088), and 2.80 and 1.50 for VEGF (P=0.031), respectively (5).
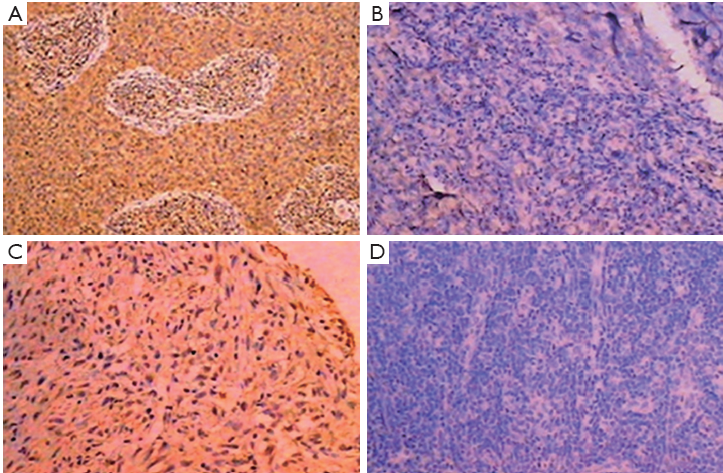
As shown as Figure 1, the VEGF immunostaining in the tumor tissues from two patients before and after intratumoral injection of rAd-p53 showed VEGF positive in 75% of cells before intratumoral injection of rAd-p53 and negative in all cells after injection of rAd-p53.
After treatment by intratumoral rAd-p53 injection combined with hyperthermia, the CR rate was 13.60% (6/44), the PR rate was 29.6% (13/44), the SD rate was 52.3% (23/44) and the PD rate was 4.5% (2/44). The responsible rate (CR + PR) was 43.2% and non-responsible rate (SD + PD) was 56.8%. Three patients in the CR patients were considered a pathologic complete response (PCR). There were 19 patients’ CT scans showed clear LDA and their CT value decreased to <30 HU. At 2-month after treatment, the samples from the LDA were almost complete coagulation or colliquation necrosis and no tumor cells appeared (Figures 2,3).
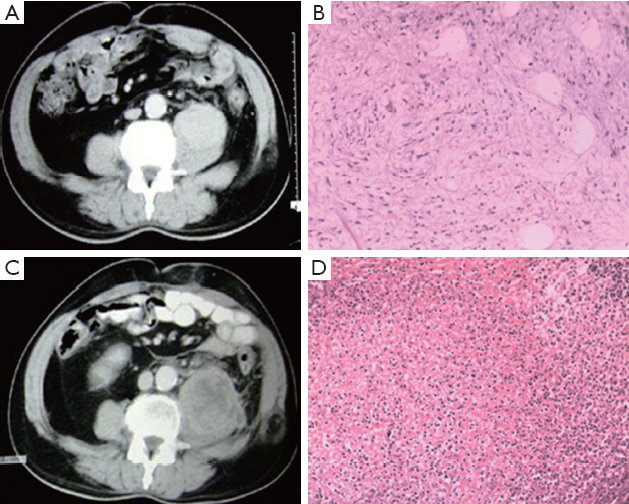
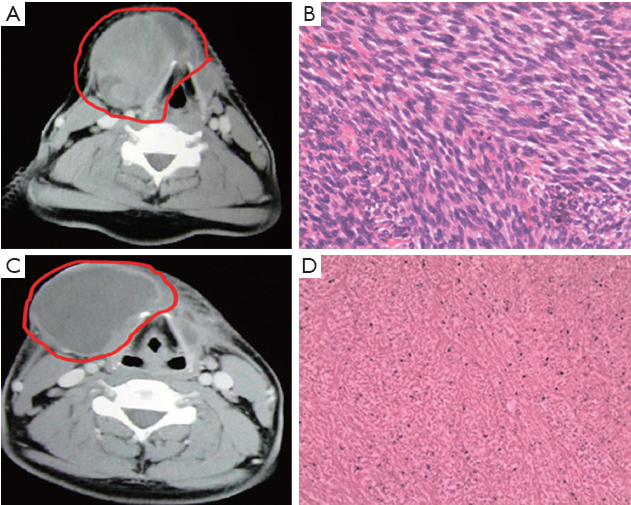
Figure 2 shows the CT scans and histopathologic examination from a typical case: 46-year-old man with recurrent mucoid liposarcoma, 6 cm in diameter, in retroperitoneum. CT value was 86 HU before treatment. The patient received intratumoral injection of rAd-p53, at a dose of 1×1012 vp once a week, for 7 times combined with 11 times of deep-seated hyperthermia and 56 Gy of irradiation. At 1-month after treatment, there was no change in tumor size but CT value went down to 25 HU. Obvious liquefaction necrosis was observed on CT image. Histopathologic examination showed that the sarcoma cells disappeared and a lot of lymphocyte cells were full of resected tissue.
Figure 3 shows the data of another typical case: 28-year-old man with recurrent spindle type sarcoma, 7.0 cm in diameter, in submaxilla. The tumor involved in right thyroid cartilage and was unresectable. The patient was treated with nine times of intratumoral injection of rAd-p53, nine times of superficial hyperthermia and 40 Gy of radiotherapy. The tumor size reduced to 4%, and CT value was reduced from 58 HU before treatment to 27 HU. Three months later, the tumor was completely removed and obvious necrosis could be observed in tumor tissue. Above 70% tumor cells were disaggregation under microscope.
OS was calculated using Kaplan-Meier method. The date of the last follow-up was December 2009 and the median follow-up time was 11.5 (range 3-76) months. The median survival time (MST) was 12.0±1 months [95% confidence interval (95% CI): 10-14 months] for the 44 patients. The 1-, 2-, 3-, 4- and 5-year OS was 43.7%, 27.2%, 21.2%, 14.1%, and 7.1%, respectively.
Adverse events
Total data showed that all the values in blood, urine and stool examination, and liver and renal function remained within normal range before and after treatment in the group receiving rAd-p53 combined with hyperthermia. Lung and heart function was normal before and after treatment. This indicates that rAd-p53 was safe and well-tolerated in the patients with advanced cancer. Additionally, rAd-p53 administration did not appear to increase the adverse effects caused by hyperthermia and radiation treatment.
All fever events were grade 1 (less than 38 °C) and grade 2 (38 to 40 °C), and were transient and self-limited. The incidence of fever among the 44 patients receiving rAd-p53 injections was 81.0% (36 of 44), including grade 1 fever (43.0%) and grade 2 fever (38.0%). Development of fever was observed as early as approximately 3 h after injection, lasting about 4 h and then disappearing spontaneously. Only slight pain and discomfort at local injection sites were felt from the repeat injections.
Discussion
rAd-p53 seems to act synergistically with conventional treatments such as chemotherapy and/or radiotherapy. In addition, this apparent synergy still exists in patients who were resistant to chemotherapy and radiotherapy. rAd-p53 therapy has been demonstrated to be safe, feasible, and efficient, including local transgenic expression and evidence of local tumor regression for patients with head and neck squamous cell carcinoma (HNSCC) (5,13,14), esophageal cancer (15), lung cancer (16,17), ovarian cancer (18), bladder cancer (19), and so on.
In this study, the enrolled patients were in advanced stage including recurrent cases failing in surgery, radiotherapy or chemotherapy, and were refractory and uncurable. After the treatment, the CR rate was 13.60% (6/44), and the PR rate was 29.6% (13/44). The responsible rate (CR + PR) was 43.2%. The MST was 12.0±1 months (95% CI: 10-14 months) for the 44 patients. The 1-, 2-, 3-, 4- and 5-year OS was 43.7%, 27.2%, 21.2%, 14.1%, and 7.1%, respectively. In addition to 6 patients with CR, 19 patients (19/38, 50.0%) had LDA of more than 50% area on CT image within tumor, indicating tumor tissue necrosis. These results demonstrated that rAd-p53 combined with hyperthermia plus or not plus radiotherapy could increase local tumor control and improve OS of patients with advanced cancer (20). Our previous study demonstrated that down-regulation of VEGF was observed in tumor tissue treated by intratumoral injection of rAd-p53 (5). Obvious necrosis was found in tumor tissues of the patients who received intratumoral injections of rAd-p53, which may be correlated with rAd-p53-induced down-regulation of VEGF. Other study showed that the overexpressed P53 protein also stimulates the expression of genes that encode proteins involved in suppressing angiogenesis (blood vessel formation). Angiogenesis is a process required for solid tumor formation and progression (21). Inhibition of the VEGF/VEGF receptor pathway is of special interest in targeting therapy for cancers. The antiangiogenesis effect of P53 protein appears to involve three possible mechanisms: (I) interfering with central regulators of hypoxia that mediates angiogenesis; (II) inhibiting production of pro-angiogenic factors; and (III) directly increasing the production of endogenous angiogenesis inhibitors. Recently, P53 has been shown to inhibit hypoxia-inducible factor-1 (HIF-1) activity, which induces angiogenic factors in response to hypoxia (22). Activation of the P53 pathway inhibits angiogenesis and suppresses tumor growth (23). rAd-p53 inhibits VEGF expression and angiogenesis, and then induces tumor necrosis in the clinical course, especially in p53 gene intratumoral injection combined with hyperthermia-based treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Zhang SW, Xiao WQ, Lu YY. Role of p53 tumor suppresspr gene in radiosensitivity of human gastric carcinoma cell lines. Zhong Hua Fang She Zhong Liu Xue Za Zhi 1999;8:116-9.
- Zhang SW, Xiao WQ, Lu YY. Enhancement effect of the tumor suppressor p53 gene on thermosensitivity on human gastric cancer cell lines. Zhong Hua Wu Li Yi Xue Yu Kang Fu Za Zhi 2000;22:94-6.
- Zhang SW, Xiao SW, Lu YY. Effect of adenovirus-mediated p53 gene transfer on apoptosis and radiosensitivity of human gastric carcinoma cell lines. Chin J Cancer Res 2003;1:14-8.
- Zhang SW, Xiao SW, Lu YY. Adenovirus-mediated p53 gene transfer increases the thermosensitivity of human gastric carcinoma cell lines (in vitro and in vivo). Chin J Cancer Res 2003;15:107-11.
- Pan JJ, Zhang SW, Chen CB, et al. Effect of recombinant adenovirus-p53 combined with radiotherapy on long-term prognosis of advanced nasopharyngeal carcinoma. J Clin Oncol 2009;27:799-804. [PubMed]
- Fujiwara T, Nishizaki M, Tanaka N. Recombinant adenovirus expressing wild-type p53 is antiagiogenic: Implication for lung cancer gene therapy. Gan To Kagaku Ryoho 2000;27:1217-24. [PubMed]
- Zhang L, Yu D, Hu M, et al. Wild-type p53 suppresses angiogenesis in human leiomyosarcoma and synovial sarcoma by transcriptional suppression of vascular endothelial growth factor expression. Cancer Res 2000;60:3655-61. [PubMed]
- Nishizaki M, Fujiwara T, Tanida T, et al. Recombinant adenovirus expressing wild-type p53 is antiangiogenic: A proposed mechanism for by-stander effect. Clin Cancer Res 1999;5:1015-23. [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. JNCI 2000;92:205-16. [PubMed]
- Hiraoka M, Akuta K, Nishimura Y, et al. Tumor response to thermoradiation therapy: use of CT in evaluation. Radiology 1987;164:259-62. [PubMed]
- Takeshita N, Tanaka Y, Matsuda T. Evaluation of CT images, tumor response and prognosis after thermoradiotherapy for deep-seated tumors. Int J Hyperthermia 1993;9:1-17. [PubMed]
- Choi H, Charnsangavej C, de Castro Faria S, et al. CT Evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 2004;183:1619-28. [PubMed]
- Clayman GL, el-Naggar AK, Lippman SM, et al. Adenovirus-mediated p53 gene transfer in patients with advanced recurrent head and neck squamous cell carcinoma. J Clin Oncol 1998;16:2221-32. [PubMed]
- Clayman GL, Frank DK, Bruso PA, et al. Adenovirus-mediated wild-type p53 gene transfer as a surgical adjuvant in advanced head and neck cancers. Clin Cancer Res 1999;5:1715-22. [PubMed]
- Shimada H, Matsubara H, Shiratori T, et al. Phase I/II adenoviral p53 gene therapy for chemoradiation resistant advanced esophageal squamous cell carcinoma. Cancer Sci 2006;97:554-61. [PubMed]
- Schuler M, Rochlitz C, Horowitz JA, et al. A phase I study of adenovirus -mediated wild-type p53 gene transfer in patients with advanced non-small cell lung cancer. Hum Gene Ther 1998;9:2075-82. [PubMed]
- Swisher SG, Roth JA, Komaki R, et al. Induction of p53-regulated genes and tumor regression in lung cancer patients after intratumoral delivery of adenoviral p53 (INGN 201) and radiation therapy. Clin Cancer Res 2003;9:93-101. [PubMed]
- Wen SF, Mahavni V, Quijano E, et al. Assessment of p53 gene transfer and biological activities in a clinical study of adenovirus-p53 gene therapy for recurrent ovarian cancer. Cancer Gene Ther 2003;10:224-38. [PubMed]
- Kuball J, Wen SF, Leissner J, et al. Successful adenovirus-mediated wild-type p53 gene transfer in patients with bladder cancer by intravesical vector instillation. J Clin Oncol 2002;20:957-65. [PubMed]
- Zhang S, Xu G, Liu C, et al. Clinical study of recombinant adenovirus-p53 (Adp53) combined with hyperthermia in advanced cancer (a report of 15 cases). Int J Hyperthermia 2005;21:631-6. [PubMed]
- Teodoro JG, Evans SK, Green MR. Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J Mol Med (Berl) 2007;85:1175-86. [PubMed]
- Sano M, Minamino T, Toko H, et al. p53-induced inhibition of Hif-1 cause scardiac dysfunction during pressure overload. Nature 2007;446:444-8. [PubMed]
- Dai F, Chen Y, Song Y, et al. A natural small molecule harmine inhibits angiogenesis and suppresses tumor growth through activation of p53 in endothelial cells. PLoS One 2012;7:e52162. [PubMed]
