Aurora-A is a novel predictor of poor prognosis in patients with resected lung adenocarcinoma
Introduction
Lung cancer is the world’s leading cause of cancer-related deaths, with nearly 1.4 million deaths and approximately 1.6 million new cases each year (1). Lung adenocarcinoma (ADC), the most common pathological type of non-small cell lung cancer (NSCLC), accounts for almost 50% of all lung cancers (2). Even though systemic management followed by surgery has progressed during the past decade, the postoperative prognosis remains poor (3) with 35-50% of early stage NSCLC patients suffering recurrence (4). Recently, promising efficacy of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) such as gefitinib and erlotinib were demonstrated in a subset of patients with EGFR mutations (1). In addition, crizotinib has been shown to yield very high response rates (>80%) and to improve survival when used in only NSCLC patients who have EML4-ALK rearrangements (5). However, these targeted agents were suitable for a minority of cases. Thus, the identification of other novel targets would contribute to development of individualized therapy and supplement the traditional tumor-node-metastasis (TNM) framework for cancer.
Aurora-A (Aur-A), also known as STK15/BTAK, was first discovered as the product of gene STK15/BTAK on chromosome 20q13 (6). A series of previous clinical studies have demonstrated that Aur-A is overexpressed and up-modulated in a wide variety of human tumors, such as esophageal cancer (7), gliomas (8), gastric cancer (9), pancreatic cancer (10), bladder cancer (11), breast cancer (12), ovarian cancer (13), and hepatocellular cancer (14). As a member of the serine/threonine kinase family, Aur-A is a key kinase in regulation of centrosome function, bipolar spindle assembly and chromosome segregation, thus playing a crucial role in mitosis (15). Activation of Aur-A, achieved through phosphorylation and overexpression in cell cycle control, accelerates cell proliferation and prolongs cell life span which may lead to a tumorigenesis (16). Such a process of tumorigenesis in NIH 3T3 cells has been demonstrated in nude mice (17). Since Aur-A is involved in multiple aspects of tumorigenesis; it is postulated that over-expression of the kinase affects patient prognosis and represents a novel target for therapy (18). The significance status of Aur-A in predicting prognosis of lung squamous cell carcinoma has been proved (19). However, there is a paucity of data in the relationship between over-expression of Aur-A and the malignancy of lung ADC. We hypothesized that Aur-A over-expression in resected lung ADCs would be associated with worse clinical outcomes.
Patients and methods
All procedures complied with the ethical guidelines for the collection of human tissue specimens and use of a laboratory study at the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Patients and specimens
In this study, 142 lung ADC patients who underwent surgery in the Thoracic Department of The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China, from 2006 to 2012 were enrolled. The cases were selected consecutively on the basis of availability of resection tissue and follow-up data. Patients with prior malignancies, a second primary tumor or those who received neoadjuvant radiotherapy and/or chemotherapy were excluded. Informed consent was obtained from all patients. Histological diagnosis was determined by hematoxylin and eosin staining according to the World Health Organization (WHO) criteria. Clinical data were reviewed retrospectively using written and electronic medical records. Patients accepted a telephone follow-up every three months until death. These data were used to analyze the relation between Aur-A expression level and tumor characteristics and clinical outcomes such as clinical stage, tumor size, lymph node status, nuclear grade, disease-free survival (DFS) rate and overall survival (OS) rate.
Western blot analysis
The lung ADC samples were cut into small pieces and dissolved into lysis solution in order to perform Western blot testing. The complex solution was homogenized and sonicated for 5 minutes on ice. Protein concentration was assessed by the Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc. California, USA). A total of 50 µg of lung ADC protein was loaded onto 10% SDS-PAGE for electroblotting. The proteins were transferred to the polyvinylidene fluoride membrane (Bio-Rad Laboratories, Inc. Singapore) by electroblotting. After being washed with PBST (PBS with 1‰ Tween) and blocked in 5% skim milk, the membranes were incubated with the Aur-A antibody (BS1860, Bioworld technology, Co.,Ltd. Nanjing, China) at 4 °C overnight on a shaker. Then the HRP-conjugated goat anti-rabbit IgG as a secondary antibody was used at room temperature for 2 hours. The membranes were washed three times with PBST and fluorescence was detected by enhanced chemiluminescence (Bio-Rad Laboratories, Inc.) and exposed to X-ray film.
Immunohistochemistry (IHC)
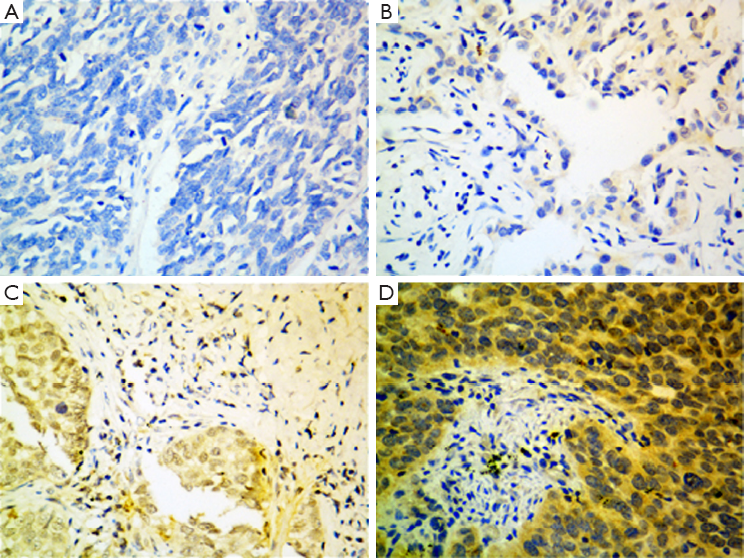
Expression of the Aur-A protein in tissue sections was measured by IHC staining. The sections were heated at 65 °C for 2 hours, deparaffinized in xylene, and rehydrated in a graded series of alcohol solutions. These sections were covered with 10mM sodium citrate buffer (PH 6.0), heated in a pressure cooker for five minutes, and treated with 1% (v/v) normal goat serum for 15 minutes. The Aur-A antibody (BS1860, Bioworld technology, Co.,Ltd. Nanjing. China) had been produced and its reliability had been confirmed by western blotting. The antibody was incubated with the sections at 4 °C overnight in a humid chamber, at a 1:200 dilution. The antibody complex was detected by incubation with an avidin-biotin-peroxidase complex solution and visualized by 3,3-diamino-benzidine. The sections were counterstained with hematoxylin for 2 min. Normal lung tissue, serving as the negative controls, and esophageal cancer tissue (known to express Aur-A) serving as positive controls, were processed in the same way.
Evaluation of IHC staining
All slides were assessed independently by two pathologists blind to patients and their clinical information. Positive staining of the cytoplasm was evaluated in at least five area 200× magnification. Aur-A IHC was initially scored into four groups according to the extent and intensity of cytoplasmic staining of the tumor cells. Staining intensity was scored as: negative [0]; weak [1]; moderate [2]; or strong [3]. Staining extent was scored according to the percentage of positive staining tumor cells seen. Slides with no staining tumor cells were scored 0; samples with <25% of tumor cells were scored 1; those with 25-50% of tumor cells were scored 2 and >50% of the cells were scored 3. The overall staining score was the result of the staining intensity score multiplied by the extent score. The specimens whose overall scores were 6 or 9 were defined as moderate-density or high-density of Aur-A protein, respectively, which were considered positive over-expression. Negative over-expression group included negative expression or low-expression samples with overall scores of 0 or 2.
Statistical analysis
Statistical analysis was carried out with the SPSS software (SPSS version 19.0; IBM SPSS Inc. Chicago, IL, USA). The correlation of Aur-A protein expression with clinic pathologic characteristic of lung ADC patients was assessed by the chi-square test. Kaplan-Meier curves were used to model OS (i.e., the time from day of surgery to death) and DFS (i.e., the time from day of surgical to recurrence or death) and the log-rank test was used to compare differences in survival between groups. Univariate and multivariate analysis of the prognostic factors were examined by Cox’s proportional hazard model. A two-side P value of <0.05 was considered statistically significant.
Results
Aur-A expression in lung ADC and normal lung tissues
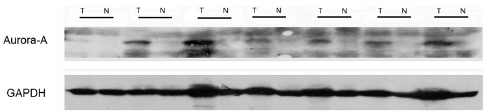
The Aur-A protein was detected by western blot and IHC in this study. A total of 12 consecutive pairs of surgically resected lung ADC tumors and normal adjacent tissues were chosen. In 10 of 12 tumor samples, Aur-A protein were elevated compared with the normal adjacent tissues by western blot analysis (Figure 1). A total of 142 lung ADC tumor sections and 20 normal lung tissue sections were detected by IHC (Figure 2). In the cohort of 142 lung ADC patients, high expression of Aur-A was detected in 98 of 142 (69.0%) patients and low expression of Aur-A was detected in 44 of 142 (31.0%) patients. The overall scores of all normal lung sections were below 2.
The relationship between Aur-A expression, pathological characteristics and survival of lung ADC patients
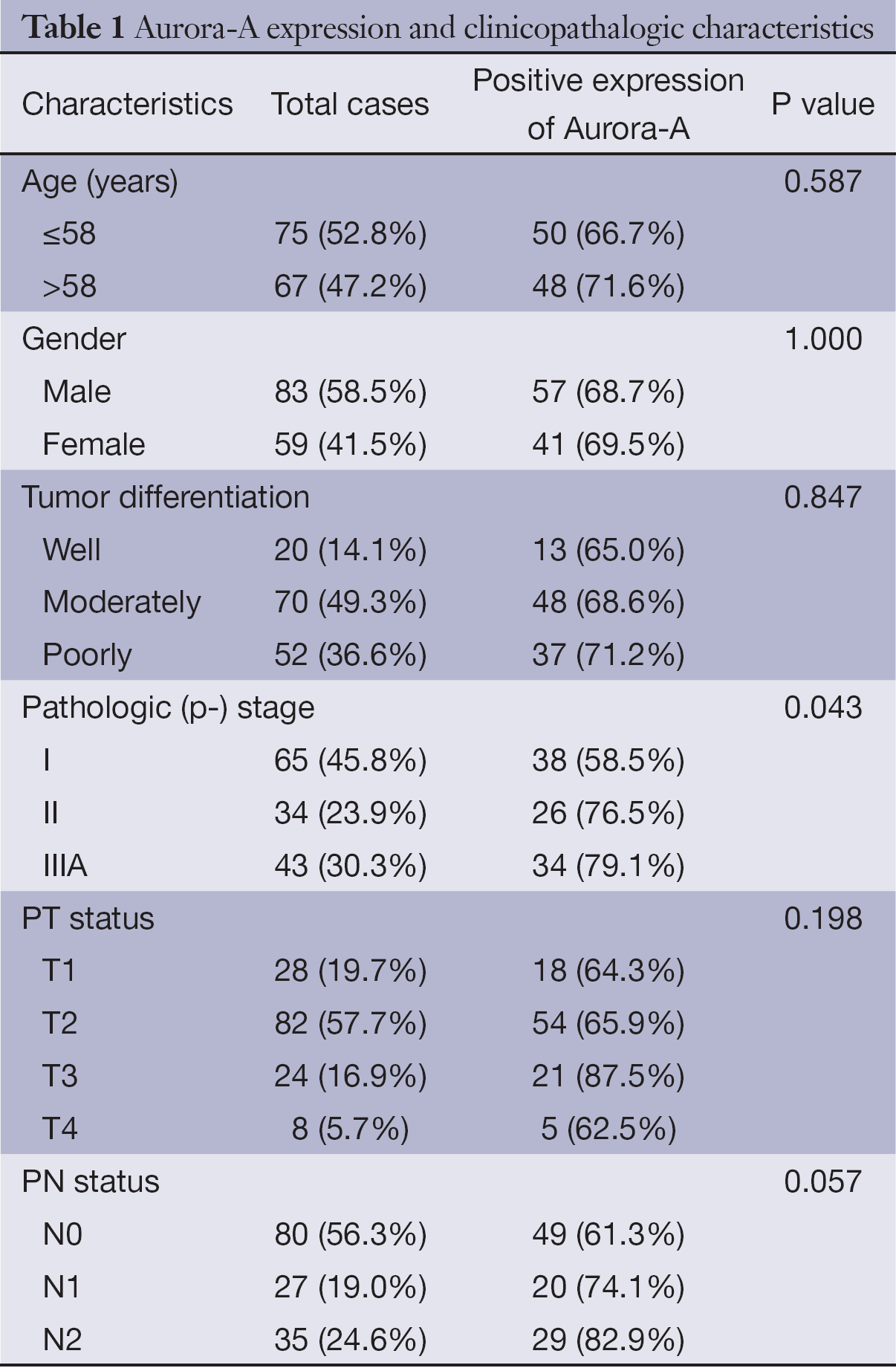
Clinical characteristics of the 142 lung ADC patients, including age, gender, tumor differentiation, pathological stage, tumor status, lymph node status, are summarized in Table 1. The age of the patients ranged from 32 to 79 years (median of 58 years), and included 83 males (58%) and 59 females (42%). The pathologic (p)-stages of patients range from I to IIIA which are reevaluated and determined according to the criteria of the WHO. The performance status (PS) of all patients is 0. The follow-up period ranged from 3 to 66 months, with a median of 27 months.
Full table
The overexpression of Aur-A protein was closely related to the pathological stage (P=0.043); positive staining was detected in 58.5% (38/65) of p-stage I patients, 76.5% (26/34) of p-stage II patients and 79.1% (34/43) of p-stage IIIA patents (Table 1). No significant association was found between Aur-A overexpression and other clinicopathologic features such as tumor size and tumor differentiation in our study (Table 1).
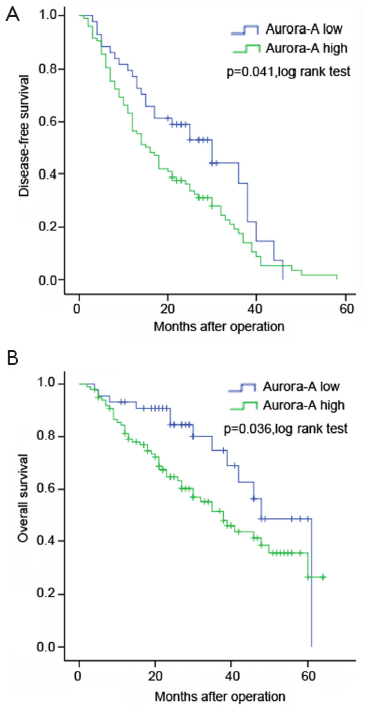
The OS of positive over-expression patients was shorter than the negative (P=0.036) (Figure 3). The median OS was 38 months for positive over-expression patients and 48 months for negative expression patients. The negative group have longer DFS compared with positive group (P=0.041) (Figure 3). The median DFS was 15 months for positive over-expression patients and 30 months for negative expression patients. In addition, our results showed that Aur-A expression might have preferentially greater impact on patients’ DFS in stage II or III than stage I (P=0.116 vs. P=0.548) (Figure 4).
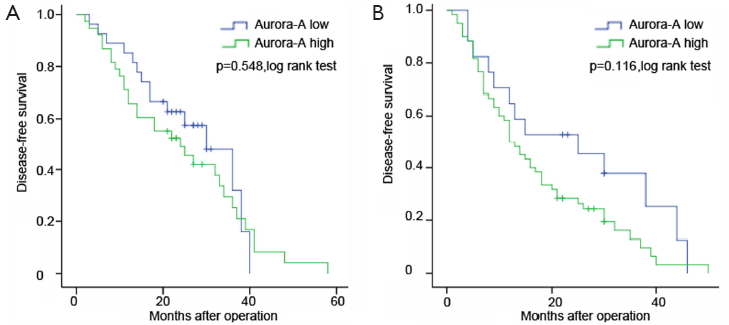
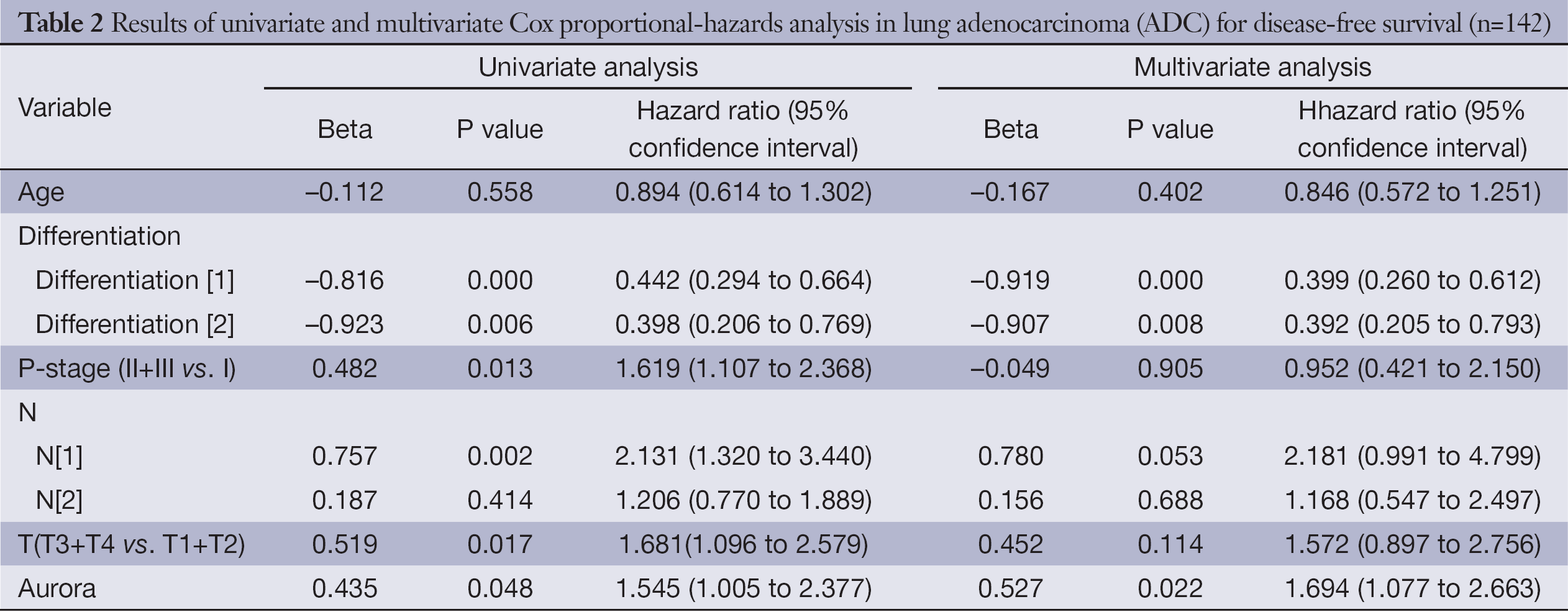
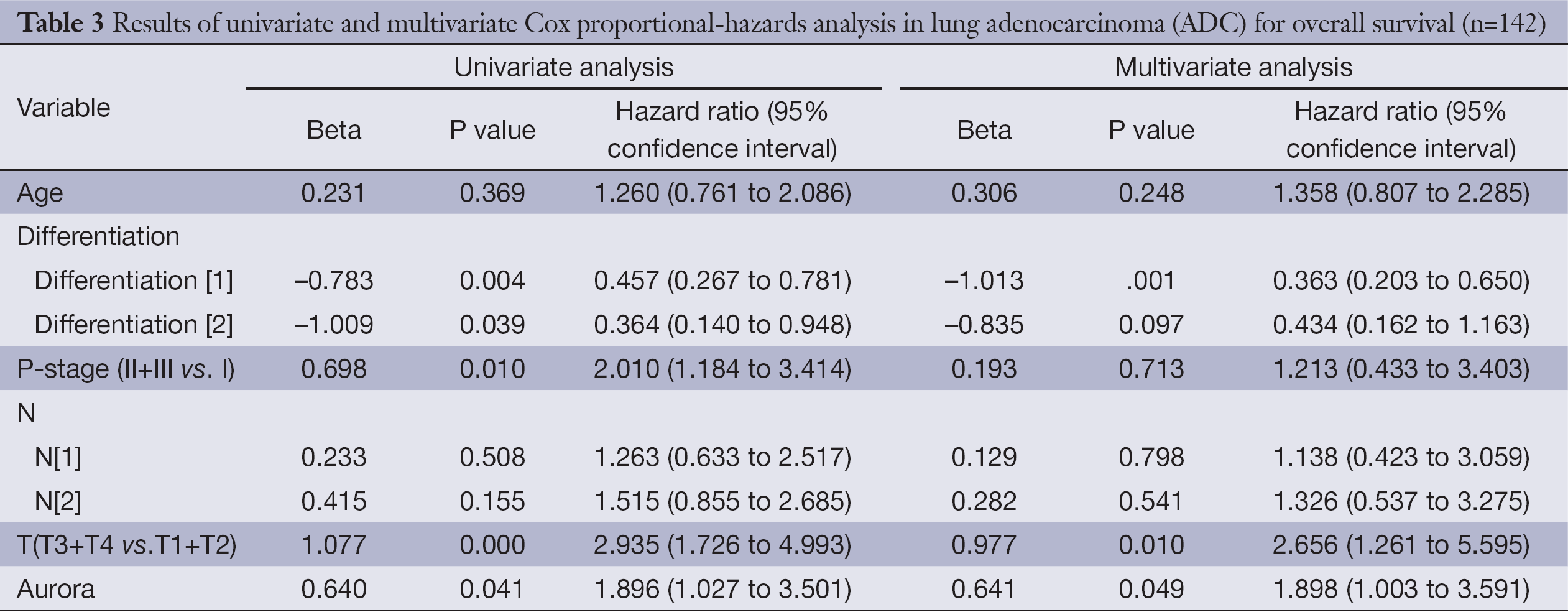
Univariate analysis demonstrated that over-expression of Aur-A adversely affected DFS (hazard ratio, 1.545; 95% CI, 1.005-2.377; P=0.048, Table 2) and OS (hazard ratio, 1.896; 95% CI, 1.027-3.501; P=0.041, Table 3) in lung ADC patients. Multivariate analysis of prognostic factors suggested that Aur-A protein was an independent prognostic factor for DFS (hazard ratio, 1.694; 95% CI, 1.077-2.663; P=0.022, Table 2) and OS (hazard ratio, 1.898; 95% CI, 1.003-3.591; P=0.049, Table 3) in the cohort of 142 resected lung ADC patients.
Full table
Full table
Discussion
This study demonstrated that Aur-A up-regulation is a common finding in lung ADC and may play a potential role in its development. Furthermore, our results suggested that there was a consistent trend that Aur-A overexpression rate was associated with the status of lymph node metastasis. Previous study showed up-regulation of Aur-A, as an early event in tumorigenesis, was more found in early stage of cancers (20). Growing evidences demonstrated that Aur-A not only plays a role in the formation of tumors, but also in the cancer progression. In this study, an ascending expression of Aur-A was in accord with the rise of the clinical stage, a validated and the most important tool to determine the progress of lung cancer (18). The promoting of cell migration and invasion is the most notable feature of the malignant behavior in cancer progression. Previously, the function of Aur-A in cellular mobility and metastasis has been reported in several studies. Wu, et al. found that the activation of RalA, as one of the downstream substrates of Aur-A, could induce cell migration and transformation through V23RalA-Ser194 phosphorylation (21). After that, another Chinese research team further reported that Aur-A could enhance LSCC (laryngeal squamous cell carcinoma) cell growth and migration mediated by up-regulation of Akt1 (22), and increased epithelial-mesenchymal transition and invasion by activation of MAPK (mitogen-activated protein kinase) in nasopharyngeal carcinoma cells (23). Next they also identified that overexpression of Aur-A could induce mammary cell migration and breast cancer metastasis by activating the Cofilin-F-actin pathway (24). All the past evidences revealed Aur-A as a vital role in the migration and invasiveness of cancer cells.
Considering the intricacy of oncogenic mechanisms seen in the vast majority of tumors, it is unlikely that Aur-A is the sole contributor to lung ADCs. Extensive investigations revealed that Aur-A can interact with a broad spectrum of other biological molecules, such as EGFR and Ki-67, which can promote the cancer cells’ growth (25,26). The result highlighted its role in cancer cell proliferation. Combined with our data, Aur-A was confirmed to prompt recurrence and poor prognosis in lung ADCs. Recently, a previous study reported that the overexpression of Aur-A protein on perimembrane was significantly correlated with poor prognosis in lung squamous cell carcinoma. However, their reports failed to show the correlation between Aur-A and prognosis in ADC (19). In our study, it was the first time to show that cytoplasmic staining of Aur-A was significantly correlated with poor prognosis in lung ADC overall and disease free survival. These conflicting results on clinical significance of Aur-A expression status might be partly due to the different evaluation system. Thus, Aur-A may serve as a novel molecular target to optimize individual therapy by determining whether a multimodality approach is need. Since the current study was based on a relatively small sample, a multicenter study in a larger population of lung ADCs is needed to confirm the results. The current study did not examine the gene amplification because the regulation mechanism of Aur-A expression are complicated. Not only the gene amplification, but also many other mechanisms can regulate the modification. Further researches need to be conducted to identify the precise signaling pathway or network of Aur-A which is ultimately involved to the pathogenesis of lung ADC.
With a deeper analysis of Aur-A overexpression performed in tumorigenesis, developing potent Aur-A kinase inhibitors (AKIs) had become a hotspot in cancer molecular therapeutic area. Some AKIs, such as MK-0457, MLN-8054, and PHA-739358 were testified to show certain abilities to inhibit tumor cell growth and to selectively kill proliferating cells. Moreover, the drugs even were involved in clinical trials in the United States and Europe (18). Recently, MK-0457, a novel kinase inhibitor, was active in patients with some phenotypes of leukemia and was well tolerated in a phase I dose study meanwhile almost half the patients with advanced solid tumors attained a stable disease (27,28). On the other hand, RNA interference technique-specific knockdown of Aur-A to induce down-regulation had been showed to suppress tumor growth in vitro pancreatic cancer cells and in vivo tumorigenicity (29). Since AKIs are emerging as a potential target drug to regulate cell circle, one might reasonably expect that they could be used to enhance the clinical treatment of cancer in the future.
In conclusion, our results showed some evidences for the concept that Aur-A acted as an independent and significant predictor for poor prognosis. Up-regulated expression of Aur-A may not only be involved in the initiation of lung ADCs, but also in the progression. The activation of Aur-A may provide some conditions for cancer to thrive. Therefore, Aur-A may serve as a promising individual therapeutic target in treatment of lung ADC, although further studies will be carried on necessarily.
Acknowledgements
We thank Dr. Wenhua Liang (State Key Laboratory of Oncology in South China, Cancer Center, Sun Yat-sen University) for critical reading of the manuscript and for helpful comments; and Zhen Zeng (Department of Nutrition,School of Public Health,Sun Yat-sen University) for invaluable advice and statistical analysis.
Funding: This work is supported by grants from the Guangdong Provincial Science &Technology Project of China (no 2009B060700036) and the Guangdong Provincial Nature Foundation for Doctor Initiative of China (no S2011040002465).
Disclosure: The authors declare no conflict of interest.
References
- Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin 2011;61:91-112. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Tanaka F, Yanagihara K, Otake Y, et al. Surgery for non-small cell lung cancer: postoperative survival based on the revised tumor-node-metastasis classification and its time trend. Eur J Cardiothorac Surg 2000;18:147-55. [PubMed]
- Kratz JR, He J, Van Den Eeden SK. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet 2012;379:823-32. [PubMed]
- Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645-53. [PubMed]
- Zhou H, Kuang J, Zhong L, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet 1998;20:189-93. [PubMed]
- Tanaka E, Hashimoto Y, Ito T, et al. The clinical significance of Aurora-A/STK15/BTAK expression in human esophageal squamous cell carcinoma. Clin Cancer Res 2005;11:1827-34. [PubMed]
- Klein A, Reichardt W, Jung V, et al. Overexpression and amplification of STK15 in human gliomas. Int J Oncol 2004;25:1789-94. [PubMed]
- Sakakura C, Hagiwara A, Yasuoka R, et al. Tumour-amplified kinase BTAK is amplified and overexpressed in gastric cancers with possible involvement in aneuploid formation. Br J Cancer 2001;84:824-31. [PubMed]
- Li D, Zhu J, Firozi PF, et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res 2003;9:991-7. [PubMed]
- Sen S, Zhou H, Zhang RD, et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst 2002;94:1320-9. [PubMed]
- Royce ME, Xia W, Sahin AA, et al. STK15/Aurora-A expression in primary breast tumors is correlated with nuclear grade but not with prognosis. Cancer 2004;100:12-9. [PubMed]
- Landen CN Jr, Lin YG, Immaneni A, et al. Overexpression of the centrosomal protein Aurora-A kinase is associated with poor prognosis in epithelial ovarian cancer patients. Clin Cancer Res 2007;13:4098-104. [PubMed]
- Jeng YM, Peng SY, Lin CY, et al. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res 2004;10:2065-71. [PubMed]
- Katayama H, Brinkley WR, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev 2003;22:451-64. [PubMed]
- Fu J, Bian M, Jiang Q, et al. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res 2007;5:1-10. [PubMed]
- Littlepage LE, Wu H, Andresson T, et al. Identification of phosphorylated residues that affect the activity of the mitotic kinase Aurora-A. Proc Natl Acad Sci USA 2002;99:15440-5. [PubMed]
- Carvajal RD, Tse A, Schwartz GK. Aurora kinases: new targets for cancer therapy. Clin Cancer Res 2006;12:6869-75. [PubMed]
- Ogawa E, Takenaka K, Katakura H, et al. Perimembrane Aurora-A expression is a significant prognostic factor in correlation with proliferative activity in non-small-cell lung cancer (NSCLC). Ann Surg Oncol 2008;15:547-54. [PubMed]
- Gritsko TM, Coppola D, Paciga JE, et al. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin Cancer Res 2003;9:1420-6. [PubMed]
- Wu JC, Chen TY, Yu CT, et al. Identification of V23RalA-Ser194 as a critical mediator for Aurora-A-induced cellular motility and transformation by small pool expression screening. J Biol Chem 2005;280:9013-22. [PubMed]
- Guan Z, Wang XR, Zhu XF, et al. Aurora-A, a negative prognostic marker, increases migration and decreases radiosensitivity in cancer cells. Cancer Res 2007;67:10436-44. [PubMed]
- Wan XB, Long ZJ, Yan M, et al. Inhibition of Aurora-A suppresses epithelial-mesenchymal transition and invasion by downregulating MAPK in nasopharyngeal carcinoma cells. Carcinogenesis 2008;29:1930-7. [PubMed]
- Wang LH, Xiang J, Yan M, et al. The mitotic kinase Aurora-A induces mammary cell migration and breast cancer metastasis by activating the Cofilin-F-actin pathway. Cancer Res 2010;70:9118-28. [PubMed]
- Xu J, Wu X, Zhou WH, et al. Aurora-A identifies early recurrence and poor prognosis and promises a potential therapeutic target in triple negative breast cancer. PLoS One 2013;8:e56919. [PubMed]
- Lai CH, Tseng JT, Lee YC, et al. Translational up-regulation of Aurora-A in EGFR-overexpressed cancer. J Cell Mol Med 2010;14:1520-31. [PubMed]
- Giles FJ, Cortes J, Jones D, et al. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL mutation. Blood 2007;109:500-2. [PubMed]
- Traynor AM, Hewitt M, Liu G, et al. Phase I dose escalation study of MK-0457, a novel Aurora kinase inhibitor, in adult patients with advanced solid tumors. Cancer Chemother Pharmacol 2011;67:305-14. [PubMed]
- Hata T, Furukawa T, Sunamura M, et al. RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res 2005;65:2899-905. [PubMed]