Severe acute pancreatitis due to tamoxifen-induced hypertriglyceridemia with diabetes mellitus
Introduction
Most cases of acute pancreatitis are related to gallstones or heavy alcohol intake. Numerous other causes are hypertriglyceridemia, hypercalcemia, abdominal trauma, drug, vasculitis and endoscopic retrograde cholangiopancreatography. Approximately 2% to 5% of cases of acute pancreatitis are drug related (1). Tamoxifen-citrate, a non-steroidal antiestrogen, is one of the most widely used hormonal treatments for patients with breast cancer. The side effects of tamoxifen are generally mild, including the effects on lipoprotein metabolism (2). After tamoxifen administration, an increased VLDL synthesis leads to increased triglyceride levels. However, there are some cases of tamoxifen-induced severe hypertriglyceridemia. Hypertriglyceridemia may occasionally produce severe, lethal pancreatitis (3-6). Here, we describe a case of tamoxifen-induced severe acute pancreatitis in patients who had dexamethasone induced type 2 diabetes mellitus and breast cancer.
Case report
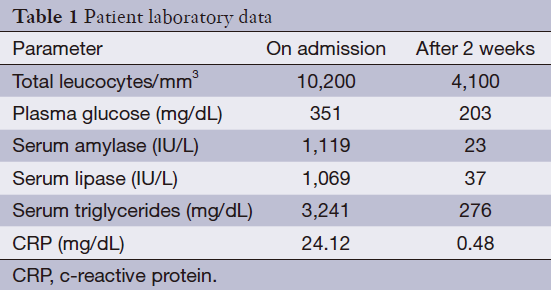
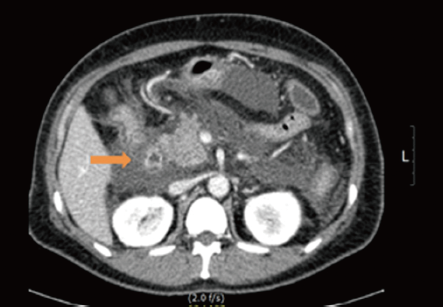
A 40-year-old pre-menopausal woman had eight courses of neoadjuvant chemotherapy with AC#4 and Docetaxel #4, followed by modified radical mastectomy (MRM) for breast cancer in February 2012 because of stage III-c invasive ductal carcinoma 1 year ago. During neoadjuvant chemotherapy period, she received a premedication consisting of dexamethasone (25 mg orally before therapy) and hydrocortisone (250 mg intravenously just before paclitaxel). After modified radical mastectomy, tamoxifen was started at a dose of 20 mg/day orally with radiation therapy thirteen times. The patient had been diagnosed with dexamethasone induced type 2 diabetes mellitus and her medication was stopped by the physician because it’s controlled without medication. She has no history of hypertriglyceridemia and her family history was negative for diabetes or hypertriglyceridemia, but her BMI on February 2012 was 30.33 kg/m2. Therefore, Clinicians recommended blood test before commencement of tamoxifen, but the patient refused it. Three months later, the patient was admitted to the emergency room with severe epigastric discomfort, nausea and vomiting. The serum amylase level was markedly increased (1,119 IU/L, normal values <100 IU/L) and a diagnosis of pancreatitis was made. There was no history of alcohol consumption, recent abdominal trauma or preceding viral syndromes. The patient’s serum triglyceride and glucose levels were markedly increased (3,241 and 351 mg/dL) leukocyte count, 10.2×109/L, with a differential count of 83.8% neutrophils and c-reactive protein (CRP) 24.12 mg/dL (Table 1). Abdominal computed tomography (CT) demonstrated bulging contour of the pancreas and loculated peripancreatic fluid collection, suggesting acute necrotizing pancreatitis (Figure 1). The patient was treated symptomatically with nothing mouth and morphine combined with demerol for pain relief. Tamoxifen was suspected as a probable cause of pancreatitis and was immediately withdrawn. The patient was commenced on oral fenofibrate 160 mg, gabexate 100 mg IV once, imipenem 500 mg IV four times and insulin infusion daily. Five days later, she was free of abdominal pain, the serum amylase was normal and the triglycerides were significantly reduced to 210 mg/dL. The evolution was favorable and the patient returned home two weeks later. Two weeks later, at the time of discharge, the patient was rechallenged with tamoxifen by herself. A follow-up one month later at the outpatient department, triglyceride was checked with the level of 102 mg/dL and goserelin acetate was started instead of tamoxifen after discharge from the hospital.
Full table
Discussion
Acute pancreatitis is a severe disease with a high rate of morbidity and mortality. Hypertriglyceridemia is a well-known risk factor for acute pancreatitis. Hypertriglyceridemia may be primary in origin or secondary to alcohol abuse, diabetes mellitus, pregnancy, or use of drugs. The drug-induced pancreatitis is suggested due to an accumulation of toxic metabolites, and to a hypersensitivity reaction to drugs or to a consequence of drug-related metabolic effects, such as hypertriglyceridemia (7).
In our case report, the cause was tamoxifen. Tamoxifen is an antagonist of estrogen and may be analogous to the chylomicronemia associated with estrogenic drugs. It mainly induces an inhibition of estrogenic activities; however, it may induce some estrogenic effects, including increased synthesis of triglycerides, very low density lipoproteins and decreased activity of lipoprotein lipase and hepatic triglyceride lipase (2). The drug caused hypertriglyceridemia not only in susceptible individuals with disturbances of triglyceride—rich lipoproteins metabolism by dexamethasone induced diabetes mellitus, familial dyslipidemia or impaired glucose tolerance, but also in normolipidemic patients. Tamoxifen use in a diabetic dyslipidemic patient may cause severe hypertriglyceridemia as seen in our case.
The mechanism of triglyceride-induced pancreatitis is thought to involve increased concentrations of chylomicrons in the blood. When triglycerides levels are elevated, chylomicrons are almost certainly present after a period of time. Presumably, the extremely high level of serum triglycerides causes ‘sludging’ in the pancreatic vasculature, resulting in ischemia and necrosis. Moreover, the degradation of triglycerides to free fatty acids by pancreatic lipases may lead to further cytotoxic injuries, which increase inflammatory mediators that may result in aggravation of the pancreatitis (8). Therefore, the effect of tamoxifen on lipid metabolism should not be ignored.
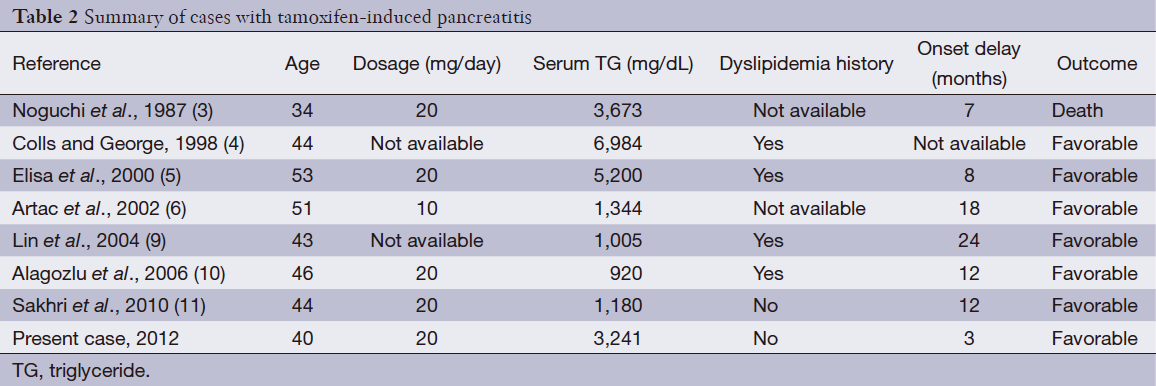
We searched for published literature and only seven cases regarding tamoxifen therapy came up (3-6,9-11). A history of hypertriglyceridemia was found in four cases and not mentioned in the other two cases. In our patient, there was no history of hypertriglyceridemia. In all of these cases, rechallenge was not performed. Our patient was rechallenged with tamoxifen by herself 2 weeks after discharge. The features of these cases are summarized in (Table 2). The outcome was favorable in all patients except one case, in which the patient died. The severity of pancreatitis did not appear to correlate with the serum triglyceride levels of these five patients. In most reported cases, the increased serum triglycerides returned to the pre-treatment values after stopping tamoxifen. Hypolipidemic drugs, namely fibrates were very effective in these patients because of their ability to decrease the serum triglyceride levels.
Full table
This patient used dexamethasone for conservative treatment of neoadjuvant chemotherapy with AC#4 and Docetaxel #4. Because her BMI was 30.33 kg/m2, she may had dyslipidemia and hypersensitivity of dexamethasone to influence the lipid metabolism (12). Dexamethasone induced type 2 diabetes mellitus, PPAR- pancreatic beta cell gene variation and genetically determined hyperlipidemia (13,14). Additionally, diabetes or impaired glucose tolerance may have contributed to the development of severe hyperlipidemia in some cases. In this case, dexamethasone presumably induced pancreas beta cell gene mutation and may be affected to reduce the threshold of pancreatitis occurrence. Therefore, this patient with dexamethasone induced diabetes mellitus will be probably more hypersensitive to tamoxifen compared with other patients.
Because short-term studies failed to observe any changes in the serum triglyceride levels and dangerous lipid abnormalities may occur months or years during tamoxifen therapy, the patient would undergo a monitoring of fasting lipids, especially in patients with endogenous dyslipidemia (familial hypertriglyceridemia, type 2 diabetes, impaired glucose tolerance, obesity, the metabolic syndrome) (15).
Clinicians should be aware of the risks of developing severe and even fatal acute pancreatitis with tamoxifen therapy. If tamoxifen is suspected as the probable causative agent, it should then be discontinued, and rechallenge should be avoided due to the risk of inducing severe acute pancreatitis and even a fatal outcome. When hyperlipidemia is detected, appropriate diet (the source of fat should consist of medium-chain triglycerides) and drug treatment (fibrates) are suggested to prevent hypertriglyceridemia-induced acute pancreatitis.
Conclusions
In our patient, triglyceride concentrations were not checked before using tamoxifen, we observed that the use of tamoxifen caused an increase in triglycerides and hypertriglyceridemic acute pancreatitis. This finding confirms that tamoxifen should be used with extreme caution in certain patients (those with familial hypertriglyceridemia, type II diabetes, impaired glucose tolerance, obesity or the metabolic syndrome, drug induced dyslipidemia including dexamethasone). These individuals should undergo pre-post tamoxifen lipid screening and careful history taking of possible drugs that may induce dyslipidemia or diabetes mellitus. (Ed note: overall, very well written; no major concerns were noticed; minor edits were made throughout the paper; well-written parts were left alone, as much as possible, to retain the originality and integrity of the original author).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lin HH, Hsu CH, Chao YC. Tamoxifen-induced severe acute pancreatitis: a case report. Dig Dis Sci 2004;49:997-9. [PubMed]
- Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med 2001;344:1997-2008. [PubMed]
- Noguchi M, Taniya T, Tajiri K, et al. Fatal hyperlipaemia in a case of metastatic breast cancer treated by tamoxifen. Br J Surg 1987;74:586-7. [PubMed]
- Colls BM, George PM. Severe hypertriglyceridaemia and hypercholesterolaemia associated with tamoxifen use. Clin Oncol (R Coll Radiol) 1998;10:270-1. [PubMed]
- Elisaf MS, Nakou K, Liamis G, et al. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol 2000;11:1067-9. [PubMed]
- Artac M, Sari R, Altunbas H, et al. Asymptomatic acute pancreatitis due to tamoxifen-induced severe hypertriglyceridemia in a patient with diabetes mellitus and breast cancer. J Chemother 2002;14:309-11. [PubMed]
- Vinklerová I, Procházka M, Procházka V, et al. Incidence, severity, and etiology of drug-induced acute pancreatitis. Dig Dis Sci 2010;55:2977-81. [PubMed]
- Ewald N, Hardt PD, Kloer HU. Severe hypertriglyceridemia and pancreatitis: presentation and management. Curr Opin Lipidol 2009;20:497-504. [PubMed]
- Lin HH, Hsu CH, Chao YC. Tamoxifen-induced severe acute pancreatitis: a case report. Dig Dis Sci 2004;49:997-9. [PubMed]
- Alagozlu H, Cindoruk M, Unal S. Tamoxifen-induced severe hypertriglyceridaemia and acute pancreatitis. Clin Drug Investig 2006;26:297-302. [PubMed]
- Sakhri J, Ben Salem C, Harbi H, et al. Severe acute pancreatitis due to tamoxifen-induced hypertriglyceridemia with positive rechallenge. JOP 2010;11:382-4. [PubMed]
- Bagdade JD, Yee E, Albers J, et al. Glucocorticoids and triglyceride transport: effects on triglyceride secretion rates, lipoprotein lipase, and plasma lipoproteins in the rat. Metabolism 1976;25:533-42. [PubMed]
- Hinds TD Jr, Stechschulte LA, Cash HA, et al. Protein phosphatase 5 mediates lipid metabolism through reciprocal control of glucocorticoid receptor and peroxisome proliferator-activated receptor-γ (PPARγ). J Biol Chem 2011;286:42911-22. [PubMed]
- Gouda HN, Sagoo GS, Harding AH, et al. The association between the peroxisome proliferator-activated receptor-gamma2 (PPARG2) Pro12Ala gene variant and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol 2010;171:645-55. [PubMed]
- Bagdade JD, Wolter J, Subbaiah PV, et al. Effects of tamoxifen treatment on plasma lipids and lipoprotein lipid composition. J Clin Endocrinol Metab 1990;70:1132-5. [PubMed]
