Prognostic significance of ERCC1 expression in postoperative patients with gastric cancer
Core tip
In this study, we explored the correlation between the expression of ERCC1 and the prognosis of gastric cancer patients after radical resection. ERCC1 expression has certain reference significance on the assessment of prognosis and prediction on sensitivity of platinum drug. Patients with ERCC1-negative expression could be sensitive to platinum-based drugs and benefit more from adjuvant chemotherapy of platinum combined with fluorouracil. As a result, the chemotherapy regimens of these patients may consider choosing platinum-containing drugs.
Introduction
Gastric cancer is one of the most common malignant tumors worldwide. Surgery and chemotherapy are important treatments for gastric cancer. So far, the prognosis of patients with gastric cancer is still poor, the overall 5-year survival rate is about 20% (1). Among patients with gastric cancer, long-term survival varied widely, if we could screen high-risk patients and give them close follow-up and appropriate intervention, survival may be prolonged.
TNM staging system (2) established by American Joint Committee on Cancer (AJCC) has played a significant role in the choice of treatment and the judgment of prognosis since it was promulgated. In recent years, some molecular biology factors affecting the prognosis of patients with gastric cancer have received attention, such as p53, PTEN, EGFR, VEGF, Survivin, HER-2, etc., but there are still divergences among the results of various researches. Therefore the clinical application of molecular biology factor as an individual prognostic marker in gastric cancer requires further research and exploration.
The latest meta-analysis on adjuvant chemotherapy of postoperative patients with gastric cancer showed that adjuvant chemotherapy, compared with surgery alone, can prolong the time to recurrence and survival time, and 5-year survival rate increased from 49.6% to 55.3% (3). However, adjuvant chemotherapy are not beneficial for all patients with gastric cancer, some patients suffer from side effects and do not really benefit. Platinum drugs as an active drug for gastric cancer, combined use of fluorouracil have become the most commonly used option of adjuvant chemotherapy for gastric cancer. Therefore, finding specific molecular markers predicting the efficacy of platinum drugs has important clinical significance.
The main target of cisplatin, oxaliplatin and other platinum-based anticancer agents is DNA. They make crosslinks within or between DNA chains to prevent its replication and transcription and promote cell apoptosis through the formation of platinum-DNA adducts. Studies have shown that the enhancement of nucleotide excision repair capacity is an important cause of drug resistance of platinum (4-7), excision repair cross-complementation group 1 (ERCC1) as a rate-limiting enzyme of the nucleotide excision repair pathway (8), the expression level reflects the capacity of nucleotide excision repair to some extent, may be related to platinum-based drug resistance. Current clinical studies on ERCC1 mainly concentrated in lung cancer as the research object. There are a few reports in head and neck cancer, bladder cancer, ovarian cancer, colorectal cancer, gastric cancer and pancreatic cancer.
This study aims to (I) explore the value of ERCC1 as a molecular marker for prognosis of gastric cancer by analyzing the relationship between the ERCC1 expression of tissue specimens from the postoperative patients with stage II and III gastric cancer and survival time; (II) explore whether ERCC1 could predict platinum drugs effect by analyzing the influence of combined chemotherapy with platinum and fluorouracil on long-term survival among gastric cancer patients with different expression of ERCC1, and to provide a reference for the choice of clinical chemotherapy regimen.
Materials and methods
Patients
A total of 605 cases of gastric cancer with gastrectomy and pathologically diagnosed as stage II or III at The First Affiliated Hospital of Nanjing Medical University from January 2005 to December 2008 were included into our study. Paraffin-embedded tissue specimens of gastric cancer were obtained. Gastric cancer staging is according to the AJCC seventh edition of the TNM staging system: 156 cases of stage II, and 449 cases of stage III. Among 605 cases of patients, 431 patients received adjuvant chemotherapy, 174 cases did not undergo adjuvant chemotherapy due to the wishes of patients. The clinical and pathological features of all patients are shown in Table 1. Case selection conditions: (I) a detailed medical history and complete pathological information; (II) histological types were adenocarcinoma; (III) surgical methods were gastrectomy combined D2 lymphadenectomy; (IIII exclude cases with deaths of no-cancer causes; (V) exclude cases with serious organic diseases. Patients with postoperative adjuvant chemotherapy still need to satisfy: (I) without preoperative neoadjuvant chemotherapy; (II) without radiotherapy before or after surgery; (III) adjuvant chemotherapy completed at least four cycles using cisplatin/oxaliplatin combined with fluorouraci. Specifically the following options: (i) cisplatin plus fluorouracil, FP regimen; (ii) cisplatin plus Xeloda, XP regimen; (iii) oxaliplatin combined with capecitabine, XELOX regimen; (iv) oxaliplatin combined with fluorouracil, FO, FOLFOX4, mFOLFOX6 regimen.
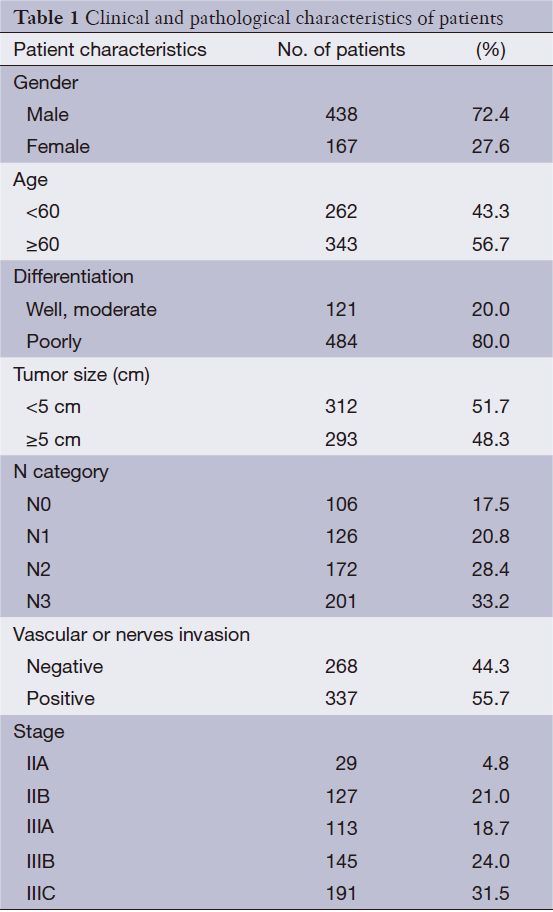
Full table
Follow-up and survival time calculations
We had conducted the follow-up by telephone or inquiring the information of the medical records of patients every 6 months. The follow-up contents included a comprehensive medical history, tumor markers and abdominal ultrasound or CT and other imaging findings. Deadline was April 30, 2013 and follow-up time between 51 to 91 months. Getting diagnosis of pathology was as starting point, and the death of cancer or cancer-related complications was as the final event. Censored data was data of patients still alive at the end of follow-up. Survival time is calculated from the date of diagnosis to death or last follow-up date.
Immunohistochemistry (IHC)
Tissue samples were formalin-fixed and paraffin-embedded; 4 mm-thick sections were cut and stained by using the avidin–biotin complex method. After that, the slides were pretreated with microwaves for antigen retrieval in 10 mm citrate buffer (pH 6.0) and incubated in the primary antibody at 4 °C overnight. The antibody used for the detection of ERCC1 was ZM-0138 (Beijing Zhongshan Biotechnology Co., Ltd., China) (1:150 dilution), and it was a mouse monoclonal antibody raised against the amino-terminal 304 amino acids of ERCC1. In addition, each case included a negative (without primary antibody) and a positive control (MCF7 cell line). If the staining was uncertain, we repeated to confirm it.
Scoring of ERCC1
A pathologist from our hospital had observed all sections (observer was blinded to the clinical data of the patients). We chose five visual fields randomly through the high power lens (×400) with Olympus optical microscope and counted 200 cells in every visual field. Score depended on the degree of cell staining and the percentage of stained cells (9). Staining Level: no colored mainly as 0, the yellow as 1, the brown as 2, dark brown as 3. The percentage of stained cells: no staining cells as 0, 1% to 9% as 0.1, 10% to 49% as 0.5, ≥50% as 1.0. The score of degree of cell staining was multiplied by the score of percentage of stained cells staining. The product of ≥1.0 was positive expression, <1.0 was negative expression.
Statistical analysis
SPSS 16.0 software was used for statistical analysis. The comparison of count data were analyzed by Chi-square test. Median survival time (MST) and the 5-year survival rate were calculated by life table analysis. The Kaplan-Meier curves were used for survival analysis. P<0.05 was considered statistically significant.
Results
ERCC1 expression in gastric cancer tissue
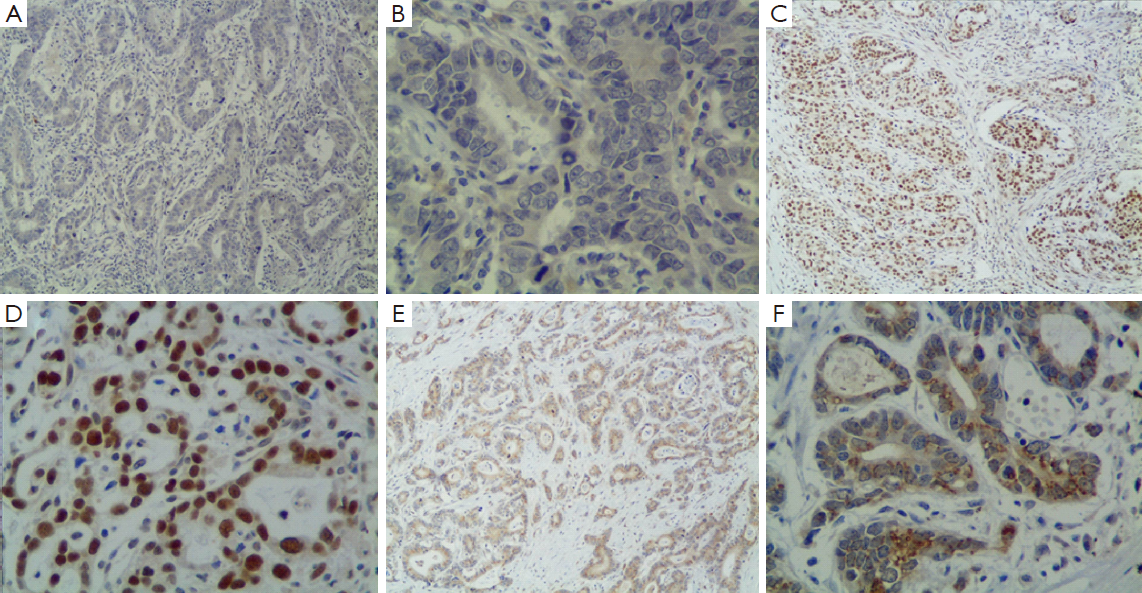
ERCC1 was mainly localized in the nucleus in gastric cancer tissue, and also expressed in cytoplasm of some tumor cells (Figure 1). A total of 412 cases are ERCC1-positive and 193 cases are ERCC1-negative, the positive rate was 68.1%. There were no correlations between ERCC1 expression and gender, age, tumor size, histological grade, depth of invasion, with or without vascular nerve involvement, lymph node metastasis and cancer stage (P>0.05) (Table 2).
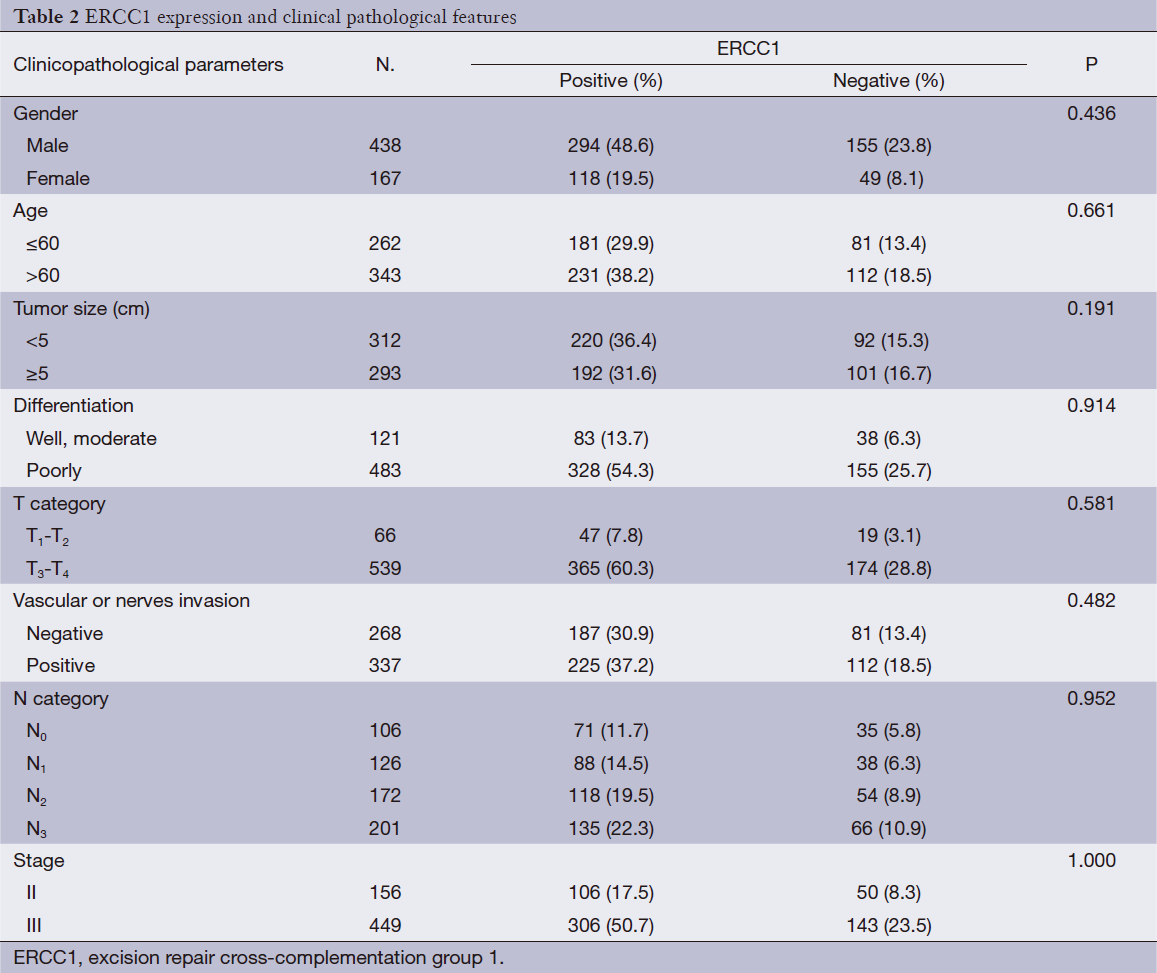
Full table
Survival and ERCC1expression
A total of 359 cases (59.3%) patients died during the follow-up period. The MST was 36.5 months, and the 5-year survival rate was 40.7%. For 412 ERCC1-positive cases, MST was 37.1 months, the 5-year survival rate was 41.3%; and for negative group (193 cases), MST was 35.4 months, and the 5-year survival rate was 39.4%. The difference was not statistically significant (P=0.455) (Figure 2A).
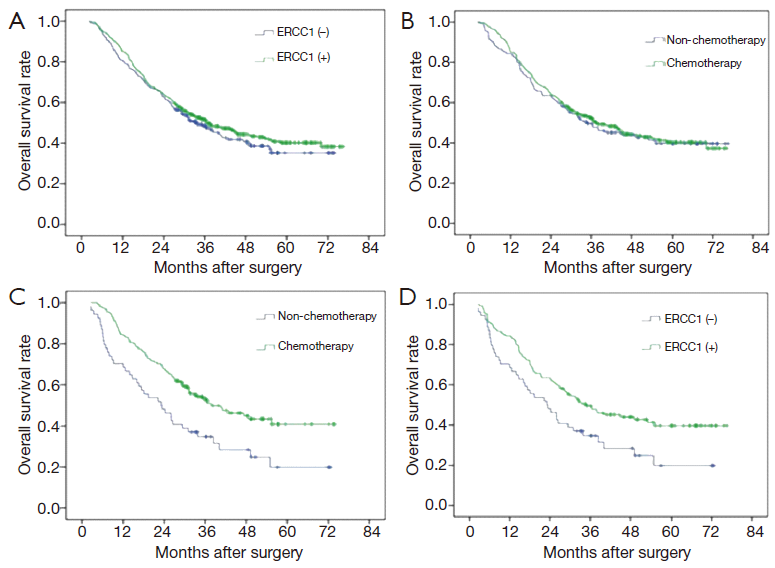
There were 122 cases without adjuvant chemotherapy and 290 cases with postoperative chemotherapy of cisplatin combined with fluorouracil in patients with ERCC1-positive expression. There was no significant difference between group with chemotherapy and group with no chemotherapy in patients with ERCC1-positive expression in terms of the MST and 5-year survival rate (MST: 40.3 vs. 32.5 months, 5-year survival rate: 43.4% vs. 36.1%, P=0.162) (Figure 2B). There were 52 patients without adjuvant chemotherapy and 141 patients with postoperative chemotherapy of cisplatin combined with fluorouracil in patients with ERCC1-negative expression. However, the MST and 5-year survival rate in patients with ERCC1-negative expression benefited more from chemotherapy (MST: 40.7 vs. 23.3 months, 5-year survival rate: 43.3% vs. 28.8%, P=0.019) (Figure 2C). Adjuvant chemotherapy can significantly improve overall survival in these patients. In the 174 patients without postoperative adjuvant chemotherapy, MST and 5-year survival rate of ERCC1-positive group (122 cases) were higher than ERCC1-negative group (MST: 32.5 vs. 23.3 months, 5-year survival rate: 36.1% vs. 28.8%, P=0.183, Figure 2D).
In the subgroup analysis, ERCC1 expression was not related with overall survival in patients with stage III gastric cancer (P>0.05) (Figure 3A). Subgroup analysis found no significant impacts of chemotherapy on the prognosis of stage II patients with different levels of ERCC1 expression. Similarly, there was no significant difference in ERCC1-positive stage III patients (Figure 3B). However, chemotherapy of platinum combined with fluorouracil can significantly improve overall survival in ERCC1-negative stage III patients (Figure 3C).
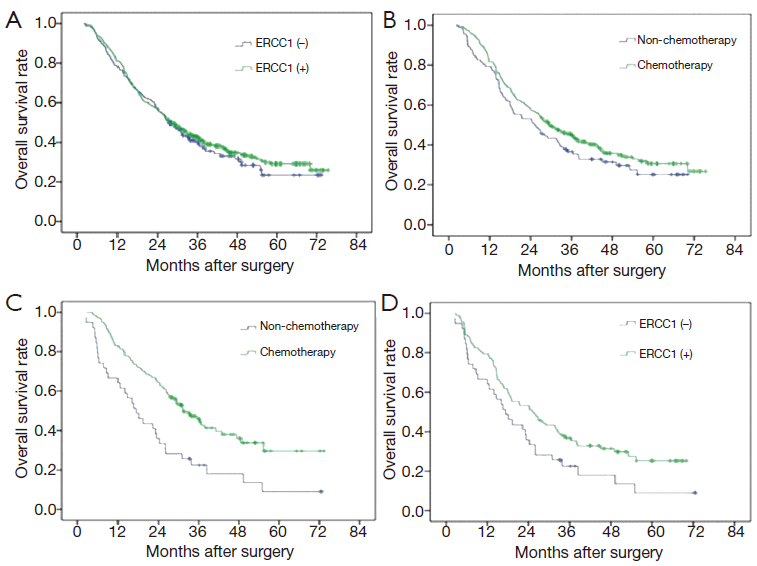
In subgroup analysis, there were no significant relationships in stage II patients without chemotherapy (48 cases). Similarly, though MST and the 5-year survival rate were higher in ERCC1-positive group than ERCC1-negative group in stage III patients without chemotherapy (MST: 25 vs. 18.1 months, 5-year survival rate: 24.7% vs. 16.2%), there was no significant difference between them (P=0.119) (Figure 3D).
Discussion
ERCC1 is a section of repair gene about 15 kb located on human chromosome 19q13.2-q13.3, containing 10 exons and encoding a protein containing 297 amino acids (10). ERCC1 protein is unstable by itself. ERCC1 and xeroderma pigmentosum group F (XPF) form heterodimers (ERCC1-XPF) to involve in nucleotide excision repair pathway (11). Experimental studies have revealed that ERCC1 gene deficient mice had serious dysplasia at birth, and died of severe liver damage soon (12). Another research reported that knockout of ERCC1 gene of mice would accelerate the aging of organs, showing a state of premature aging, and was closely related to brain damage, liver failure and died shortly after birth (13). Jaspers et al. (14) reported the first case of human ERCC1 genetic defect. Due to the lack of ERCC1 gene, the patient had allergy to UV and mitomycin, and the patient had serious fetal and postnatal growth retardation, showing brain, face, eyes, bones and other serious clinical symptoms. So, ERCC1 plays an important role in the repair of damaged DNA and maintaining the integrity of genetic information.
Therefore, theoretically ERCC1 expression levels in tumors to some extent reflect the tumor intrinsic ability to repair DNA damage. The disturbance of DNA damage repair system cause an increase in genetic instability, causing accelerated tumor growth and more malignant manifestations, leading to poor prognosis. In the study of lung cancer in 2006, Olaussen et al. (9) found that ERCC1= positive expression had better prognosis through the analysis of the survival of 372 cases of lung cancer of stage I-III without chemotherapy after radical resection. There were a few experiments concluding ERCC1-positive patients of lung cancer patients had better prognosis, and ERCC1 was an independent prognostic factor in patients with lung cancer (15,16). But there had not had gastric cancer-related research reported yet. In East Asia, the united gastrectomy with D2 lymph node dissection is the standard curative treatment for gastric cancer, and a number of studies have shown that lymph node dissection has a direct impact on the prognosis of patients. Therefore this study adopted cases united gastrectomy with D2 lymph node dissection into research.
Chemotherapy is one of the main treatments for gastric cancer. But there is a big difference in sensitivity to chemotherapeutic drugs of different individual. Therefore, finding molecular markers predicting sensitivity to chemotherapeutic drugs to guide effective choice of drugs for patients and to reduce unnecessary adverse reaction, is hotspot of individual chemotherapy of tumor research. Removal of platinum-DNA adducts and increased capacity of repairing crosslinking within or between DNA chains are important reasons led to drug resistance of platinum. Studies have shown that nucleotide excision repair pathway plays a vital role in the repair of platinum-induced DNA damage (4-7). ERCC1 as one of the critical protein of the pathway, has significance in the repair of platinum-induced DNA damage and resulting in drug resistance of platinum. The high expression of ERCC1 mRNA and the relationship between ERCC1 mRNA and drug resistance of platinum has been initially verified in lung cancer, gastric cancer, ovarian cancer, esophageal cancer, colorectal cancer and other cancer researches (17-20).
In this study, we found that for ERCC1-negative patients, platinum-containing chemotherapy can significantly improve overall survival and 5-year survival rate; while ERCC1-positive patients showed no prolonged survival from chemotherapy. It can be inferred that ERCC1-negative patients with gastric carcinoma are suitable for platinum-based chemotherapy, while the ERCC1-positive patients may not benefit from platinum-based drugs. In the subgroup analysis, patients of gastric cancer of stage III showed similar results and statistical significance as the overall data, but patients of stage II did not obtain a statistically significant result. This study enrolled patients using drugs of the same type, but chemotherapy regimen is not consistent, and different drug sensitivity to fluorouracil in patients may exist. There may be a certain impact on the accuracy of the experimental results. In a study of advanced gastric cancer, Kwon et al. (21) found that among 64 patients with routine FOLFOX4 chemotherapy, ERCC1 expression was positive in 70.3% of patients, and overall response rate of treatment was 39.1%, and response can be better achieved in ERCC1-negative tumor, and time to tumor progression (TTP) was almost twice that ERCC1-positive patients had, and the survival period was also longer than the ERCC1-positive patients. But Yun et al. (22) thought ERCC1 could not predict sensitivity to cisplatin nor be overall survival time indicator. Kim et al. (23) had a research on 149 cases with FP chemotherapy with gastric cancer of stage II ~ III and came to the opposite result: ERCC1-positive patients lived longer. Most findings suggest that patients with lower expression of ERCC1 were more efficient with platinum-based chemotherapy and had higher survival time, and there are a few experiments did not receive a positive result, or come to the opposite conclusion. Therefore, relationship between ERCC1 expression and sensitivity to platinum drug needs a large sample of multi-level experiments to verify.
In summary, ERCC1 expression has certain reference significance on the assessment of prognosis and prediction on sensitivity of platinum drug. Patients with ERCC1-negative expression could be sensitive to platinum-based drugs and benefit more from adjuvant chemotherapy of platinum combined with fluorouracil. As a result, the chemotherapy regimens of these patients may consider choosing platinum-containing drugs.
Acknowledgements
The authors are grateful to the fund support by the National Natural Science Foundation of China (Grant number: 81171908, 81100274 and 81201705) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Disclosure: The authors declare no conflict of interest.
References
- Hartgrink HH, Jansen EP, van Grieken NC, et al. Gastric cancer. Lancet 2009;374:477-90. [PubMed]
- Washington K.7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010;17:3077-9.
- Paoletti X, Oba K, Burzykowski T, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA 2010;303:1729-37. [PubMed]
- Reardon JT, Sancar A. Nucleotide excision repair. Prog Nucleic Acid Res Mol Biol 2005;79:183-235. [PubMed]
- Gillet LC, Schärer OD. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem Rev 2006;106:253-76. [PubMed]
- Gossage L, Madhusudan S. Current status of excision repair cross complementing-group 1 (ERCC1) in cancer. Cancer Treat Rev 2007;33:565-77. [PubMed]
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 2007;33:9-23. [PubMed]
- Evans E, Moggs JG, Hwang JR, et al. Mechanism of open complex and dual incision formation by human nucleotide excision repair factors. EMBO J 1997;16:6559-73. [PubMed]
- Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 2006;355:983-91. [PubMed]
- Altaha R, Liang X, Yu JJ, et al. Excision repair cross complementing-group 1: gene expression and platinum resistance. Int J Mol Med 2004;14:959-70. [PubMed]
- Biggerstaff M, Szymkowski DE, Wood RD. Co-correction of the ERCC1, ERCC4 and xeroderma pigmentosum group F DNA repair defects in vitro. EMBO J 1993;12:3685-92. [PubMed]
- McWhir J, Selfridge J, Harrison DJ, et al. Mice with DNA repair gene (ERCC-1) deficiency have elevated levels of p53, liver nuclear abnormalities and die before weaning. Nat Genet 1993;5:217-24. [PubMed]
- Prasher JM, Lalai AS, Heijmans-Antonissen C, et al. Reduced hematopoietic reserves in DNA interstrand crosslink repair-deficient Ercc1-/- mice. EMBO J 2005;24:861-71. [PubMed]
- Jaspers NG, Raams A, Silengo MC, et al. First reported patient with human ERCC1 deficiency has cerebro-oculo-facio-skeletal syndrome with a mild defect in nucleotide excision repair and severe developmental failure. Am J Hum Genet 2007;80:457-66. [PubMed]
- Lee KH, Min HS, Han SW, et al. ERCC1 expression by immunohistochemistry and EGFR mutations in resected non-small cell lung cancer. Lung Cancer 2008;60:401-7. [PubMed]
- Seyhan EC, Altòn S, Cetinkaya E, et al. Prognostic significance of ERCC1 expression in resected non small cell lung carcinoma. Ann Thorac Cardiovasc Surg 2011;17:110-7. [PubMed]
- Joshi MB, Shirota Y, Danenberg KD, et al. High gene expression of TS1, GSTP1, and ERCC1 are risk factors for survival in patients treated with trimodality therapy for esophageal cancer. Clin Cancer Res 2005;11:2215-21. [PubMed]
- Lord RV, Brabender J, Gandara D, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res 2002;8:2286-91. [PubMed]
- Reed E, Dabholkar M, Thornton K, et al. Evidence for in the appearance of mRNAs of nucleotide excision repair genes, in human ovarian cancer tissues. Oncol Rep 2000;7:1123-8. [PubMed]
- Shirota Y, Stoehlmacher J, Brabender J, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol 2001;19:4298-304. [PubMed]
- Kwon HC, Roh MS, Oh SY, et al. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol 2007;18:504-9. [PubMed]
- Yun J, Kim KM, Kim ST, et al. Predictive value of the ERCC1 expression for treatment response and survival in advanced gastric cancer patients receiving cisplatin-based first-line chemotherapy. Cancer Res Treat 2010;42:101-6. [PubMed]
- Kim KH, Kwon HC, Oh SY, et al. Clinicopathologic significance of ERCC1, thymidylate synthase and glutathione S-transferase P1 expression for advanced gastric cancer patients receiving adjuvant 5-FU and cisplatin chemotherapy. Biomarkers 2011;16:74-82. [PubMed]
