Synchronous primary cancers of trachea and esophagus and ventricular tachycardia
Introduction
Primary tumors of the trachea are rare, which account for less than 0.1% of tumors happened in human (1). In adults, 90% of primary tracheal tumors are malignant, with squamous cell carcinoma and adenoid cystic carcinoma accounting for two thirds. Squamous cell carcinoma is the most common histological type of malignant tracheal tumor, followed by adenoid cystic cancer. The remaining part are distributed widely in a heterogeneous group, covering adenocarcinoma, poorly differentiated (or undifferentiated) carcinoma, and neuroendocrine tumors including small cell cancer (1,2). Small cell cancers mostly are pulmonary in origin, extra-pulmonary cases contribute to 2.5% to 5% (3), and the most common anatomical sites are esophagus (18%), other gastrointestinal tract (15%), genitourinary (20%), head and neck (11%), and breast (10%) (4). The incidence of small cell cancer is 9.7% (2) in primary trachea cancer, associated with a cumulative survival of 32% and 24% at 12 and 33 months respectively (5). More than that, multiple primary cancer involving trachea is even rarer, little information has been found in English literatures in regard to its clinical features and treatment.
Herein, we report a case of synchronous primary tracheal small cell cancer co-existing with squamous cell carcinoma in situ of upper thoracic esophagus, and with a special symptom of paroxysmal palpitation, so as to share the diagnostic and treatment experience of this extremely uncommon synchronous double primary cancer.
Case report
A 70-year-old woman with no history of smoking and alcohol drinking or heart disease complained of dry cough and paroxysmal palpitation in the night for more than one month. On baseline examination, there was nothing remarkable on chest radiograph. Her dynamic electrocardiography (DCG) showed ventricular tachycardia (ventricular premature beats of 6,040, in 24 hours), which could explain her sensation of frequent palpitation. Her coronary arteriography and heart color ultrasound were negative. With an initial diagnosis of acute bronchitis and ventricular arrhythmia, she was treated with antibiotic and antitussive for two weeks. Unfortunately, her cough aggravated with dyspnea.
On physical examination, a 1.0 cm sized hard, fixed and painless node was palpated behind her right clavicle. Laboratory tests revealed her tumor marker neuron-specific enolase (NSE) was 23.9 ng/mL (normal value: below 15.0 ng/mL), the other blood tests were all within normal range. Further examination on her showed normal pulmonary function; negative bronchial provocation test and tuberculosis-antibody (TB-Ab) test. She had no family history of tumors or similar diseases before, and denied any other noteworthy medical history.
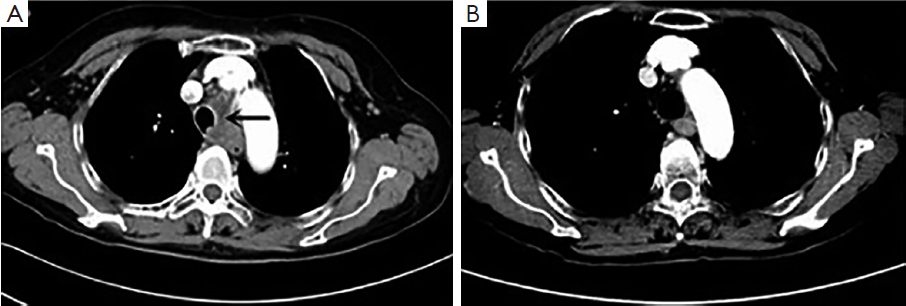
Enhanced chest CT scan showed a mass, 3.7 cm × 2.3 cm, in the left lower trachea (Figure 1) and an enlarged right supraclavicular lymph node, confirming our finding in physical examination. No visible mass was found in lung fields, neither in enhancing head MRI, abdomen CT and bone scan.
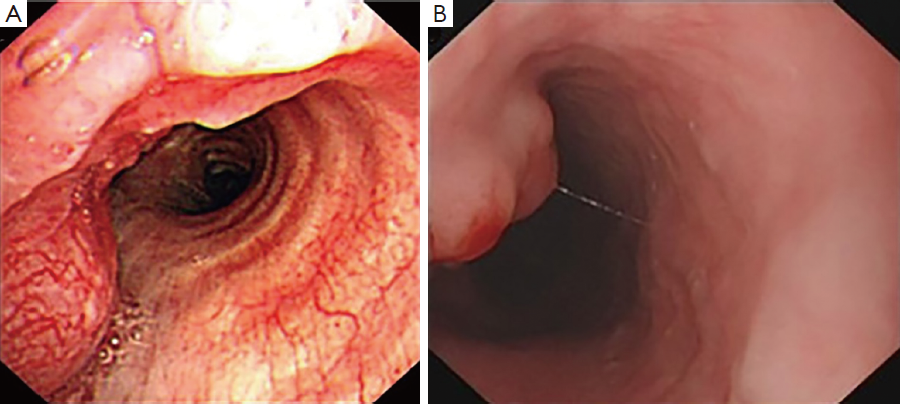
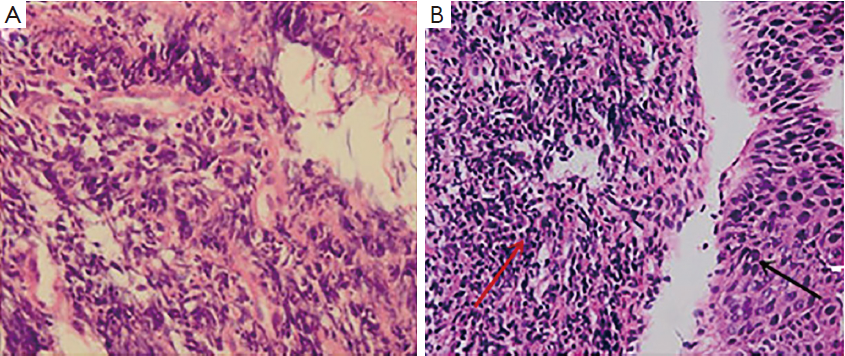
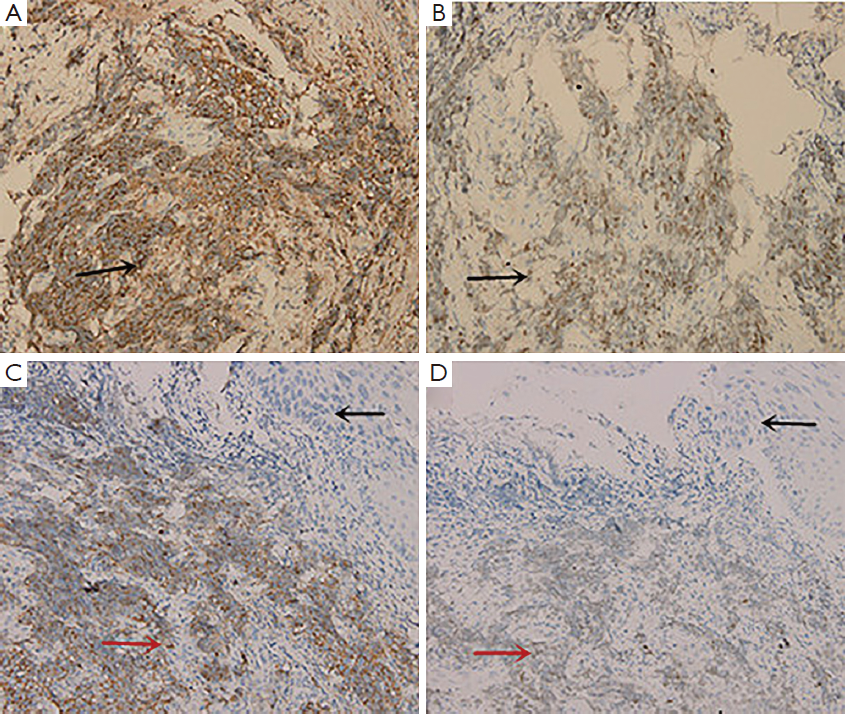
Examination with fiberoptic bronchoscope and gastroscope revealed polypoid neoplasm in trachea (Figure 2A) and upper thoracic esophagus (Figure 2B) respectively. Biopsies of the two neoplasm areas and right supraclavicular lymph node were performed. Hematoxylin-eosin (HE) staining of the trachea tumor was shown in Figure 3. Pathological section of tracheal biopsy (Figure 3A) was squeezed seriously, but still can demonstrate the characteristics of small cell cancer: multiple nests with small hyperchromatic nuclei and scanty cytoplasm. Hyperchromatic tumor cells from the metastatic lymph node were of typical small cell cancer morphology. Immunohistochemistry (IHC) confirmed the pathologic type, with synaptophysin (Figure 4A) and thyroid transcription factor-1 (TTF-1) (Figure 4B) positive in both trachea tumor and lymph node. The expression of synaptophysin or chromogranin A (CgA) are typical markers for neuroendocrine tumors, and often positive in small cell cancer. In the esophageal biopsy, a few squamous cancer cells and severe atypical dysplasia were found in the mucosa (Figure 3B) in which synaptophysin (Figure 4C) and TTF-1 (Figure 4D) were negative. Additional sections of the esophageal sample revealed a mass invading the lamina propria and submucosa. The mass consisted of poorly differentiated cancer cells which resembled the morphology of tumor cells from the tracheal tumor and lymph node and were also positive for synaptophysin (Figure 4C) and TTF-1 (Figure 4D), suggesting direct invasion of esophagus by the tracheal tumor from outside.
Based on diagnostic criteria of primary multiple cancer described by Waren and Gates: (I) the tumors must be clearly malignant on histological examination; (II) they must be separated by normal mucosa; and (III) the possibility that the second tumor represents a metastasis must be excluded (6); and in combination with the features of histology, IHC and negative findings of her lungs in the enhanced CT, the patient was diagnosed with synchronous double primary cancers: primary small cell cancer of lower trachea with right supraclavicular lymph node metastasis and upper thoracic esophageal squamous cell carcinoma in situ.
With the consideration of possible toxicities of chemotherapy and her severe symptoms of dry cough and arrhythmia, sequential chemoradiation was administered to the patient. At first, four cycles of systemic chemotherapy with etoposide and carboplatin (EC) regimen (etoposide 100 mg/m2 d1-3 and carboplatin: AUC =5, d1) were given. Concomitant medication was administered to her, with bisoprolol fumarate to control heart rate and codeine hydrochloride to relieve severe dry cough. After two cycles, she had a significant relief of dry cough and dyspnea, therefore quitted cocaine hydrochloride. Four cycles later, the patient received a general evaluation. Her feeling of palpitation disappeared and bisoprolol fumarate was quitted. The tumor marker NSE dropped to normal level. Chest enhancing CT scan showed her tracheal tumor almost unidentifiable. However, her right supraclavicular lymph node was stable and the wall of esophagus was thicker than normal. Radiotherapy was arranged immediately, covering the primary tracheal and esophageal tumor, the mediastinum and the right supraclavicular metastatic lymph node to a total dose of 60 Gy in 30 fractions. The treatment was well tolerated. At two months following the completion of radiotherapy, an enhancing chest CT was performed (Figure 1B), which showed complete response of tracheal tumor, right supraclavicular lymph node, and no progression in the esophagus. At the follow up of 12 months after treatment, no recurrence was detected based on chest CT.
Discussion
Primary tracheal small cell cancer co-existing with esophageal cancer is extremely rare. Usually, primary multiple cancer is associated with exposure to carcinogenic agents or occurs in people who has multiple sites of genetic abnormality, and it sometimes results from the carcinogenicity of therapeutic agents applied to the first primary cancer (6,7). The incidence of primary multiple cancer in cancer patients is about 4.3% (7). On the contrary, the occurrence of primary multiple cancers in esophagus is relatively a well-known phenomenon, with reported incidence ranging from 9.5% to 20.7% in patients with esophagus cancer. However, among these patients, synchronous cancers are much fewer, as the proportion reported was 6.9%. The frequencies, from high to low, of other organ cancers associated with esophageal cancer are cancers of the stomach, head and neck, lung and others (6). In a large sample study, the authors found an inverse site-correlation of synchronous primary multiple cancer between (trachea, bronchus and lung) and esophagus. The incidence of respiratory system tumors (trachea, bronchus, lung) co-exiting with synchronous esophagus tumor was only 0.4% (7). We failed to find out information about primary tracheal small cell cancer in association with cancer of other organs in English literatures. It is widely accepted that smoking is a risk factor of respiratory tract tumor and upper gastrointestinal tract tumor is often associated with alcohol drinking. Therefore, a case of synchronous primary tracheal small cell cancer and esophageal squamous cell cancer with no history of smoking and alcohol drinking or tumor related family history is a clinical rarity.
Common symptoms of primary tracheal carcinoma are cough, dyspnea, hemoptysis, stridor, hoarseness (1), and paroxysmal tachycardia is unusual. Paroxysmal arrhythmia is common in the elderly, which has various causes including organic heart disease or drugs, etc. Interestingly, in our case the patient presented with an initial symptom of paroxysmal ventricular tachycardia without preexisting cardiovascular disease, and her symptom of palpitation disappeared gradually with chemotherapy. Through English literature search, we only found one case that reported a dedifferentiated adenoid cystic carcinoma of the trachea with a history of paroxysmal supraventricular tachycardia (8). Whether the arrhythmia was relevant to tracheal cancer awaits more clinical data to clarify.
The diagnosis and treatment of multiple primary cancers is a clinical challenge. Multiple cancers are often misdiagnosed as metastatic tumor. In this case the patient had two different cancers in trachea and esophagus, and the diagnosis of metastatic tracheal cancer could be precluded because of no evidence of primary tumor in the lung based on imaging and pathologic results. Though TTF-1 positive expression in adenocarcinoma indicates lung origin, extra pulmonary small cell cancer may also have TTF-1 positive expression (9).
Treatment of multiple primary cancers should take into account of all individual primary sites. In non-small cell lung cancer or esophagus cancer associated multiple primary cancers, it seems aggressive surgical approach offers greater chance for long-term survival (7,10). The treatment methods for tracheal cancer are surgery, radiotherapy and chemotherapy, and early diagnosis is most important for a better survival (2). In patients with tracheal cancer only, previous literatures showed an improved survival for undergoing all types of surgery in comparison to patients on whom no cancer directed surgery was performed (2,11). However, small cell cancer usually grows and disseminates very rapidly, surgery is recommended for few patients with early stage (11). Instead, chemotherapy with radiotherapy is often applied in patients with tracheal small cell cancer. There is lack of standard chemotherapy regimen for tracheal small cell cancer, choosing of drugs is often referring to the practice of small cell cancer of the lung in which cisplatin plus etoposide is effective regimen (11,12). Radiotherapy of 60 Gy is recommended for tracheal small cell carcinoma (1,12).
And for esophagus squamous cell carcinoma in situ, surgical resection or endoscopic mucosal resection (EMR) is recommended. The goal of EMR or surgery is to remove all the early malignancy completely.
However, in our case, the trachea small cell cancer already invaded the esophageal wall with tumor seeded within the lamina propria and submucosa underneath the site of esophageal squamous carcinoma in situ. Therefore, curative surgery was not applicable for tracheal small cell cancer and so was EMR for esophageal squamous cell carcinoma in situ. The patient was treated with a priority for the more aggressive tracheal small cell cancer. At present no clinical data was found for radiotherapy of esophageal carcinoma in situ. However, both tumors respond well to sequential EC chemotherapy and irradiation with complete response. At one year’s follow up, the patient was well and had no evidence of recurrence.
In summary, synchronous double primary tracheal and esophageal cancer is extremely rare. Our case presents the uncommon symptom of arrhythmia which may shed new insight in guiding the diagnosis of primary tracheal carcinoma, especially when nothing found from routine clinical diseases. Our experience implies that sequential chemotherapy with EC regimen followed by thoracic irradiation is effective for senile synchronous double primary trachea small cell cancer and esophageal cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Macchiarini P. Primary tracheal tumours. Lancet Oncol 2006;7:83-91. [PubMed]
- Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol 2011;34:32-7. [PubMed]
- Walenkamp AM, Sonke GS, Sleijfer DT. Clinical and therapeutic aspects of extrapulmonary small cell carcinoma. Cancer Treat Rev 2009;35:228-36. [PubMed]
- Wong YN, Jack RH, Mak V, et al. The epidemiology and survival of extrapulmonary small cell carcinoma in South East England, 1970-2004. BMC Cancer 2009;9:209. [PubMed]
- Gelder CM, Hetzel MR. Primary tracheal tumours: a national survey. Thorax 1993;48:688-92. [PubMed]
- Lee GD, Kim YH, Kim JB, et al. Esophageal Cancer Associated with Multiple Primary Cancers: Surgical Approaches and Long-term Survival. Ann Surg Oncol 2013;20:4260-6. [PubMed]
- Kaneko S, Yamaguchi N. Epidemiological analysis of site relationships of synchronous and metachronous multiple primary cancers in the National Cancer Center, Japan, 1962-1996. Jpn J Clin Oncol 1999;29:96-105. [PubMed]
- Ishida M, Okabe H. Dedifferentiated adenoid cystic carcinoma of the trachea: a case report with respect to the immunohistochemical analyses of mammalian target of rapamycin pathway proteins. Hum Pathol 2013;44:1700-3. [PubMed]
- Agoff SN, Lamps LW, Philip AT, et al. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol 2000;13:238-42. [PubMed]
- Jung EJ, Lee JH, Jeon K, et al. Treatment outcomes for patients with synchronous multiple primary non-small cell lung cancer. Lung Cancer 2011;73:237-42. [PubMed]
- Yang KY, Chen YM, Huang MH, et al. Revisit of primary malignant neoplasms of the trachea: clinical characteristics and survival analysis. Jpn J Clin Oncol 1997;27:305-9. [PubMed]
- Lan CC, Yang MC, Lee CH, et al. Solitary primary tracheal small-cell lung cancer causing acute respiratory failure: diagnosis and treatment. Respir Care 2010;55:929-32. [PubMed]
