TPX2 knockdown suppressed hepatocellular carcinoma cell invasion via inactivating AKT signaling and inhibiting MMP2 and MMP9 expression
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignant cancer and ranks third as a cause of cancer-related death worldwide, and approximate 50% new HCC cases and deaths occur in China, duo to the high frequency of hepatitis B virus (HBV) infection (1). Furthermore, as the rising morbidity of metabolic syndrome and hepatitis C virus (HCV) infection, recent studies have shown that the incidence of HCC is increasing in many western countries (2,3). More than 600,000 new HCC patients were diagnosed annually, and the HCC-related deaths exceeded 500,000 in 2008 (4,5). In the past decades, development of liver resection and transplantation, which act as curative treatment, has improved the prognosis of HCC patients, but it remains poor (6,7). Therefore, exploring the pathogenesis of HCC and developing novel prognostic biomarkers and therapeutic targets could be important for HCC treatment.
Targeting protein for Xenopus kinesin-like protein 2 (TPX2) is a nuclear proliferation-related protein and affects spindle assembly in mammalian cells, which is primarily identified by Heidebrecht and colleagues (8). TPX2, which is regulated by cell cycle, is essential for the stability of spindle pole and the activity of TPX2 is required for proper spindle assembly during mammalian cells mitosis (9). As a well-known activator protein for Aurora A, TPX2 is also necessary for targeting Aurora A to the microtubules of the mitotic spindle (9). Moreover, high-expression of TPX2 induces the amplification of centrosome and leads to DNA polyploidy (10). Recently, several studies have revealed the correlation between TPX2 and malignant tumors (11). Strong evidences indicate that TPX2 overexpression may play a key role in the invasion and progression of human cancers (12). Furthermore, aberrant expression of TPX2 has been found in various types of cancers including colon, bladder and lung, and elevated TPX2 expression was correlated with the aggressiveness of ovarian and salivary gland cancer (13-17). Notably, TPX2 could up-regulate matrix metalloproteases (MMPs) levels through activating AKT signaling in colon cancer (14). It is well known that MMPs degrade components of extracellular matrix (ECM) and high levels of MMPs are important for HCC invasion and metastasis (18). Otherwise, MMPs overexpressions effectively reflect the aggressiveness of HCC cells and are associated with poor prognosis in HCC patients. Meanwhile, previous studies have revealed an important molecular mechanism in which phosphorylated AKT (p-AKT) up-regulates MMP2 and MMP9 expression via AKT signaling pathway (18,19). AKT, an important serine/threonine kinase, affects key cellular processes by regulating its downstream effectors in HCC (19,20). Hence, we hypothesized that high-expression of TPX2 could up-regulate MMP2 and MMP9 levels by activating AKT signaling in HCC cells.
Subtle changes in TPX2 expression may favor tumor development in vivo (21). Recently, Satow et al. confirmed TPX2 overexpression in HCC by combined functional genome survey, but the role and mechanisms of TPX2 in HCC were poorly understood (22). In our study, we found that TPX2 expression in normal hepatocytes was significantly lower as compared with that in HCC cells. Furthermore, TPX2 knockdown suppressed cell invasion with AKT pathway inactivation and MMP2/9 downregulation in HCC cells. Our results suggest that TPX2 may promote cell invasion by activating AKT pathway and subsequently up-regulating MMP2/9 expression in HCC.
Materials and methods
Cell culture
The immortalized normal human liver cell line L02 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Six HCC cell lines including SMMC-7721, BEL-7402, Huh-7, HepG2, Hep3B and SKHep1 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All the cells were maintained in Dulbecco’s modified Eagle medium (DMEM, Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Gibco, USA) with 100 units/mL penicillin and 100 µg/mL streptomycin (Sigma, St-Louis, MO, USA) and cultured in a humidified 5% CO2 incubator at 37 °C.
RNA isolation and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cell lines using TRIzol reagent (Invitrogen, USA). For the purpose of avoiding any DNA contamination, isolated RNA was treated with RNase-free DNase I (Invitrogen, USA) and qualified by spectrophotometry. The RNA samples were measured by optical density at 260 nm, and then reverse transcribed into cDNA with a RevertAid Premium First Strand cDNA Synthesis Kit (Fermentas, Canada). qRT-PCR was done in an ABI 7,500 system using the SYBR® Premix Ex TaqTM II (Tli RNaseH Plus) (TakaRa, Japan). TPX2 primers: forward 5'-ACCTTGCCCTACTAAGATT-3'; reverse 5'-AATGTGGCACAGGTTGAGC-3'. β-actin primers: forward 5'-GGGAAATCGTGCGTGACAT-3'; reverse 5'-CTGGAAGGTGGACAGCGAG-3'. The reaction conditions for the PCR program were as follows: initial melting at 95 °C for 30 sec followed by 40 cycles at 95 °C for 5 sec, 60 °C for 64 sec. Analysis of melting curve for the primers was conducted to confirm the specificity of the PCR product, and the threshold cycle (Ct) value for triplicate reactions was averaged. Three experimental replicates were performed. The relative expression of TPX2 mRNA for each sample was calculated as follows: ΔCt = Ct (TPX2) Ct (β-actin), –ΔCt (sample) = ΔCt (sample) –ΔCt (calibrator), and the fold changes in mRNAs were calculated through relative quantification (2–ΔΔCt).
Protein extraction and western blot
The primary rabbit anti-TPX2 polyclonal antibody (sc-32863), rabbit anti-AKT polyclonal antibody (sc-8312, which can be used to detect total proteins of AKT1, AKT2 and AKT3), rabbit anti-p-AKT polyclonal antibody (sc-293095, which can be used to detect phosphorylated AKT1, AKT2 and AKT3), rabbit anti-MMP2 polyclonal antibody (sc-10736), rabbit anti-MMP9 polyclonal antibody (sc-10737) and rabbit anti-β-actin polyclonal antibody (sc-130656) were purchased from Santa Cruz Biotechnology (USA). The secondary goat anti-rabbit antibody (sc-2004) was also obtained from Santa Cruz Biotechnology (USA).
Total protein was extracted from the cultured cells. Briefly, the protein was extracted from the collected cells using radioimmunoprecipitation lysis buffer (Beyotime, China). Protein lysates were quantified using a BioRad kit (Hercules, USA). The denatured protein samples (30 µg) were separated by polyacrylamide gel electrophoresis (PAGE). Then proteins were electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were incubated in 5% bovine serum albumin in phosphate buffered saline (PBS) for 2 h, and then serially with different primary antibodies, respectively, at 4 °C overnight. Then blots were examined with the secondary antibody conjugated with HRP and reactions were visualized using the HyGLO HRP detection kit from Denville (USA).
siRNA transfection
A specific siRNA against TPX2 (sc-37653) and control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology (USA). HCC tumor cells were seeded at the concentration of 2×105 per well in a six-well plate and cultured for overnight. Then cells were transfected with 100 nM siRNAs using Lipofectamine RNAi MAX Reagent (Invitrogen, USA) according to the manufacturer’s protocol. Further experiments were performed 48 h after transfection.
Cell invasion and proliferation assays
The invasion of HCC tumor cells was assessed using Millicell invasion chambers (8 mm pore size, Millipore, USA). The Millicell invasion chambers were precoated with basement membrane Matrigel (Millipore, USA). HCC tumor cells were grown in serum-free medium for 24 hours and then added into the upper chamber at a concentration of 5×104 cells/mL. Simultaneously, the upper chamber was supplemented with serum-free medium and meanwhile medium with 20% FBS was added into bottom chamber. These tumor cells were cultured at the normal condition for 24 h. At the end of the experiment, cells in the top surface of Matrigel membranes were carefully swabbed and membrane was fixed with 4% paraformaldehyde for 10 min. The tumor cells in the membrane were stained by crystal violet solution and counted under microscope (200×). Five fields were randomly chosen to get the mean invaded cells in each membrane.
The proliferation of HCC tumor cells was determined using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Tumor cells were seeded in a 96-well plate. At each time point, cells were stained with 100 µL sterile MTT dye (0.5 mg/mL) at 37 °C for 4 h, followed by removal of the culture medium and addition of 150 µL of dimethyl sulphoxide (DMSO). The absorbance of the samples was measured at a wavelength of 570 nm at 24, 48 and 72 h. Three experimental replicates were performed.
Statistical analysis
Statistical analysis was carried out using the SPSS 16.0 statistical software package (SPSS Inc., Chicago, IL, USA). Data were showed as the Mean ± SEM. Differences between groups were compared with the Mann-Whitney test or Student-t test. P<0.05 was considered to be statistical significance.
Results
Expression of TPX2 in cell lines
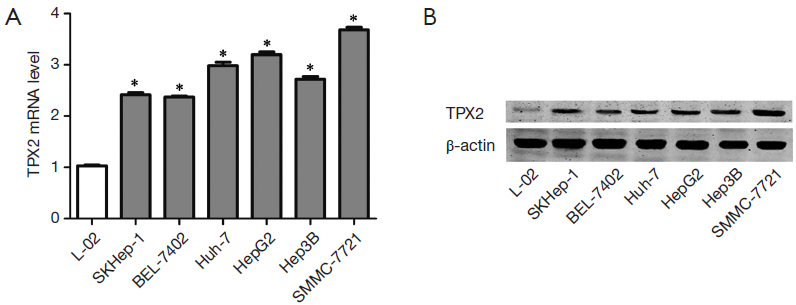
To determine the expression of TPX2 in cell lines, we first investigated the expression of TPX2 mRNA in L02, SMMC-7721, BEL-7402, Huh-7, HepG2, Hep3B and SKHep1 cell lines using qRT-PCR. We found that the expression of TPX2 mRNA in normal liver cell line LO2 was significantly lower than that in HCC cell lines including SMMC-7721, BEL-7402, Huh-7, HepG2, Hep3B and SKHep1 (P<0.05, respectively, Figure 1A). Furthermore, SMMC-7721 expressed the highest level of TPX2 mRNA in HCC cell lines and the TPX2 expression of HepG2 was the second highest among the six HCC cell lines (Figure 1A). Next, we examined the expression of TPX2 protein in normal hepatocytes and HCC cells. Our date indicate that TPX2 protein level in LO2 cells was obviously lower as compared with that in HCC cells, which was consistent with TPX2 mRNA (Figure 1B). These results suggest that TPX2 may function as an oncogene in HCC.
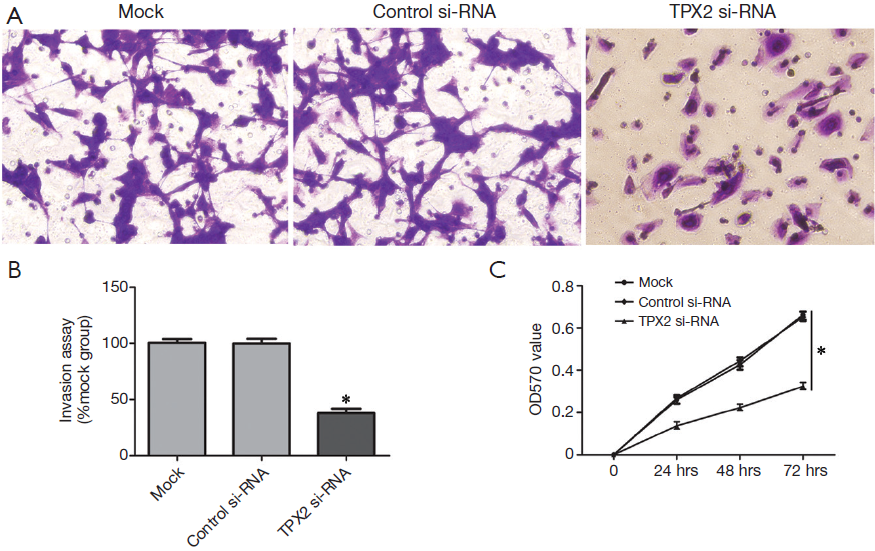
TPX2 knockdown suppresses cell invasion and proliferation in SMMC-7721 cells
To further identify the role of TPX2 in HCC, we investigated the effect of TPX2 knockdown on cell invasion and proliferation using transwell cell invasion and MTT assays in TPX2 siRNA transfected SMMC-7721 cells. As compared with the control-siRNA group (control siRNA transfected SMMC-7721 cells), we found that TPX2 knockdown significantly reduced the number of invaded cell in SMMC-7721 cells (P<0.05, Figure 2A,B). We next evaluated cell proliferation using MTT assay in SMMC-7721 cells with TPX2 or control siRNA transfection. As expected, TPX2 knockdown by a specific siRNA prominently decreased cell proliferation in SMMC-7721 cells (P<0.05, Figure 2C). These results indicate that TPX2 may promote cell proliferation and invasion in HCC cells.
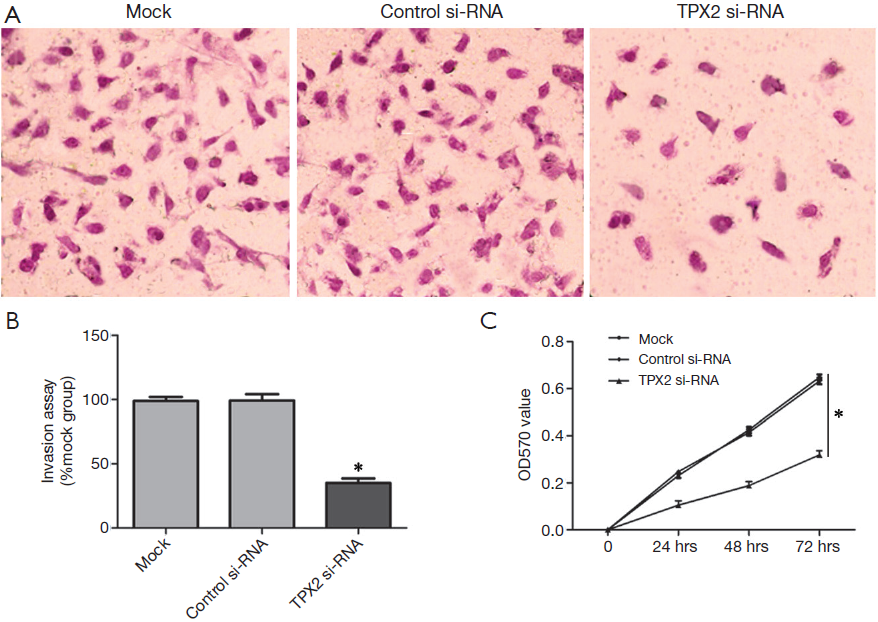
TPX2 knockdown suppresses cell invasion and proliferation in HepG2 cells
The TPX2 expression level of HepG2 is lower than that in SMMC-7721 cells, but its TPX2 expression was the second highest among the six HCC cell lines. Therefore, we also investigated the effect of TPX2 knockdown on cell invasion and proliferation in TPX2 siRNA transfected HepG2 cells. In this experiment, we found that TPX2 knockdown significantly reduced the number of invaded cell in HepG2 cells (P<0.05, Figure 3A,B). We next detected cell proliferation using MTT assay in HepG2 cells with TPX2 or control siRNA transfection. As expected, TPX2 knockdown by a specific siRNA evidently decreased cell proliferation in HepG2 cells (P<0.05, Figure 3C). These results also indicate that TPX2 may have a key role in the proliferation and invasion of HCC cells.
TPX2 knockdown inhibits AKT signaling pathway
Since TPX2 acts as an oncogenic protein and up-regulates MMPs expression through activating AKT signaling in colon cancer cells (14). To identify the underlying mechanisms by which TPX2 promotes cell invasion and proliferation in HCC cells, we evaluated the effect of TPX2 knockdown on AKT signaling pathway. SMMC-7721 cells that were transfected with TPX2 or control siRNA were subjected to WB for p-AKT. As shown in Figure 4A, TPX2 knockdown obviously reduced the level of p-AKT in SMMC-7721 cells (P<0.05). Thus, TXP2 may promote tumor progression by modulating the activation of AKT signaling in HCC.
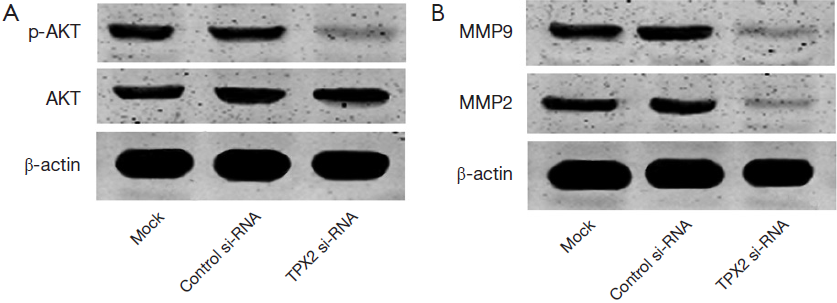
TPX2 knockdown reduces MMP2 and MMP9 expression
Since AKT signaling activation plays an important role in promoting MMPs expression including MMP2 and MMP9 (23). To better understand the downstream molecules involved in TPX2 induced-AKT signaling activation in HCC cells, we determined whether TPX2 knockdown affected MMP2 and MMP9 expression in SMMC-7721 cells. Immunoblotting was performed to assess MMP2 and MMP9 expression in TPX2 or control siRNA transfected SMMC-7721 cells. We found that TPX2 knockdown markedly decreased the protein levels of MMP2 and MMP9 in SMMC-7721 cells (P<0.05, Figure 4B). Altogether, TPX2 may promote cell invasion and proliferation by activating AKT signaling and subsequently increasing MMP2/9 expression in HCC cells.
Discussion
Since the high frequency of metastasis, a lot of HCC patients are diagnosed at advanced stage and not suitable to receive curative therapy, which leads to poor prognosis of HCC (6). Recently, many biomarkers have been identified to be associated with the carcinogenesis and progression of HCC, but most of them do not turn out to be benefit for the patients of HCC (24). Thus, it is important to develop effective biomarkers and target agents of HCC and explore the molecular mechanism of HCC progression (25). TPX2, which is located in chromosome 20q 11.2, is considered to be implicated in regulating multiple aspects of mitotic spindle and chromosome isolation (10,11). Recently, several studies have indicated that TPX2 over-expression is commonly observed in human cancers including colon, ovarian and prostate cancer (11,14). Importantly, multiple researches found that TPX2 was a potent oncogenic protein in the progression of human cancer (11). Furthermore, recent study demonstrated that TPX2 induced AKT signaling activation and up-regulated MMP2 expression, which finally resulted in colon cancer cell invasion (14).
TPX2 expression and its role have been investigated in several human cancers. Satow et al. found that TPX2 expression in HCC tissues was significantly higher as compared with those in nontumorous tissues (22). However, the role TPX2 and its related mechanisms in HCC were unknown. In this study, we first examined the expression of TPX2 mRNA and protein in a normal liver cell line LO2 and six HCC cell lines, such as SMMC-7721, BEL-7402, Huh-7, HepG2, Hep3B and SKHep1. We found that TPX2 expression in normal liver cells was lower than that in HCC cells. Our data indicate that TPX2 was expressed highly in cultured HCC cells and the TPX2 expression level in normal liver cells was low.
Carcinogenesis is characterized by uncontrolled cell growth and cancer formation is associated with alterations in molecules, which are related to the modulation of cell proliferation and death (26). Therefore, to identify the molecules implicated in the carcinogenesis events is very important to developing effective therapeutic strategies. In the previous studies, AKT signaling pathway has been considered to promote tumor cell invasion and proliferation (23,27). AKT signaling pathway is aberrantly hyper-activated in tumor cells by distinct mechanisms including down-regulation of phosphatidylinositol-3-kinase interacting protein 1 (PI3KIP1) and high-expression of COX2 (27). It has been reported that AKT signaling pathway suppresses apoptosis and promotes cell proliferation in HCC (27). Moreover, strong evidences show that AKT signaling pathway plays a critical role in promoting MMP2 and MMP9 expression (23). Otherwise, activated AKT signaling can up-regulate MMP2 and MMP9 expression and subsequently promote cell invasion in HCC (18). As two important members of the MMP family, MMP2 and MMP9 are critical factors in the invasion and metastasis of HCC cell (28). In this study, we selected SMMC-7721 and HepG2, two HCC cell lines with high basal TPX2 expression, for TPX2 knockdown experiment. Notably, the tumor cell invasion and MTT assays showed that TPX2 knockdown by a specific siRNA significantly attenuated cell invasion and proliferation in SMMC-7721 and HepG2 cells. Subsequent experiments showed that TPX2 knockdown led to inactivation of AKT signaling and down-regulation of MMP2 and MMP9 expression in SMMC-7721 cells. These data indicate that TXP2 promote cell proliferation and invasion with AKT signaling pathway activation and MMP2/9 up-regulation in HCC cells.
Due to aberrant signaling in several types of tumors, AKT signaling pathway has been targeted for inhibition to block malignant tumor growth. In our study, we observed that TPX2 expression was closely associated with phosphorylated protein level of AKT in HCC, suggesting that TPX2 could be very important in AKT signaling modulation. Although phosphatidylinositol-3-OH kinase (PI3K) is well identified as a major regulator of AKT signaling activation, recent researches highlight other molecules that activate AKT signaling pathway directly to promote its pro-proliferative functions (18,27). Here we found that down-regulation of TPX2 expression could diminish the phosphorylated protein level of AKT. Hence, our results suggest that TPX2 may be an important alternate AKT activator.
In conclusion, we demonstrated that elevated levels of TPX2 are observed in HCC cells as compared with normal liver cells. TPX2 knockdown inhibited cell proliferation and invasion in HCC cells. Furthermore, we figure out that TPX2 knockdown inactivates AKT signaling and decreases MMP2/9 expression in HCC. Altogether, we hypothesize that TPX2 overexpression contributes to hepatocarcinogenesis, possible through activating AKT signaling and subsequently increasing MMP2/9 expression. By addressing this pathway, we identified TPX2 as a potential therapeutic target for HCC.
Acknowledgements
Author contributions: Qingguang Liu and Yingmin Yao were responsible for the design of research. Qingquan Liu and Hongyong Zhang conducted the experiments. Qingquan Liu and Pinghua Yang drafted the manuscript. Kangsheng Tu and Xin Zheng participated in the data analysis.
Funding: This study was supported by grants from the National Natural Science Foundation of China (81272645 and 81301743).
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 2003;139:817-23. [PubMed]
- Venook AP, Papandreou C, Furuse J, et al. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010;15 Suppl 4:5-13. [PubMed]
- Alazawi W, Cunningham M, Dearden J, et al. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther 2010;32:344-55. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [PubMed]
- Wang C, Ren R, Hu H, et al. MiR-182 is up-regulated and targeting Cebpa in hepatocellular carcinoma. Chin J Cancer Res 2014;26:17-29. [PubMed]
- Heidebrecht HJ, Buck F, Steinmann J, et al. p100: a novel proliferation-associated nuclear protein specifically restricted to cell cycle phases S, G2, and M. Blood 1997;90:226-33. [PubMed]
- Kufer TA, Silljé HH, Körner R, et al. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J Cell Biol 2002;158:617-23. [PubMed]
- Gruss OJ, Wittmann M, Yokoyama H, et al. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat Cell Biol 2002;4:871-9. [PubMed]
- Neumayer G, Belzil C, Gruss OJ, et al. TPX2: of spindle assembly, DNA damage response, and cancer. Cell Mol Life Sci 2014;71:3027-47. [PubMed]
- Chang H, Wang J, Tian Y, et al. The TPX2 gene is a promising diagnostic and therapeutic target for cervical cancer. Oncol Rep 2012;27:1353-9. [PubMed]
- Li Y, Tang H, Sun Z, et al. Network-based approach identified cell cycle genes as predictor of overall survival in lung adenocarcinoma patients. Lung Cancer 2013;80:91-8. [PubMed]
- Wei P, Zhang N, Xu Y, et al. TPX2 is a novel prognostic marker for the growth and metastasis of colon cancer. J Transl Med 2013;11:313. [PubMed]
- Yan L, Li S, Xu C, et al. Target protein for Xklp2 (TPX2), a microtubule-related protein, contributes to malignant phenotype in bladder carcinoma. Tumour Biol 2013;34:4089-100. [PubMed]
- Cáceres-Gorriti KY, Carmona E, Barrès V, et al. RAN nucleo-cytoplasmic transport and mitotic spindle assembly partners XPO7 and TPX2 are new prognostic biomarkers in serous epithelial ovarian cancer. PLoS One 2014;9:e91000. [PubMed]
- Shigeishi H, Ohta K, Hiraoka M, et al. Expression of TPX2 in salivary gland carcinomas. Oncol Rep 2009;21:341-4. [PubMed]
- Gao J, Ding F, Liu Q, et al. Knockdown of MACC1 expression suppressed hepatocellular carcinoma cell migration and invasion and inhibited expression of MMP2 and MMP9. Mol Cell Biochem 2013;376:21-32. [PubMed]
- Wang YH, Dong YY, Wang WM, et al. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-κB pathways induced by paracrine cytokines. J Exp Clin Cancer Res 2013;32:51. [PubMed]
- Leng J, Han C, Demetris AJ, et al. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology 2003;38:756-68. [PubMed]
- Aguirre-Portolés C, Bird AW, Hyman A, et al. Tpx2 controls spindle integrity, genome stability, and tumor development. Cancer Res 2012;72:1518-28. [PubMed]
- Satow R, Shitashige M, Kanai Y, et al. Combined functional genome survey of therapeutic targets for hepatocellular carcinoma. Clin Cancer Res 2010;16:2518-28. [PubMed]
- Chien CS, Shen KH, Huang JS, et al. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell Biochem 2010;333:169-80. [PubMed]
- Scaggiante B, Kazemi M, Pozzato G, et al. Novel hepatocellular carcinoma molecules with prognostic and therapeutic potentials. World J Gastroenterol 2014;20:1268-88. [PubMed]
- Welker MW, Trojan J. Antiangiogenic treatment in hepatocellular carcinoma: the balance of efficacy and safety. Cancer Manag Res 2013;5:337-47. [PubMed]
- Sieber O, Heinimann K, Tomlinson I. Genomic stability and tumorigenesis. Semin Cancer Biol 2005;15:61-6. [PubMed]
- Zheng X, Gai X, Ding F, et al. Histone acetyltransferase PCAF up-regulated cell apoptosis in hepatocellular carcinoma via acetylating histone H4 and inactivating AKT signaling. Mol Cancer 2013;12:96. [PubMed]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010;141:52-67. [PubMed]
