Maintenance treatment of trastuzumab for patients with advanced breast cancer to achieve long term survival: two case reports and literature review
Introduction
Metastatic breast cancer (MBC) is an incurable disease, but it can be effectively managed with appropriate treatment strategies. The aims of therapy in this setting include prolongation of survival with good quality of life and symptom control (1). In particular, inadequate evidence is available on optimal duration of chemotherapy and the number of administered cycles is generally based on patient responsiveness and individual tolerability as well as physician preferences. It has been a topic of concern for clinical oncologists to improve the quality of life of patients with advanced breast cancer using various kinds of treatment methods, prolong their survival time as far as possible and eventually make breast cancer become a chronic disease, especially for patients with HER-2 positive breast cancer who have relatively poor prognosis and shorter survival time after metastasis. Trastuzumab is a humanized hybrid monoclonal antibody that selectively binds to the extracellular domain of HER2. Its antitumor action is not fully understood but possible mechanisms include inhibition of PI3K/AKT and mammalian target of rapamycin (mTOR), induction of apoptosis, and antibody-dependent cell-mediated cytotoxicity (2,3). The maintenance treatment of trastuzumab was used in our research on two patients with HER-2 positive MBC and relatively long term survival was then achieved. We also hypothesized that continuation of trastuzumab monotherapy in a maintenance fashion after attaining disease control with chemotherapy induction may also be beneficial in patients with breast cancer. Herein, we report two cases of MBC that received validated combination regimens with trastuzumab. They all went on to receive trastuzumab monotherapy for maintenance.
Cases report
Case 1
The patient visited to doctor first time complaining of a mass in her right breast in August 2008. The pathological diagnosis obtained from biopsy which was performed in another hospital showed invasive ductal carcinoma on the right breast and immunohistochemical examination had not been performed. From August 9th, 2008 to November 6th, 2008 she underwent the neoadjuvant chemotherapy of TE plan for four periods, specifically including epirubicin 75 mg/m2 for d1 and docetaxel 75 mg/m2 for d2, q21d. Evaluation of efficacy showed clinically complete remission (cCR). Modified radical mastectomy was performed on the right breast on November 6th, 2008. Postoperative pathological examination (after chemotherapy on the right breast) showed residual cancer, obvious cell degeneration, uninvolved papilla skin and metastases of axillary lymph nodes (3/12). Immunohistochemical examination results were: ER (–), PgR (–), HER-2 (–), TOPII 10% and P53 50%. Two cycles of the original chemotherapy plan continued after operation. The conventional radiotherapy was performed on the left chest wall and left supraclavicular area (50 Gy/25 f) in January 2009, followed by regular reexamination.
Recurrent metastasis and treatment: a gradually enlarging mass in the left supraclavicular area was found in June 2010. On August 26th, 2010, she was diagnosed as postoperative metastasis of right breast cancer to lymph nodes, bone and brain after a comprehensive review. Pathological biopsy on left supraclavicular lymph nodes showed poorly differentiated invasive adenocarcinoma in the left supraclavicular fibrous connective tissue, which was morphologically consistent with breast origin. Immunohistochemical test showed ER (–), PgR (–), HER-2 (++), and Ki67 40%. The results of fluorescence in situ hybridisation (FISH) test showed HER-2 gene amplification with a ratio of 6.0. The patient refused to receive trastuzumab treatment for lack of money. Six cycles of chemotherapy [vinorelbine, 25 mg/m2 for d1 and d8, cisplatin for d1-2, q21d (NP)] were performed from September 1st, 2010 to January 2011, and meanwhile zoledronic acid (4 mg, q28d) was used to treat bone metastases. From September 10th to October 6th, 2010,whole brain radiotherapy (40 Gy/20 f) was performed. After two cycles of chemotherapy, the lymph nodes in the left supraclavicular completely disappeared. Reexamination of head MRI 1 month after radiotherapy showed decreased volume of brain metastases. The evaluation of efficacy on NP therapy showed partial remission (PR). However, the treatment had been discontinued by the patient for more than 4 months since January 2011.
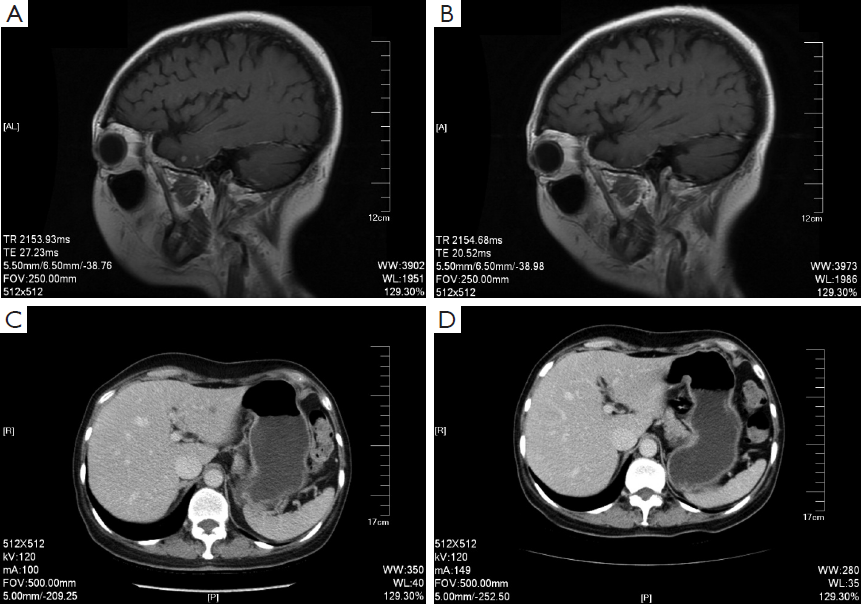
The head MRI reexamination on return visit (June 1st, 2011) showed progressive multiple brain metastases. Bone scanning showed new tumor lesions. The CDU and CT of abdomen suggested hepatic metastases. The patient insisted on rejecting trastuzumab treatment for financial problem. And from June 2nd to September 22nd, 2011, five cycles of oral capecitabine (1,500 mg, bid, q21d) were performed and reexamination was conducted every two cycles. Meanwhile zoledronic acid (4 mg, q28d) was used to treat bone metastasis. The results of reexamination showed decreased volume of hepatic metastases and brain lesions (Figure 1). The evaluation of efficacy showed PR. Since September 22nd, 2011, the treatment had been discontinued again by the patient for 3 months.
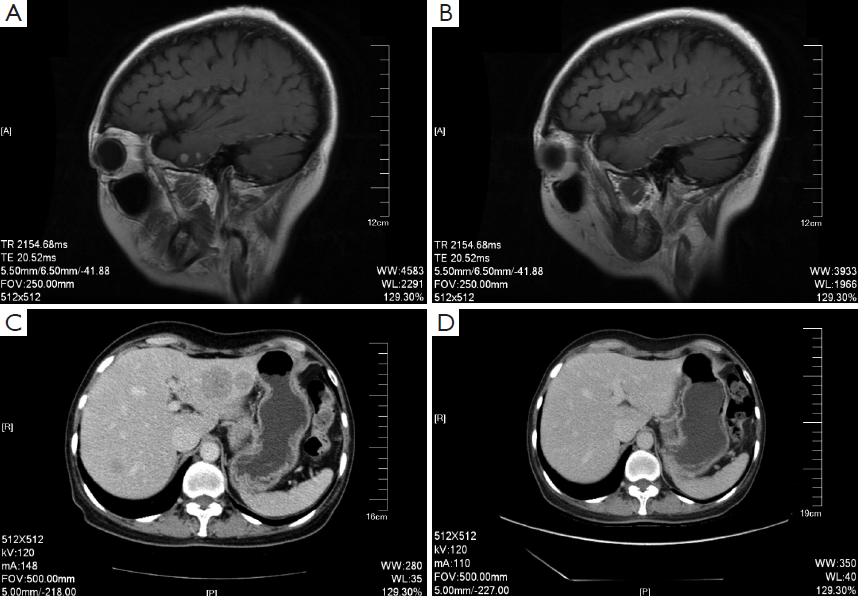
On December 26th, 2011 reexamination of the abdominal CT scan showed progression in liver and brain metastases (Figure 2), and liver biopsy showed invasive adenocarcinoma/metastasis in the liver. Immunohistochemical results revealed ER (–), PgR (–), HER-2 (3+), and Ki67 30%. At that time a patient assistant program (PAP) Project was conducted by China Charity Federation funded by the Roche, which lowered the cost of trastuzumab treatment and thus made it affordable for the patient. From December 29th, 2011 to October 15th, 2012 the patient received combination treatment of capecitabine tablets (1,500 mg, bid, d1-14, q21d) with trastuzumab, and reexamination was performed every 2 to 3 cycles, which showed obvious shrink of metastases in the liver and the brain (Figure 2) and improvement of bone metastases. The evaluation of efficacy revealed PR. Capecitabine tablets were discontinued because of grade II hand-foot syndrome. From October 15th, 2012 to February 8th, 2014 maintenance treatment of trastuzumab (6 mg/kg, q21d) was performed. Reexamination showed that liver and brain metastases continued to decrease and eventually disappeared, while bone metastasis was stable. Evaluation of efficacy showed PR. Treatment and follow-up are being continued at present. During the treatment of trastuzumab, examination on cardiac function was performed every 3 months, with normal results.
The patient has survived for over 41 months after brain metastasis, and the remission of disease has continued for more than 25 months as a result of the trastuzumab treatment (both combination and single administration). Meanwhile, trastuzumab treatment has significantly prolonged the survival time of the patient with the effect on the progressive brain metastasis after radiotherapy. The patient could work and live a normal life with good tolerance to drugs.
Case 2
The patient underwent modified radical mastectomy on her right breast in a local hospital on September 17th, 2009. vPostoperative pathological examination showed invasive ductal breast carcinoma in the right breast with the size of 5×5×4.5 and 3.5×3×3 cm3 respectively, with no invasive carcinoma on the skin and surgical margin. Examination of axillary lymph node samples showed metastases (15/18) with no invasive carcinoma between the pectoralis major and the pectoralis minor. The immunohistochemical examination showed ER (–), PgR (–), and HER-2 (+). Five cycles of postoperative TE chemotherapy were performed, specifically including docetaxel 80 mg for d1 and epirubicin 90 mg for d1, q28d. Radiotherapy was not performed.
In June 2010 the patient noticed soybean-shaped nodes on her right chest wall and local hot compress was applied on the chest wall by herself. Subsequently the nodes progressed into pieces with the emergence of local red swelling. Physical examination conducted in the local hospital on August 13th, 2010 revealed swelling lymph nodes in the right clavicle with a size of 2 cm × 2 cm. There was red swelling in the chest wall around the original operative incision, with an area of about 6 cm × 3 cm. The biopsy of local excision of the right chest wall showed invasive carcinomas in the skin and soft tissues. The radical operation of recurrent cancer on the right chest wall was performed with lymph node clearance in the right armpit and under the right clavicle. Postoperative pathological examination demonstrated invasive carcinoma in the chest wall skin, no metastasis in the right armpit (0/3), invasive carcinoma in the pectoralis and metastases in right subclavian lymph nodes (6/6). Poorly healing and dehiscent postoperative incisions were observed.
On September 13th, 2010 the patient visited our department. A comprehensive review showed no obvious distant metastasis and she was diagnosed as postoperative right breast cancer, with postoperative recurrent carcinoma in the right chest wall and metastases in the infraclavicula lymph nodes. Consultation for the pathological sections of primary tumor suggested invasive ductal carcinoma in the right breast with partial mucinous adenocarcinoma and invasive carcinoma within lymph nodes. Immunohistochemical test showed ER (–), PgR (–), HER-2 (2+), and Ki67 20%. FISH test indicated HER-2 gene amplification (Ratio =5.0). The patient rejected to receive trastuzumab treatment for financial problem and underwent 6 cycles of consolidation chemotherapy with NP regimen from September 16th, 2010 to January 7th, 2011, which specifically included vinorelbine 25 mg/m2 for d1 and d8 and cisplatin 75 mg/m2 for d1-3, q3w. No recurrence in the chest wall was found during this period. However, the operative incision dehisced with a size of 8 cm × 4 cm and the wound surface failed to heal, for which reason free skin graft of the right chest wall was performed and uneventful healing of the postoperative wound was thus achieved. From February 1st to February 28th, 2011, radiotherapy on the right chest wall and right supraclavicular area was performed (50 Gy).
In April 2011 (more than one month after radiotherapy), the patient presented with progressive increase of red area on her right chest wall with pain. On June 9th, 2011, she visited our department for another time and the physical examination showed red swelling and thickening on the skin with a large area starting from the right chest wall to the clavicle upward, to the 10th front rib downward, to the right sternum horizontally and to the dorsal midline backward. Computerized tomography of the chest suggested exacerbation of the thickening soft tissues in the right anterior chest wall with multiple metastatic nodes and swelling lymph nodes in the left axilla. She continued to refuse trastuzumab treatment and underwent five cycles of chemotherapy with TX regimen (docetaxel 75 mg/m2 for d1, capecitabine 1,000 mg/m2 bid po d1-14, q21d) from June 13th to October 2011, after which significant reduction of symptoms and obvious decrease of tumor lesions were observed. The overall efficacy evaluation was PR. After five cycles, the patient discontinued chemotherapy due to grade I gastrointestinal reaction, grade III WBC decline and requirement for hospitalization.
Due to the PAP program conducted by China Charity Federation which reduced the average fee of trastuzumab treatment and made it affordable for the patient, she started trastuzumab treatment on October 15th, 2011. Regular reexamination showed progressive improvement of tumor lesions in the chest wall, which completely disappeared 5 months later (in March 2012). And the efficacy evaluation was cCR. Now (February 2014), the patient remains in the condition of complete remission with no adverse events, while treatment and follow-up are still ongoing.
The patient has survived for more than 44 months after recurrence and metastasis, and the maintenance treatment of trastuzumab has brought the patient with up to 28 months of remission period. The patient lives a normal life.
Discussion
In the current study, the two patients benefited obviously from the combination chemotherapy of trastuzumab and the maintenance of mono-therapy after multiple distant metastases and brain metastasis was found. With further research performed and the improvement of treatment methods, the five-year survival rate of patients with early breast cancer has increased to over 85%. However, recurrent MBC is still found in about 20-30% of patients and the aim of treatment for this incurable disease is to alleviate symptoms, improve the quality of life and prolong the survival term. The International Consensus Guidelines for Advanced Breast Cancer (ABC 1) lays stress on the treatability of advanced breast cancer despite of its incurability, which means patients can survive with tumors for a long term (4). Clinical researches confirmed that longer survival time could be achieved with maintenance treatment on advanced breast cancer. The GEICAM 2001-01 study was carried out to evaluate the efficacy of maintenance treatment of pegylated liposomal doxorubicin (PLD) on advanced breast cancer, in which doxorubicin or epirubicin with sequential paclitaxel was used as first-line treatment, followed by dividing patients into maintenance group and observation group (5).
The research showed that the PLD maintenance treatment significantly prolonged the median progression-free survival (16.04 vs. 9.96 months, P=0.0001). The research of KCSG-BR 0702 selected combination treatment of gemcitabine with paclitaxel (PG) as the maintenance treatment. Compared with the 6 cycles of combination chemotherapy of PG in the control group, the maintenance treatment group showed obviously improved progression free survival (PFS) (7.5 vs. 3.8 months, HR =0.73, P=0.026) and overall survival (OS) (32.3 vs. 23.5 months, HR =0.65, P=0.047) (6). A recent Meta-analysis collected 11 randomized clinical control researches with a total number of 2,269 patients and analyzed the effect of the duration of first-line chemotherapy on the PFS and OS of patients with MBC. The results showed that longer time of first-line chemotherapy could significantly improve the OS with an obviously improved PFS (HR =0.64, P<0.001) and reduce the cancer related mortality by 9% (HR =0.91, P=0.046) (7).
The maintenance treatment consists of consecutive maintenance treatment and transformational maintenance treatment. Consecutive maintenance treatment is defined as a long-term treatment with extension of the time of first-line treatment or one of the drugs, after achieving curative effect [CR, PR and Stable Disease (SD)], until the disease progresses, as demonstrated in Case 1. Transformational maintenance treatment is defined as after CR, PR and SD, a long-term treatment with transformation to another kind of medication with milder adverse reaction until the disease progresses, as demonstrated in Case 2. The heterogeneity of cancer patients is manifested not only in different reactions to the treatment but also in different symptoms of adverse reactions. The quality of life had nearly been neglected by earlier clinical researches on maintenance treatment. Therefore, the duration of chemotherapy was restricted by adverse reactions and quality of life, although maintenance treatment could bring about survival benefit. The discontinuation of drugs due to intolerance to adverse reactions caused by chemotherapy was recorded both in the two cases given above. It was concluded that emphasis should be put on the selection of applicable regimen for appropriate patients in terms of maintenance treatment.
Breast cancer was divided into five subtypes by St. Gallen in 2011 according to the molecular biological characteristics of HR, HER-2 and Ki67: Luminal A, Luminal B, HER-2-positive, triple negative, and other special subtypes (8). So the treatment for MBC might as well be classified according to the molecular subtypes. However, despite of the conventional knowledge of consistency in receptor types of metastatic lesions and primary lesions, more and more researches revealed inconformity in the expression of receptors between them (9-11). The inconformity in HR was more common than that in HER-2, which was sufficient to affect the selection of regimen, especially for patients with transformation of HER-2 negative to positive. A Meta-analysis involving 26 researches with 2,520 patients revealed an inconformity rate of 5.5% in HER-2 (3.6-8.5%), which was probably caused by the progression in tumor (gene flow), detection techniques, subjectivity in detection and heterogeneity of HER-2 expression in tumors (12). Both the cases mentioned above were triple negative in primary tumor lesions with HER-2-positive in metastatic tumor lesions, suggesting the necessity of retesting on metastatic tumor lesions.
MBC can be treated with endocrine therapy, chemotherapy or targeted treatment according to its molecular typing. However, various reasons could result in the inaccessibility to undelayed optimal regimen. As demonstrated in both cases mentioned above, financial problems made it impossible for them to receive first-line targeted treatment. However, good therapeutic effect of trastuzumab in multi-line treatment was demonstrated by effective disease control with multiple chemotherapy followed by trastuzumab treatment.
Breast cancer is the second most common solid malignancy that metastasizes to the central nervous system (CNS), which ranked only second to lung cancer. The HER-2 positive subtype is most likely to develop brain metastasis (13,14). As brain metastasis often appears in advanced stage where multiple organ metastases occur, brain metastasis has been put on little emphasis and such cases are often excluded from clinical trials. Due to better prognosis of HER-2+ breast cancer on account of targeted treatment, especially the emergence of anti-HER-2 drugs, the incidence of brain metastasis has increased with the improvement of OS term. And thus brain metastasis in HER-2 positive breast cancer has become the focus of researches.
A single-arm phase II study by LANDSCAPE on lapatinib plus capecitabine recruited 45 patients with previously untreated brain metastases from breast cancer. It found post-treatment alleviation in brain metastases (which was defined as more than 50% shrink in the size of tumor) in 29 patients (65.9%, 95% CI: 50.1-79.5%) (15). But the results should be verified in a future phase III clinical trial. A Meta-analysis concluded that anti-HER-2 treatments including trastuzumab and lapatinib could result in better prognosis of postponing CNS metastasis in patients with breast cancer (16). A research by Musolino et al. found that the median time of brain metastasis after the initial extracranial recurrence was 9.8 and 11.3 months for HER-2-negative and positive patients who received adjuvant therapy of trastuzumab, respectively. It also concluded that trastuzumab treatment could delay brain metastasis in HER-2-positive MBC patients with relatively equivalent time to develop brain metastasis compared with HER-2-negative ones (17).
The RegistHER retrospective study suggested that patients with advanced breast cancer and brain metastases benefited more from trastuzumab treatment than those in the control group (17.5 vs. 3.8 months, HR =0.33, P<0.001) (18). The 1st International Consensus Guidelines for Advanced Breast Cancer indicated that anti-HER2 treatment could positively control the extracranial tumor lesions of patients with brain metastases from HER2 positive breast cancer to bring about survival benefits for them (4). At present the routine treatment for brain metastasis includes stereotactic radiosurgery (SRS) and whole-brain radiotherapy (WBRT). With the development of comprehensive therapy, progress has also been made in chemotherapy and targeted therapy. The efficacy of treatment for brain metastasis could be enhanced with the combination of WBRT and SRS. The first case in this paper achieved remission for a certain time with WBRT. Good therapeutic effect and tolerance had been obtained with mono-therapy of targeted treatment when brain metastases progressed after whole brain radiotherapy, which was a worthwhile treatment. This was likely because radiotherapy opened the blood-brain barrier to make it easier for trastuzumab to pass through the barrier, thus reaching sufficient concentration in brain tissue.
Clinical researches indicated that, contrary to the chemotherapy treatment strategy, patients with progression after trastuzumab treatment could still benefit from continued trastuzumab treatment (19,20). A research by Hermine et al. demonstrated that the median PFS was 10.1 months in patients who continued trastuzumab treatment after progression compared with that of 7.1 months in those who discontinued the treatment after progression. There was a significant extension in the median OS of patients with continued trastuzumab treatment, which had not been reached 27.8 months after follow-up, while the median OS was only 16.8 months in patients with discontinuation of treatment. The median OS was 21.3 and 4.6 months respectively for patients in the two groups after progression (19). In the study of GBG26, HER-2-positive MBC patients with progression after trastuzumab treatment were randomly divided into two groups: mono-therapy of capecitabine and the combination chemotherapy of capecitabine with trastuzumab. The results showed that patients could still achieve longer PFS with continued trastuzumab treatment after progression (20). Therefore, the National Comprehensive Cancer Network (NCCN) Guideline suggested that even patients with HER-2 positive breast cancer who had progression after anti-HER-2 treatment could still continue to receive anti-HER-2 treatment combined with other chemotherapy regimen (21). Based on the researches above, a subsequent treatment option for the two cases in this paper was suggested, that is, the continued administration of trastuzumab treatment until progression and combination of trastuzumab with chemotherapeutics after progression.
In conclusion, trastuzumab-based therapy is defined as the standard treatment for HER-2-positive MBC patients. Further anti-HER-2 treatment after progression as a result of trastuzumab-exposed could still benefit patients. As the reactivity of different patients to the treatment is different such as efficacy and adverse reaction, the individualized maintenance treatment for patients with advanced breast cancer is worthy of further research.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lohmann AE, Saini KS, Metzger-Filho O. Pertuzumab in metastatic breast cancer: unanswered questions. Transl Cancer Res 2012;1:122-4.
- Nelson V, Rademaker A, Kaklamani V. Paradigm of polyendocrine therapy in endocrine responsive breast cancer: the role of fulvestrant. Chin Clin Oncol 2013;2:10.
- Christinat A, Di Lascio S, Pagani O. Hormonal therapies in young breast cancer patients: when, what and for how long? J Thorac Dis 2013;5:S36-46. [PubMed]
- Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast 2012;21:242-52. [PubMed]
- Alba E, Ruiz-Borrego M, Margelí M, et al. Maintenance treatment with pegylated liposomal doxorubicin versus observation following induction chemotherapy for metastatic breast cancer: GEICAM 2001-01 study. Breast Cancer Res Treat 2010;122:169-76. [PubMed]
- Park YH, Jung KH, Im SA, et al. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol 2013;31:1732-9. [PubMed]
- Gennari A, Stockler M, Puntoni M, et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol 2011;29:2144-9. [PubMed]
- Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: Summary of the Consensus Discussion. Breast Care (Basel) 2011;6:136-141. [PubMed]
- Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol 2009;20:1953-8. [PubMed]
- Dieci MV, Barbieri E, Piacentini F, et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann Oncol 2013;24:101-8. [PubMed]
- Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol 2012;30:593-9. [PubMed]
- Houssami N, Macaskill P, Balleine RL, et al. HER2 discordance between primary breast cancer and its paired metastasis: tumor biology or test artefact? Insights through meta-analysis. Breast Cancer Res Treat 2011;129:659-74. [PubMed]
- Chien AJ, Rugo HS. Emerging treatment options for the management of brain metastases in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat 2013;137:1-12. [PubMed]
- Yan M, Lü HM, Liu ZZ, et al. High risk factors of brain metastases in 295 patients with advanced breast cancer. Chin Med J (Engl) 2013;126:1269-75. [PubMed]
- Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 2013;14:64-71. [PubMed]
- Larsen PB, Kümler I, Nielsen DL. A systematic review of trastuzumab and lapatinib in the treatment of women with brain metastases from HER2-positive breast cancer. Cancer Treat Rev 2013;39:720-7. [PubMed]
- Musolino A, Ciccolallo L, Panebianco M, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer 2011;117:1837-46. [PubMed]
- Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res 2011;17:4834-43. [PubMed]
- Extra JM, Antoine EC, Vincent-Salomon A, et al. Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study. Oncologist 2010;15:799-809. [PubMed]
- von Minckwitz G, Schwedler K, Schmidt M, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer 2011;47:2273-81. [PubMed]
- Duffy MJ, Crown J. Monitoring response to therapy in patients with cancer: is circulating DNA the answer? Ann Transl Med 2013;1:24.