In vitro effect of iASPP on cell growth of oral tongue squamous cell carcinoma
Introduction
Oral tongue squamous cell carcinoma (OTSCC) is one of the most common head and neck squamous cell carcinomas. Each year, approximately 10,990 new cases of tongue cancer are diagnosed in the United States, accounting for approximately 30% of all oral cavity and pharynx cancers (1). Because of its specific histologic structure, the tongue is not equipped to protect itself from tumor invasion and metastasis (2).
The tumor suppressor p53 plays a central role in regulating cell cycle arrest, apoptosis, and DNA repair in a variety of cells (3). However, p53 is either absent or mutated in over half of human cancers. The discovery of the ASPP protein family as specific regulators of p53 provides a new mechanism by which the apoptotic function of p53 is regulated (4-6).
The ASPP family consists of three proteins, ASPP1, ASPP2 and iASPP, that interact with and/or modulate the behavior of p53 (5,7,8). ASPP1 and ASPP2 enhance the apoptotic function of p53, whereas iASPP inhibits p53-dependent apoptosis (9,10). Binding assays reveal differential binding of the C-terminal domains of iASPP and ASPP2 to the core domains of the p53 family members p53, p63, and p73 (11).
iASPP is located at the chromosomal region 19q13.3, which is coded by the PPP1R13L gene in humans and the ape-1 gene in C. elegans (12). iASPP can specifically inhibit p53-mediated cell death. iASPP’s regulatory function of p53 is conserved from worms to humans (13). In addition, iASPP is an oncoprotein that cooperates with Ras, E1A, and E7, but not mutant p53, to transform cells in vitro (13). iASPP was originally described as RelA-associated inhibitor (RAI), which binds to the NF-κB subunit p65(RelA) to inhibit its transcriptional activity (14-16). Overexpression of iASPP is observed in types of human cancers, such as breast carcinoma, acute leukemia, hepatocellular carcinoma, and lung cancer (17-21). However, no information is available in the literature regarding the role of iASPP expression in OTSCC. In the present study, we first detected iASPP expression in OTSCC tissues. Next, we used small interfering RNA (siRNA) to knock-down iASPP expression in OTSCC cell lines (SCC25 and UM1). We used this system to investigate the effects of iASPP decrease on cell proliferation, apoptosis and cell cycle progression as well as to probe the feasibility of gene therapy for OTSCC.
Ethics statement
This study was approved by the Ethics Committee of First Affiliated Hospital of Sun Yat-Sen University.
Material and methods
Tissue collection
All archived formalin-fixed, paraffin-embedded tissue specimens from OTSCC, oral premalignant dysplasia (OPD) and normal tongue biopsies (NTB) were collected from the Department of Oral and Maxillofacial Surgery at the First Affiliated Hospital of Sun Yat-sen University from 1998-2007. The samples were comprised of 71 OTSCC, 20 OPD and 16 NTB cases. NTB samples were used as normal controls and obtained from normal tongue tissue close to the OTSCC lesion in OTSCC patients. None of the OTSCC patients received any form of adjuvant therapy prior to surgery. The tumor extent and tumor grade was classified according to the TNM system by UICC and the WHO classification of histological differentiation, respectively.
Immunohistochemistry analysis
Tissues sections (5-µm thick) were dewaxed, and rehydrated in descending alcohol dilutions. Heat-mediated antigen retrieval was performed in 0.01 M citrate buffer (pH6.0) in a water bath for 30 minutes. Endogenous peroxidase activity in the sections was inhibited by incubation in 0.3% hydrogen peroxide in methanol for 10 minutes at room temperature.
After treatment with 5% normal buffer serum for 10 minutes to block non-specific sites, the sections were incubated with an anti-iASPP rabbit antibody (Abnova, Taipei, Taiwan) at a dilution of 1:1,000 overnight at 4 °C. The sections were then incubated with a horse radish peroxidase-conjugated anti-rabbit antibody for 30 minutes at 37 °C. Color was then developed by incubation with horseradish per oxidase substrate solution containing 3,3’-diaminobenzidine typo chromogen reagent. The sections were counterstained with hematoxylin and then mounted with neutralbalsam.
To determine iASPP immunoreactivity, each case was assessed in three different fields at 400× magnification, and cytosolic staining of yellowish or brownish granules was graded as follows: 0 for background staining, 1 for faint-staining, 2 for moderate staining and 3 for strong staining. In addition, the amount of positive staining areas from the entire tissue section was graded as follows: 1 for <1/3, 2 for 1/3-2/3, and 3 for >2/3. The results obtained were multiplied together, yielding a scale with the values of 0, 1, 2, 3, 4, 6 and 9. From this scale, 0-4 were considered low-staining, and 6 or 9 were considered high-staining. This multiplied scale subclassification system was previously described by Waltregny et al. (22).
Cell culture and antibodies
OTSCC cell lines (SCC-25and UM1) were maintained in DMEM/F12 supplemented with 10% FBS, 100 IU/mL penicillin and 100 µg/mL streptomycin (Gibco, NY, USA). Cells were cultured overnight at 37 °C under humidified conditions of 5% CO2 and 95% air.
siRNA and transient transfection
Sense and antisense siRNA oligonucleotides corresponding to the target sequence for the human iASPP gene (GenBank NM_006663.3, Table 1) were designed and synthesized by RiboBio (RiboBio, Guangzhou, China). Non-targeting siRNA was used as a control for the off-target effects caused by RNA interference (RNAi). Cells were plated to 30-50% confluence in DMEM containing 10% FBS without antibiotics. Transfections were performed 24 hours later. Lipofectamine 2000 (Invitrogen, CA, USA) was diluted in Opti-MEM (Gibco, NY, USA) and incubated for 5 minutes. The iASPP siRNAs were diluted in Opti-MEM to a concentration of 50 nM. The two dilutions were mixed and incubated for 20 minutes at room temperature before transfection. Cells were analyzed 72 hours after transient transfection, and the protein expression was determined by western blotting.
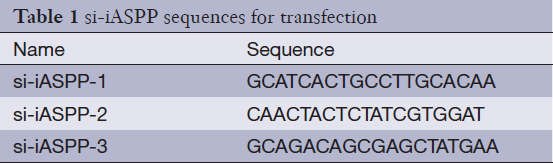
Full table
Western blot
Western blots were performed as described previously (23), using antibodies specific to iASPP (Abnova, Taipei, Taiwan). Briefly, protein samples prepared from the cells were subjected to SDS-PAGE, transferred to PVDF membranes (Millipore, CA, USA) and detected using appropriate primary antibodies followed by horseradish peroxidase-conjugated goat, anti-rabbit IgG. The intensities of the western blot bands were quantified by the image analysis software Quantity One (Bio-Rad, CA, USA).
Quantitative real-time PCR
Quantitative RT-PCR assays were used to detect iASPP expression as previously described by Chen et al. (21). Total RNA was extracted from the cells using the Trizol reagent (Invitrogen, CA, USA) and then reverse transcribed into cDNA using M-MLV-RTase (Promega, WI, USA). The resulting cDNA was used for PCR using the SYBR-Green Master PCR Mix in triplicate. The qRT-PCR primers are listed in Table 2. Melting curve analyses were performed to ensure the specificity of the quantitative RT-PCR reactions. The relative quantification value for the iASPP gene compared with the target calibrator is expressed as 2–(Ct-Cc) (Ct and Cc are the mean threshold cycle differences after normalizing to GAPDH).
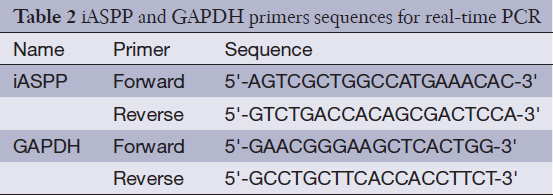
Full table
Cell proliferation assays
After transfection, the transfection medium in each well was replaced with 100 µL fresh serum-free medium with 10 µL MTS. After incubation at 37 °C for 4 hours, the absorbance was measured at 490 nm. Each assay was performed in triplicate. All tests were repeated three times.
Flow cytometry-based apoptosis and cell cycle analysis
Cells were grown in 6-well plates to approximately 30-50% confluence and transiently transfected with the desired iASPP siRNA. Flow cytometry-based apoptosis and cell cycle analyses were performed as described previously (24). For cell cycle analysis, the cells were harvested and resuspended in PBS, and the samples were then fixed in ethanol at –20 °C overnight. The cells were washed with PBS and resuspended in Staining Solution (50 µg/mL propidium iodide, 100 µg/mL RNase A, 0.2% Triton X-100 in PBS). The stained cells (1×105 cells per well) were then analyzed with a flow cytometer (FACScan, Bectone Dickinson, CA, USA). For apoptosis measurement, the cells were harvested and washed twice in PBS. The cells were resuspended in 500 mL PBS plus AnnexinV-PE and 7-AAD (Annex-inV-PE/7-AAD staining kit, BioVision, Mountain View, CA). The stained cells (1×105) were then analyzed with a flow cytometer (FACScan, Becton-Dickinson, CA, USA).
Statistical analysis
Statistical analysis was carried out using SPSS (version 16.0). Fisher’s Exact test was used to analyze the relationship between gene expression and the clinicopathologic features of OTSCC patients. One-way ANOVA was used to compare the differences between groups. For all statistical analyses, P<0.05 was considered statistically significant.
Results
iASPP expression in OTSCC and OPD
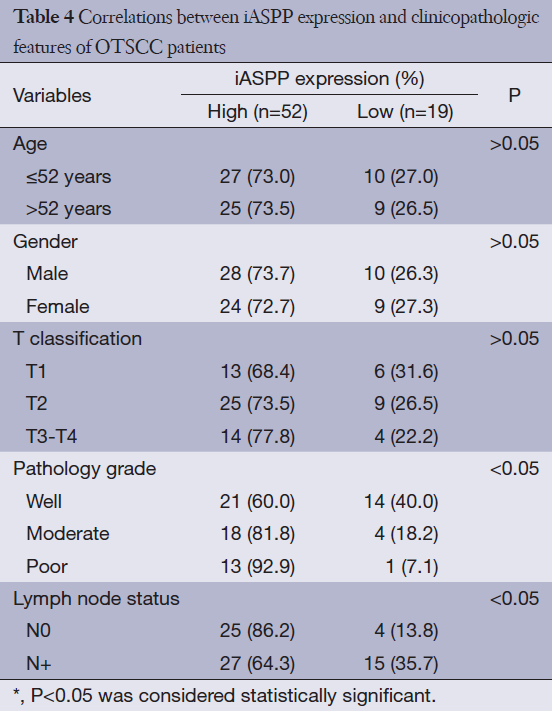
Given that research regarding iASPP expression in human OTSCC tissues has not been reported to date, we performed immunohistochemical analysis to elucidate the expression of iASPP in OTSCC tissues. Representative immunohistochemical staining indicated that iASPP is primarily expressed in the cytoplasm with minimal staining in the nucleus. Compared with very low expression of iASPP in NTB (Figure 1A), it has more extensive and intense expression in both cytoplasmic and nuclear compartments of OPD (Figure 1B) and OTSCC tissues (Figure 1C,D). iASPP expression was significantly increased in OTSCC cases compared with OPD and NTB, and iASPP expression is significantly higher in OTSCC compared to OPD tissues (P<0.001, Table 3). These results indicate that iASPP is markedly upregulated in OTSCC tissues. The relationship between iASPP expression and clinicopathologic features of 71 OTSCC tissues is displayed in Table 4. iASPP expression is significantly associated with pathological stage and lymph node status (P<0.05), and these characteristics have enhanced proliferative and metastatic potential. However, iASPP expression is not associated with patient age, gender, or T classification (P>0.05).
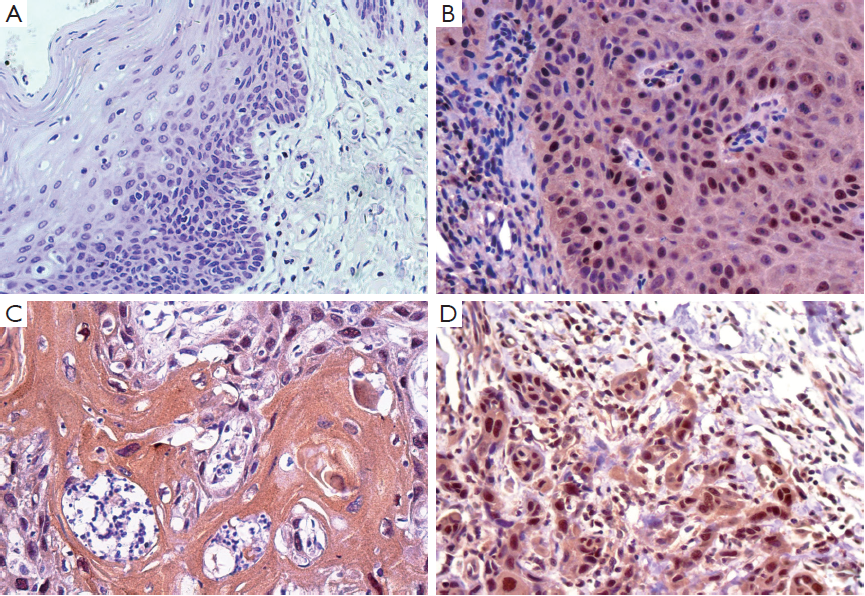
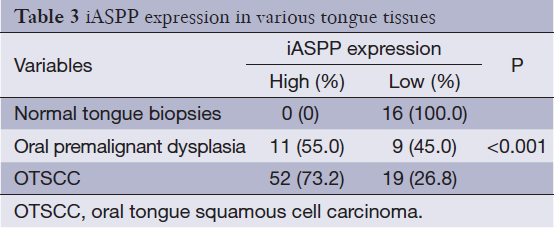
Full table
Full table
Silencing of the expression of iASPP in OTSCC cells
To further examine the role of iASPP in OTSCC, iASPP was silenced by RNAi in the OTSCC cell line SCC25. Cells were divided into three groups as follows: non-infected cells (CON), non-silenced control cells (NC) and siRNA-infected cells. We selected three siRNA sequences from the iASPP region for evaluation. The siRNAs were transfected into SCC25 cells. Figure 2A,B showed that si-iASPP-3 had the highest interference efficiency among the three siRNAs 72 hours after transfection; thus, si-iASPP-3 was chosen for use in the following experiments (P<0.05). To further determine the effect of RNAi on iASPP expression in SCC25 and UM1 OTSCC cells, iASPP mRNA (3 days post transduction) and protein levels (3 days post transduction) were analyzed. The si-iASPP-3 group showed lower expression of iASPP mRNA and protein than the CON or NC groups (Figure 2C-E; P<0.05).
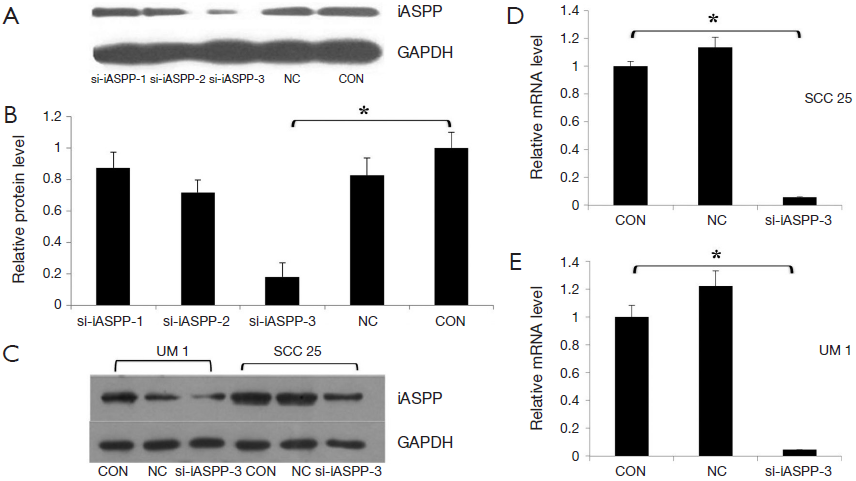
However, no significant difference in iASPP protein expression was observed between the CON and NC groups. These results indicate that iASPP expression could be significantly reduced by si-iASPP-3 in SCC25 and UM1 cells.
Down-regulation of iASPP expression inhibits OTSCC cell proliferation
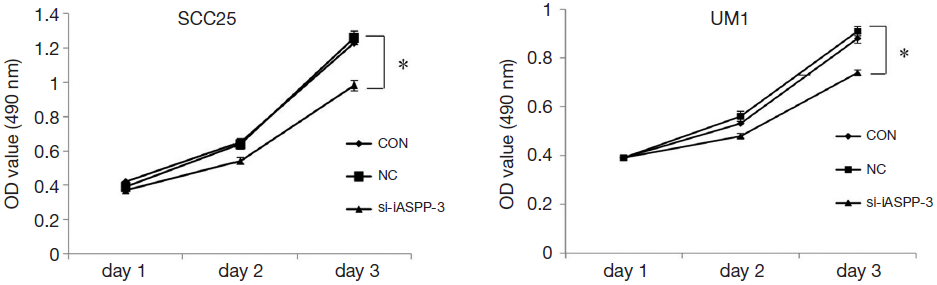
To investigate whether iASPP RNAi affects OTSCC cell proliferation, the MTS cell proliferation assay was used. As shown in Figure 3, the growth pattern was altered from the second to the third day in OTSCC cells, and the proliferation rate in the si-iASPP-3 group decreased by 22.3% in SCC25 and 18.7% in UM1 72 hours after transduction. In addition, the si-iASPP-3 group growth rate was significantly reduced compared with the CON or NC groups (P<0.05). These results revealed that knockdown of iASPP expression by si-iASPP-3 siRNA in OTSCC cells inhibited OTSCC cells growth and proliferation.
Down-regulation of iASPP expression induces cell cycle arrest in OTSCC cells
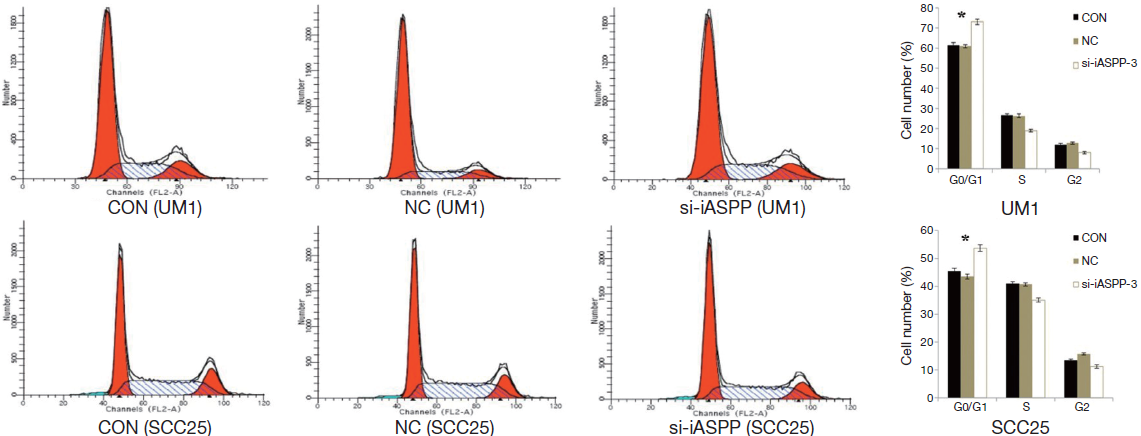
We performed flow cytometry to detect whether si-iASPP-3 affects OTSCC cell cycle 48 hours after transduction. These results showed that the proportion of OTSCC cells significantly increased in the G1/G0 phase and decreased in the G2/M phase of the si-iASPP-3 group compared with the CON or NC group (Figure 4; P<0.01). Thus, our results suggest that knockdown of iASPP expression by si-iASPP-3 siRNA in OTSCC cells inhibits OTSCC cell proliferation through inhibition of the cell cycle.
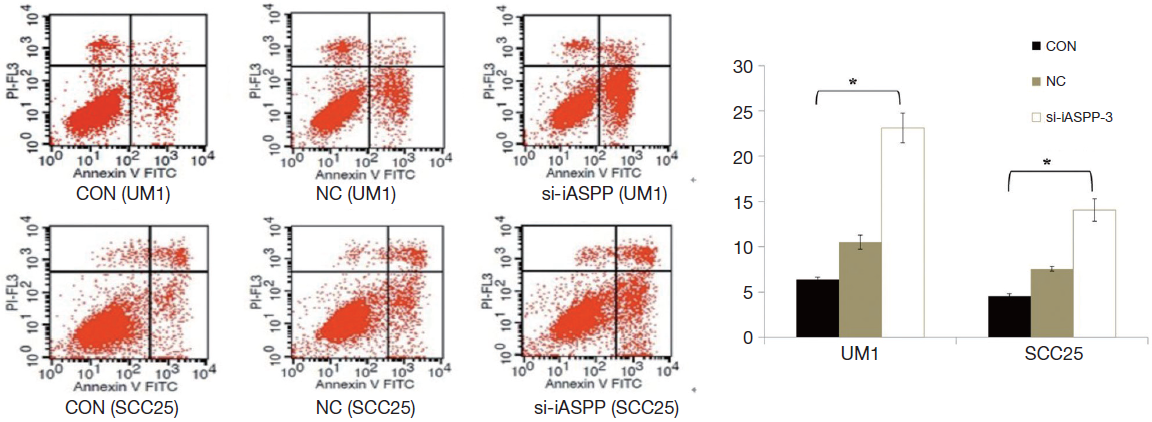
Down-regulation of iASPP promotes apoptosis of OTSCC cells
Further investigations of apoptosis elucidated a strong role for iASPP in cell apoptosis or survival. Apoptotic analysis by flow cytometry showed an increased apoptotic rate in the si-iASPP-3 group compared with NC or CON group (Figure 5; P<0.01). These results suggested that iASPP expression in OTSCC may inhibit cellular apoptosis.
Discussion
To the best of our knowledge, our present study is the first report on the expression and role of iASPP in OTSCC. iASPP expression could reflect the degree of OTSCC malignancy because iASPP expression increases with pathological grade. In addition, the relationship between iASPP expression and lymph node metastasis indicates that iASPP might serve as a potential target for OTSCC treatment. Consistent with previous studies, our investigations indicate that iASPP is localized to the cytoplasm and nucleus of epithelial cells (12,25,26). However, in other malignancies, such as hepatocellular carcinoma (20), non-small cell lung cancer (21), and prostate cancer (27), iASPP expression is exclusively localized to the cytoplasm. Liu et al. attributed these finding to the different specificity of the anti-iASPP antibodies. Liu et al. also found that ASPP expression is elevated in head and neck squamous cell carcinoma tissues and cell lines and serves as a novel prognostic marker and a potential therapeutic target in HNSCC (28). Furthermore, Jiang et al. found that increased iASPP expression is significantly associated with reduced disease-free survival of ovarian cancer patients and that iASPP expression is an independent prognostic factor (26). All these findings indicate that iASPP plays an important role in mediating the malignant progression of OTSCC and may serve as a prognostic marker predictive of OTSCC clinical outcome.
In the present study, we demonstrate that inhibition of iASPP expression in UM1 and SCC25 cell lines induces G0/G1 arrest and cell apoptosis as well as reduces cell proliferation. Recent reports have indicated that iASPP down-regulation inhibits proliferation and induces cell apoptosis (4,18,21,29,30). Therefore, it is speculated that iASPP contributes to tumorigenesis. Over-expression of iASPP may be involved in tumor establishment and progression, whereas iASPP down-regulation may inhibit tumor development.
As a negative regulator of p53-activating apoptosis, iASPP may serve as a good candidate for targeted cancer therapy (31). UM1 and SCC25 cells express wild-type p53, and the effect of iASPP on apoptosis may be dependent on p53. In addition to its involvement with p53, iASPP participates in the p63-mediated epithelial integrity program by regulating the expression of genes essential for cell adhesion, thereby suggesting that iASPP is a key regulator of epithelial homeostasis (32). In addition, Chikh et al. found that the p53-related protein p73 has similar functions but is rarely lost or mutated in cancer (32). Other reports suggested that iASPP might promote carcinogenesis by other mechanisms in addition to p53 inhibition (19,33). iASPP may act as a novel NF-κB-binding protein, repressing transcriptional activity and preventing the survival of pre-cancerous cells (34). However, further studies are needed to dissect the proapoptotic function and mechanism of iASPP knockdown in OTSCC cells.
In summary, we described the expression pattern of iASPP in OTSCC. iASPP overexpression correlates with malignant tumor progression. siRNA-mediated iASPP down-regulation inhibits proliferation and induces G0/G1 cell cycle arrest and cell apoptosis. Thus, our results suggest a critical role for iASPP in OTSCC tumorigenesis. iASPP may serve as a novel therapeutic target for OTSCC.
Acknowledgements
This work was supported by grants from the Science and Technology Planning Project of Guangdong (2009B060700037, 2009B080701009, 2011B080701014).
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Kim SH, Cho NH, Kim K, et al. Correlational of oral tongue cancer inversion with matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF) expression. J Surg Oncol 2006;93:330-7. [PubMed]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000;408:307-10. [PubMed]
- Bergamaschi D, Samuels Y, Sullivan A, et al. iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53. Nat Genet 2006;38:1133-41. [PubMed]
- Samuels-Lev Y, O’Connor DJ, Bergamaschi D, et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell 2001;8:781-94. [PubMed]
- Liu ZJ, Lu X, Zhong S. ASPP--Apoptotic specific regulator of p53. Biochim Biophys Acta 2005;1756:77-80.
- Bergamaschi D, Samuels Y, O’Neil NJ, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet 2003;33:162-7. [PubMed]
- Slee EA, Lu X. The ASPP family: deciding between life and death after DNA damage. Toxicol Lett 2003;139:81-7. [PubMed]
- Sullivan A, Lu X. ASPP: a new family of oncogenes and tumour suppressor genes. Br J Cancer 2007;96:196-200. [PubMed]
- Ahn J, Byeon IJ, Byeon CH, et al. Insight into the structural basis of pro- and antiapoptotic p53 modulation by ASPP proteins. J Biol Chem 2009;284:13812-22. [PubMed]
- Robinson RA, Lu X, Jones EY, et al. Biochemical and structural studies of ASPP proteins reveal differential binding to p53, p63, and p73. Structure 2008;16:259-68. [PubMed]
- Slee EA, Gillotin S, Bergamaschi D, et al. The N-terminus of a novel isoform of human iASPP is required for its cytoplasmic localization. Oncogene 2004;23:9007-16. [PubMed]
- Bergamaschi D, Samuels Y, O’Neil NJ, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet 2003;33:162-7. [PubMed]
- Yang JP, Hori M, Sanda T, et al. Identification of a novel inhibitor of nuclear factor-kappaB, RelA-associated inhibitor. J Biol Chem 1999;274:15662-70. [PubMed]
- Trigiante G, Lu X. ASPP [corrected] and cancer. Nat Rev Cancer 2006;6:217-26. [PubMed]
- Slee EA, Lu X. The ASPP family: deciding between life and death after DNA damage. Toxicol Lett 2003;139:81-7. [PubMed]
- Nexø BA, Vogel U, Olsen A, et al. Linkage disequilibrium mapping of a breast cancer susceptibility locus near RAI/PPP1R13L/iASPP. BMC Med Genet 2008;9:56. [PubMed]
- Liu ZJ, Cai Y, Hou L, et al. Effect of RNA interference of iASPP on the apoptosis in MCF-7 breast cancer cells. Cancer Invest 2008;26:878-82. [PubMed]
- Zhang X, Wang M, Zhou C, et al. The expression of iASPP in acute leukemias. Leuk Res 2005;29:179-83. [PubMed]
- Lu B, Guo H, Zhao J, et al. Increased expression of iASPP, regulated by hepatitis B virus X protein-mediated NF-κB activation, in hepatocellular carcinoma. Gastroenterology 2010;139:2183-94. [PubMed]
- Chen J, Xie F, Zhang L, et al. iASPP is over-expressed in human non-small cell lung cancer and regulates the proliferation of lung cancer cells through a p53 associated pathway. BMC Cancer 2010;10:694. [PubMed]
- Waltregny D, Bellahcène A, Van Riet I, et al. Prognostic value of bone sialoprotein expression in clinically localized human prostate cancer. J Natl Cancer Inst 1998;90:1000-8. [PubMed]
- He Q, Zhou X, Li S, et al. MicroRNA-181a suppresses salivary adenoid cystic carcinoma metastasis by targeting MAPK-Snai2 pathway. Biochim Biophys Acta 2013;1830:5258-66.
- Ding X, Zhang N, Cai Y, et al. Down-regulation of tumor suppressor MTUS1/ATIP is associated with enhanced proliferation, poor differentiation and poor prognosis in oral tongue squamous cell carcinoma. Mol Oncol 2012;6:73-80. [PubMed]
- Liu Z, Zhang X, Huang D, et al. Elevated expression of iASPP in head and neck squamous cell carcinoma and its clinical significance. Med Oncol 2012;29:3381-8. [PubMed]
- Jiang L, Siu MK, Wong OG, et al. iASPP and chemoresistance in ovarian cancers: effects on paclitaxel-mediated mitotic catastrophe. Clin Cancer Res 2011;17:6924-33. [PubMed]
- Zhang B, Xiao HJ, Chen J, et al. Inhibitory member of the apoptosis-stimulating protein of p53 (ASPP) family promotes growth and tumorigenesis in human p53-deficient prostate cancer cells. Prostate Cancer Prostatic Dis 2011;14:219-24. [PubMed]
- Liu WK, Jiang XY, Ren JK, et al. Expression pattern of the ASPP family members in endometrial endometrioid adenocarcinoma. Onkologie 2010;33:500-3. [PubMed]
- Liu ZJ, Cai Y, Hou L, et al. Effect of RNA interference of iASPP on the apoptosis in MCF-7 breast cancer cells. Cancer Invest 2008;26:878-82. [PubMed]
- Liu ZJ, Xin HM, Chen J, et al. A new strategy to resume the apoptosis activity of p53 in leukemia cell lines retaining wild-type p53. Leuk Res 2007;31:1156-8. [PubMed]
- Cai Y, Qiu S, Gao X, et al. iASPP inhibits p53-independent apoptosis by inhibiting transcriptional activity of p63/p73 on promoters of proapoptotic genes. Apoptosis 2012;17:777-83. [PubMed]
- Chikh A, Matin RN, Senatore V, et al. iASPP/p63 autoregulatory feedback loop is required for the homeostasis of stratified epithelia. EMBO J 2011;30:4261-73. [PubMed]
- Bell HS, Ryan KM. iASPP inhibition: increased options in targeting the p53 family for cancer therapy. Cancer Res 2008;68:4959-62. [PubMed]
- Liu ZJ, Zhang Y, Zhang XB, et al. Abnormal mRNA expression of ASPP members in leukemia cell lines. Leukemia 2004;18:880. [PubMed]
