LRP5 polymorphism—A potential predictor of the clinical outcome in advanced gastric cancer patients treated with EOF regimen
Background
Gastric cancer, the fourth most common and the second most deadly cancer worldwide, is highly prevalent in developing nations with almost two-thirds of the cases occur in developing countries and 42% in China alone (1). Despite the availability of chemotherapeutic agents that may improve survival or quality of life, there is no universally accepted standard chemotherapy regimen for the first-line treatment of patients with advanced and metastatic gastric cancer (MGC) and the median overall survival (OS) remains less than one year. Among palliative treatments for MGC, an anthracycline-containing triplet (ECF and its modifications) (2-4), a docetaxel-containing triplet [DCF regimen: docetaxel, cisplatin (CDDP), and 5-FU] (5), and a platinum and 5-FU doublet are the most common combinations (6).
Despite numerous efforts to identify suitable predictive markers, there is still a lack of accurate biomarkers to discriminate between patients who are likely to respond to combination chemotherapy and those who are not. In recent decades, studies have found that single nucleotide polymorphisms (SNPs) may influence the clinical outcomes of gastric cancer patients (7-9).
Low-density lipoprotein-related receptor 5 (LRP5) is a protein with key functions in canonical Wnt signaling (10). The Wnt/β-catenin signaling pathway is essential for embryonic development and adult tissue homeostasis, consequently mutation of many of the components result in human birth defects, cancer and other diseases. Wnts bind to a member of the Frizzled family of seven-transmembrane proteins and to either LRP5 or LRP6 leading to down-regulation of GSK-3 activity and the initiation of the canonical Wnt/beta-catenin signaling cascade to regulate cell proliferation, differentiation and survival (11-13). The initiation and progression of gastric cancer have been linked to the aberrantly activated Wnt/β-catenin signaling pathway (14-16).
It has been demonstrated that expression of LRP5 is a common event in osteosarcoma, and may serve as a potential novel marker for disease progression in high-grade osteosarcoma (17). Analysis of gene expression profiles also revealed that LRP5 was overexpressed in metastatic primary pancreatic endocrine neoplasms when compared with the nonmetastatic samples, suggesting that LRP5 may also promote tumor metastasis (18). But its role in gastric cancer has not been investigated.
We therefore presumed that variants within the LRP5 gene might be related with chemosensitivity and survival among patients with gastric cancer. To examine this assumption, we retrospectively investigated genetic polymorphism of rs3736228 located in LRP5 gene in advanced Chinese gastric cancer patients who received first-line chemotherapy of EOF regimen.
Methods
Study population
In the present retrospective study, 107 consecutive patients with advanced gastric cancer (AGC) who received first-line chemotherapy of EOF regimen (Intravenous epirubicin 50 mg/m2 was given on day 1, combined with a 2-h intravenous infusion of oxaliplatin 130 mg/m2, and followed by 5-FU 375-425 mg/m2/day as a 24-h continuous infusion for 5 days) were enrolled. Treatment cycles were repeated every 3 weeks until disease progression, unacceptable toxicity, or withdrawal of consent. The tumor responses were evaluated according to the response evaluation criteria in solid tumors (RECIST) guidelines. The blood samples of the patients were collected before treatment and stored in tissue bank of Fudan University Shanghai Cancer center. The study was in compliance with the principles of the Helsinki Accord and all patients signed informed consent form of providing blood sample to the tissue bank.
Genotyping
Genomic DNA was prepared from venous blood using standard phenol chloroform extrac¬tion. We genotyped genetic polymorphism of rs3736228 located at 11:68201295 in LRP5 gene from the HapMap project database (hPFS://www.hapmap.org) and dbSNP (hPFS://www.ncbi.nlm.nih.gov/SNP/). We genotyped the SNP by the TaqMan® assay method using the ABI 7900 DNA detection system (Applied Biosystems, Foster City, California, USA). All probes and primers were designed by the Assay-on-Design service of Applied Biosystems. The standard PCR was performed using the Taqman® Universal PCR Master Mix (Applied Biosystems) reagent.
Statistical analysis
We used SHEsis (19) to analyze allelic and genotypic distributions (20). This online software integrates efficient analysis tools for association studies and implements a Monte Carlo simulation strategy (21). The dis-crepancies of allele and genotype frequency between between disease control [complete response (CR), partial response (PR), stable disease (SD), CR + PR + SD], and progressive disease (PD) patients were compared using χ2 test. Power calculations were performed using the G*Power program (19). All the P values in the study were two-tailed and the significance level was set at P=0.05. Time-dependent variables progression free survival (PFS) and OS were estimated by the Kaplan-Meier method. Log-rank tests were used in univariate analysis for PFS and OS, and a multivariate analysis of prognostic factors using the Cox proportional hazard model was performed.
Results
Patient characteristics
Between May 2009 and June 2012, One hundred and seven unrelated Chinese Han patients with AGC and available blood samples were recruited from Fudan University Shanghai Cancer Center. Patients’ age ranged from 23 to 74 years old, and there were more men than women. Most of the patients had 3 or more tumor sites. For rs3736228, the percentage of patients with CC, CT and TT genotype were 68.2%, 29.0% and 2.8%, respectively. Detailed baseline descriptions of demographic and clinical features stratified by rs3736228 genotype are shown in Table 1.
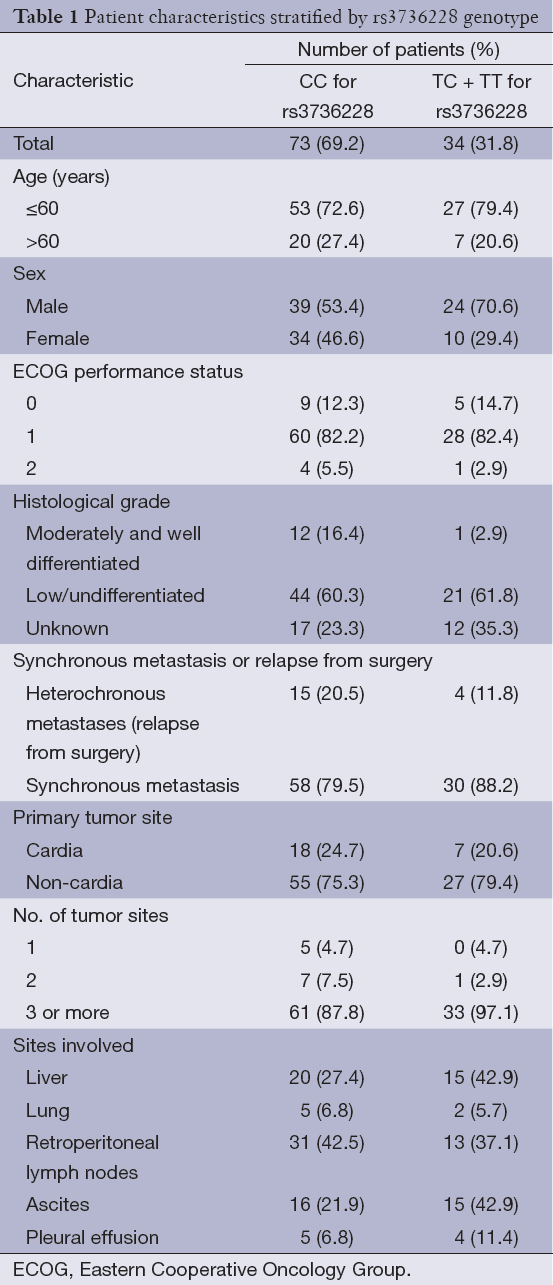
Full table
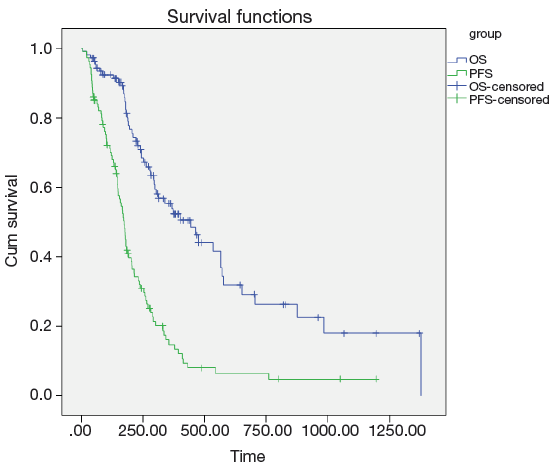
Among all the 107 gastric cancer patients in our study, the responses to the first-line EOF combination chemotherapy were as follows: CR (n=1, 0.9%), PR (n=40, 37.4%), SD (n=47, 43.9%), and PD (n=19, 17.8%). At the median follow-up duration of 28.4 (range, 7.7-69.0) months, the median PFS and OS for all the patients were 5.8 (range, 5.1-6.4) months and 14.8 (range, 10.2-19.4) months, respectively (Figure 1).
Genotype frequency and effects on response to chemotherapy
Genotype distributions of the SNP showed no significant deviations from Hardy-Weinberg equilibrium in either disease control (CR + PR + SD) or PD patients. The allele and genotype frequencies of the SNP are listed in Table 2. For genetic polymorphism of rs3736228, there were statistically significant differences in allele frequencies between the 88 disease control patients and the 19 disease progressive patients (P=0.002). The C allele and CC genotype of rs3736228 were significantly less common in the disease progressive group compared to the disease control group (allele, 65.8% versus 86.4%, OR 0.304, 95% CI: 0.137-0.673, P value <0.001; genotype, 31.6% versus 76.1%, P value =0.002). The CC genotype was significantly correlated with a higher disease control rate to the combination chemotherapy when compared to the CT and TT genotypes (89.3% for CC genotype vs. 61.8% for CT and TT genotypes, P value <0.001).
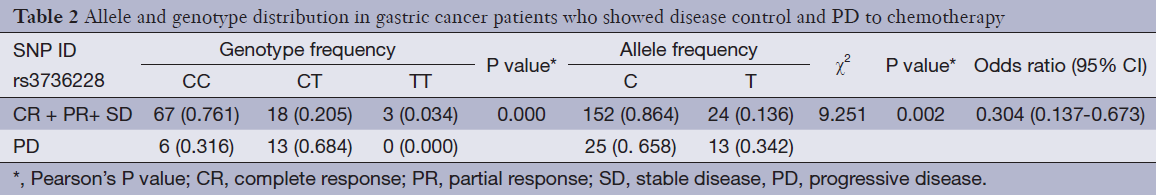
Full table
Clinicopathological features, genotype and survival analysis
Among the clinical features [age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, histological grade, synchronous or heterochronous metastases, primary tumor site, liver or lung metastasis, retroperitoneal lymphonode metastasis, ascites, pleural effusion and No. of tumor sites], univariate analysis showed that histological grade, retroperitoneal lymphonode metastasis, ascites and number of tumor sites were the factors associated with the PFS. Liver metastasis, ascites and pleural effusion were the factors associated with the OS (Table 3).
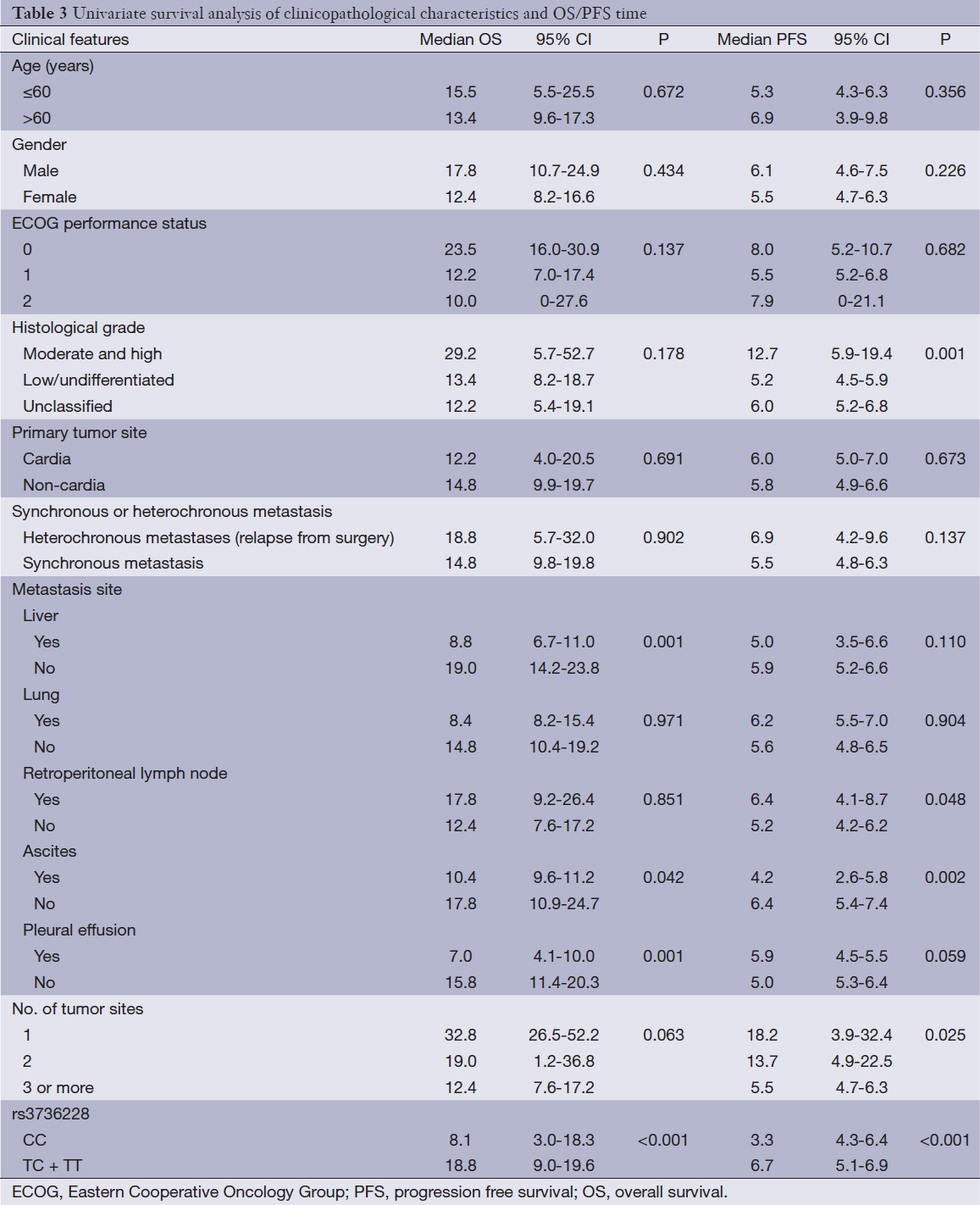
Full table
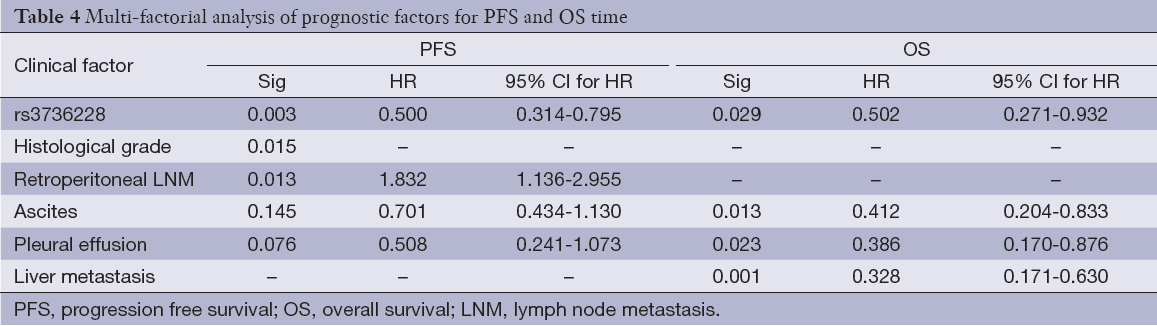
Univariate analysis also showed that the LRP5 polymorphism was significantly associated with the PFS and OS (Figure 2). For rs3736228, the PFS for the patients with the TC and TT genotypes were worse than for the patients with the CC genotype (3.3 months and 6.7 months, respectively, Hazard ratio =0.454, 95% CI: 0.289-0.714, log-rank P value <0.001). Moreover, the OS for the patients with the TC and TT genotypes of rs3736288 were worse than for the patients with the CC genotype (8.1 months and 18.8 months, respectively, Hazard ratio =0.363, 95% CI: 0.203-0.649, log-rank P value <0.001).
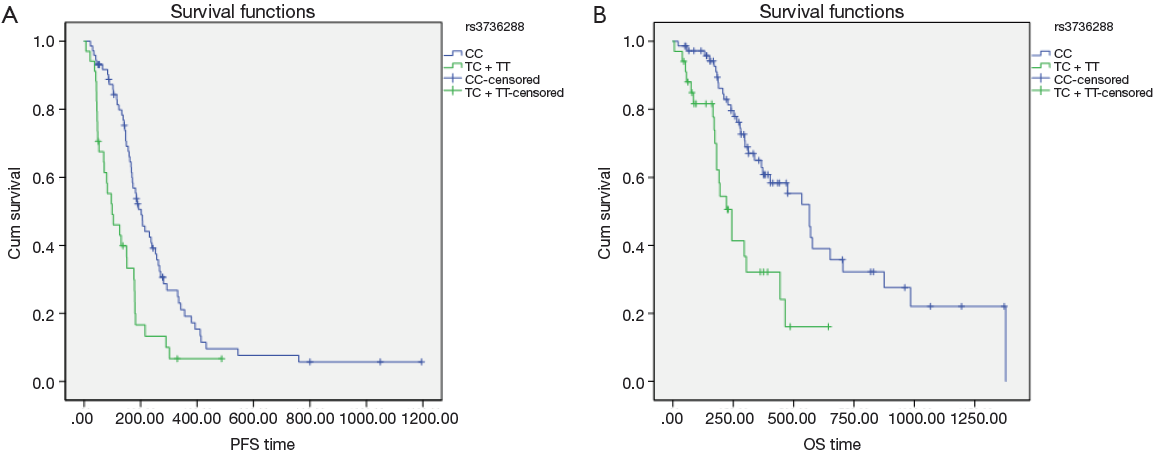
The multivariate model included rs3736228 and the clinicopathologic features listed in Table 4. For there were significant interactions among number of metastatic sites and sites of metastases, we did not include number of metastatic sites in the final multivariate model. The multivariate analysis also showed that rs3736228 was significantly associated with the PFS (Hazard ratio =0.500, 95% CI: 0.314-0.795, P value =0.003) and OS (Hazard ratio =0.502, 95% CI: 0.271-0.932, P value =0.029). Among the clinical and the pathologic features, histological grade and retroperitoneal lymphonode metastasis also showed the association with PFS, liver metastasis, ascites and pleural effusion also showed the association with OS. There were no violations of the proportional hazard assumptions in the Cox model.
Full table
Discussion
To the best of our knowledge, this is the first study of LRP5 gene polymorphism on treat-ment response and survival in AGC patients from the Chinese mainland. Our findings suggested that LRP5 polymorphism have a predictive effect on the response to first-line chemotherapy of EOF regimen, and associate with the PFS and OS, even after adjusting for clinical features. And this result has not been reported before.
Deregulation of the WNT signalling pathway has been implicated in numerous cancers; however, only in colorectal cancer (CRC) it has been shown to be the driving mutation. More than 90% of colorectal tumors (colon cancer cells) harbor activating mutations in APC or CTNNB1 (β-catenin) (21,22). In contrast to CRC, the mutations that drive gastric cancer are much more unclear. Although mutations of APC and β-catenin are frequently observed in CRC, they are rare in gastric cancer.
Recently, it has been demonstrated that LRP5 and 6 are indispensable coreceptors of the canonical Wnt pathway by interacting with several key components of the Wnt signaling pathway. It has been shown that LRP5 is required for Wnt1-induced mammary tumor formation (23), and that LRP5 is overexpressed in some types of malignant tissues (17,18). Furthermore, it has been established that LRP5/6 antagonist Dkk1 is a proapoptotic tumor suppressor (24,25). These data support the notion that LRP5 and 6 are potential oncogenic proteins. However, the molecular and cellular mechanisms underlying LRP5/6-induced Wnt/β-catenin signaling and tumorigenesis remain to be elucidated. And the role of LRP5 in gastric cancer is unknown.
SNP rs3736228 lies in chromosome 11 and was located on LRP5 gene. rs3736228 was reported to associated with bone mineral density (26,27). The correlation between rs3736228 and cancer has not been investigated before. Our work showed that the CC genotype of rs3736228 was significantly correlated with a higher disease control rate, a longer PFS and OS time compared with the patients with the TC and TT genotypes of rs3736228. Additionally, these effects also remained in stratification analyses. The results suggest that the polymorphism of LRP5 gene play an important role in the chemosensitivity and clinical outcome among AGC patients. Clearly, LRP5 pathway harbors great potential for future applications in gastric cancer diagnostics, staging, and therapy.
Gastric cancers are deadly cancers that are often diagnosed at a stage when treatment options are limited and have limited effectiveness, therefore identifying genetic variants that predict survival and chemotherapy response for this cancer is particularly important. Although our results do not establish the biological mechanism by which LRP5 affects chemosensitivity and survival, our results suggest that LRP5 genetic variants should be considered as promising prognostic and predictive factors for gastric cancer, and warrant further study. rs3736228 may serve as a novel biomarker for the efficacy of first-line chemotherapy with EOF regimen in AGC patients. Moreover, LRP5 can serve as potential novel targets for developing anti-cancer drugs.
It is well known that some clinicopathological characteristics, such as tumor stage, PS status and No. of tumor sites, are associated with prognosis of cancer patients. Among the clinical features, histological grade and retroperitoneal lymphonode metastasis had been identified as independent prognostic factors of the PFS in our study, liver metastasis, ascites and pleural effusion had been identified as independent prognostic factors of the OS, which had been confirmed in other studies (20,28).
The present study has some limitations. First, our sample size is relatively small and it’s a retrospective study, which may lead to statistical bias in the analyses. Replicating studies with larger samples and with prospective design will be necessary to clarify the correlation between LRP5 gene and gastric cancer. Second, we didn’t identify the exact mechanism by which LRP5 SNP influence response and gastric cancer survival. Basic researches are needed to elucidate the molecular and cellular mechanisms underlying LRP5-induced Wnt/β-catenin signaling and tumorigenesis and by which LRP5 affects chemosensitivity in gastric cancer cells. Third, we used the only one SNP in our investigation, which did not include all representative SNPs in the entire gene. The effect of a single SNP on death risk or clinical outcomes may be limited as one would expect, and some other functional SNPs, which may influence survival, may have been missed and need to be investigated in larger studies. Forth, genetic studies to date have focused on subjects of Asian ethnicity, and further research needs to be undertaken in other ethnic groups. Fifth, there was no independent replication population readily available for the present study, which should be done in the future to validate our findings.
Conclusions
In conclusion, our results firstly suggest that LRP5 polymorphisms of SNP rs3736228 might be predictive of the response to chemotherapy and correlate with the PFS and OS in patients with AGC in the Chinese Han population treated with first-line chemotherapy of EOF regimen. The results of this study indicate a genetic profile that influences the response rate and survival of patients with advanced disease in addition to known clinical and pathologic features. This information does not simply address the analysis of a novel predictive marker, but rather, it is strictly related to the possible development and optimization of target therapies that exploit the LRP5 pathway. Replication and confirmation of our findings in independent series would open new perspectives in the management and the treatment of patients with advanced gastric cancer.
Acknowledgements
We appreciate the contribution of the members participating in this study. This work was supported by the Natural Science Foundation of Shanghai (Grant No. 13ZR1408200).
Disclosure: The authors declare no conflict of interest.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [PubMed]
- Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 2002;20:1996-2004. [PubMed]
- Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997;15:261-7. [PubMed]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. [PubMed]
- Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009;20:666-73. [PubMed]
- Bashash M, Shah A, Hislop G, et al. Genetic polymorphisms at TIMP3 are associated with survival of adenocarcinoma of the gastroesophageal junction. PLoS One 2013;8:e59157. [PubMed]
- Li Y, Liu Z, Liu H, et al. ERCC1 and ERCC2 variants predict survival in gastric cancer patients. PLoS One 2013;8:e71994. [PubMed]
- Wang Z, Chen JQ, Liu JL, et al. Polymorphisms in ERCC1, GSTs, TS and MTHFR predict clinical outcomes of gastric cancer patients treated with platinum/5-Fu-based chemotherapy: a systematic review. BMC Gastroenterol 2012;12:137. [PubMed]
- Joiner DM, Ke J, Zhong Z, et al. LRP5 and LRP6 in development and disease. Trends Endocrinol Metab 2013;24:31-9. [PubMed]
- Moon RT, Kohn AD, De Ferrari GV, et al. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 2004;5:691-701. [PubMed]
- Tamai K, Semenov M, Kato Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature 2000;407:530-5. [PubMed]
- Wehrli M, Dougan ST, Caldwell K, et al. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 2000;407:527-30. [PubMed]
- Cai C, Zhu X. The Wnt/β-catenin pathway regulates self-renewal of cancer stem-like cells in human gastric cancer. Mol Med Rep 2012;5:1191-6. [PubMed]
- Li H, Mo J, Jia G, et al. Activation of Wnt signaling inhibits the pro-apoptotic role of Notch in gastric cancer cells. Mol Med Rep 2013;7:1751-6. [PubMed]
- Mao J, Fan S, Ma W, et al. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis 2014;5:e1039. [PubMed]
- Hoang BH, Kubo T, Healey JH, et al. Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer 2004;109:106-11. [PubMed]
- Hansel DE, Rahman A, House M, et al. Met proto-oncogene and insulin-like growth factor binding protein 3 overexpression correlates with metastatic ability in well-differentiated pancreatic endocrine neoplasms. Clin Cancer Res 2004;10:6152-8. [PubMed]
- Benjamini Y, Drai D, Elmer G, et al. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125:279-84. [PubMed]
- Chau I, Norman AR, Cunningham D, et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 2004;22:2395-403. [PubMed]
- Cebrat M, Strzadała L. Role of Wnt signaling and APC protein in the etiology of colorectal cancer. Postepy Hig Med Dosw 2001;55:513-24. [PubMed]
- Scholer-Dahirel A, Schlabach MR, Loo A, et al. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A 2011;108:17135-40. [PubMed]
- Lindvall C, Evans NC, Zylstra CR, et al. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem 2006;281:35081-7. [PubMed]
- González-Sancho JM, Aguilera O, García JM, et al. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene 2005;24:1098-103. [PubMed]
- Mao B, Wu W, Davidson G, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 2002;417:664-7. [PubMed]
- Canto-Cetina T, Polanco Reyes L, González Herrera L, et al. Polymorphism of LRP5, but not of TNFRSF11B, is associated with a decrease in bone mineral density in postmenopausal Maya-Mestizo women. Am J Hum Biol 2013;25:713-8. [PubMed]
- Tran BN, Nguyen ND, Eisman JA, et al. Association between LRP5 polymorphism and bone mineral density: a Bayesian meta-analysis. BMC Med Genet 2008;9:55. [PubMed]
- Lee SS, Lee JL, Ryu MH, et al. Combination chemotherapy with capecitabine (X) and Cisplatin (P) as first line treatment in advanced gastric cancer: experience of 223 patients with prognostic factor analysis. Jpn J Clin Oncol 2007;37:30-7. [PubMed]
