Prognostic prediction in gastric cancer patients without serosal invasion: comparative study between UICC 7th edition and JCGS 13th edition N-classification systems
Introduction
There are currently two main staging systems for gastric cancer, the Japanese Classification of Gastric Carcinoma (JCGC) and the Union International Cancer Control (UICC-TNM), which define disease stage mainly according to tumor stage (T-stage) and lymph node stage (N-stage). Many studies have shown that N-stage and T-stage are the two most important factors for assessing the extent of disease, determining prognosis, and providing guidance for therapeutic strategies in gastric cancer patients (1-5).
The prognosis of gastric cancer patients with serosal invasion is very poor. Even after radical resection of primary tumors, approximately 20% of these patients die due to recurrence (4-6). Peritoneal dissemination represents the most common type of recurrence (5). Peritoneal metastasis is known to be the most common non-curative factor, and serosal invasion is a critical predisposing factor for peritoneal metastasis in advanced gastric cancer (7). The main mechanism underlying peritoneal metastasis is thought to involve free cancer cells that are exfoliated from tumor cells in the gastric serosa, and the frequency of peritoneal metastasis therefore increases once tumor cells penetrate the serosa (8,9).
Lymph node involvement is the most important independent prognostic factor for gastric cancer (2-4). The JCGC (13th edition) N-classification system is based on the sites of involved lymph nodes, whereas the new TNM (7th edition) N-staging system is based on the number of metastatic lymph nodes (10,11). The purpose of this study was to compare N-classification systems (TNM 7th ed. vs. JCGC 13th ed.) and to evaluate the prognostic value of the N-number of the TNM 7th ed. We also analyzed the effect of serosal invasion on prognosis and attempted to clarify the impact of lymph node metastasis on prognosis in gastric cancer patients.
Materials and methods
Clinical samples
A total of 1,115 patients with primary gastric cancer who underwent curative gastric resection with D2 lymphadenectomy at The First Affiliated Hospital of Dalian Medical University between January 2000 and January 2008 were included in this study (Table 1). The surgical procedure was defined as curative resection (R0, absence of residual tumor as determined both macroscopically and microscopically). All of the patients provided written, informed consent to participate based on a document approved by our institutional ethics committee. None of the patients received preoperative adjuvant therapy. To reduce any effects directly related to surgery, patients whose preoperative examination revealed distant metastasis or those who died within 6 months after surgical resection were excluded from this study. All surgical resections were performed by a single experienced surgical team. All clinicopathologic variables were classified according to the JCGC (13th ed.) and TNM (7th ed.). The clinicopathologic data and long-term survival of the two groups were analyzed retrospectively. The follow-up period was calculated from the date of surgery until January 2013, and all patients were followed up for at least 5 years after surgery. The mean follow-up period was 71 months (range of 13-142 months). The follow-up data were obtained from census registry certificates and outpatient records. Only cancer-related deaths were considered in survival analysis in this study.
Follow-up evaluation consisted of assessments of patient history, physical examination, laboratory tests, including tumor marker tests (carcinoembryonic antigen, alpha-fetoprotein, carbohydrate antigen 12-5 and carbohydrate antigen 19-9), chest radiography, endoscopy, computed tomography (CT), and bone scintigraphy. These assessments were repeated every 6 months until the third postoperative year, and then every year thereafter for at least 5 years. Magnetic resonance imaging, CT of the brain or chest, and positron emission tomography were performed only when indicated.
Statistical analysis
Group differences were statistically analyzed using the χ2 test and t-test. Cumulative survival curves were constructed by the Kaplan-Meier method, and the differences between the curves were analyzed by the log-rank test. Multivariate analysis was based on the Cox regression model (the N-stages according to the JCGC13th and TNM7th systems were analyzed separately). Statistical procedures were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
The clinicopathologic features of the patients with and without serosal invasion are shown in Table 1. Serosal invasion was identified in 212 of 1,115 patients (19.0%), and it was significantly associated with lymph node metastasis according to the JCGC13th (P<0.001) and TNM7th (P<0.001) systems. However, age, gender, tumor location, tumor size, Borrmann type and histological type did not significantly differ (Table 1).
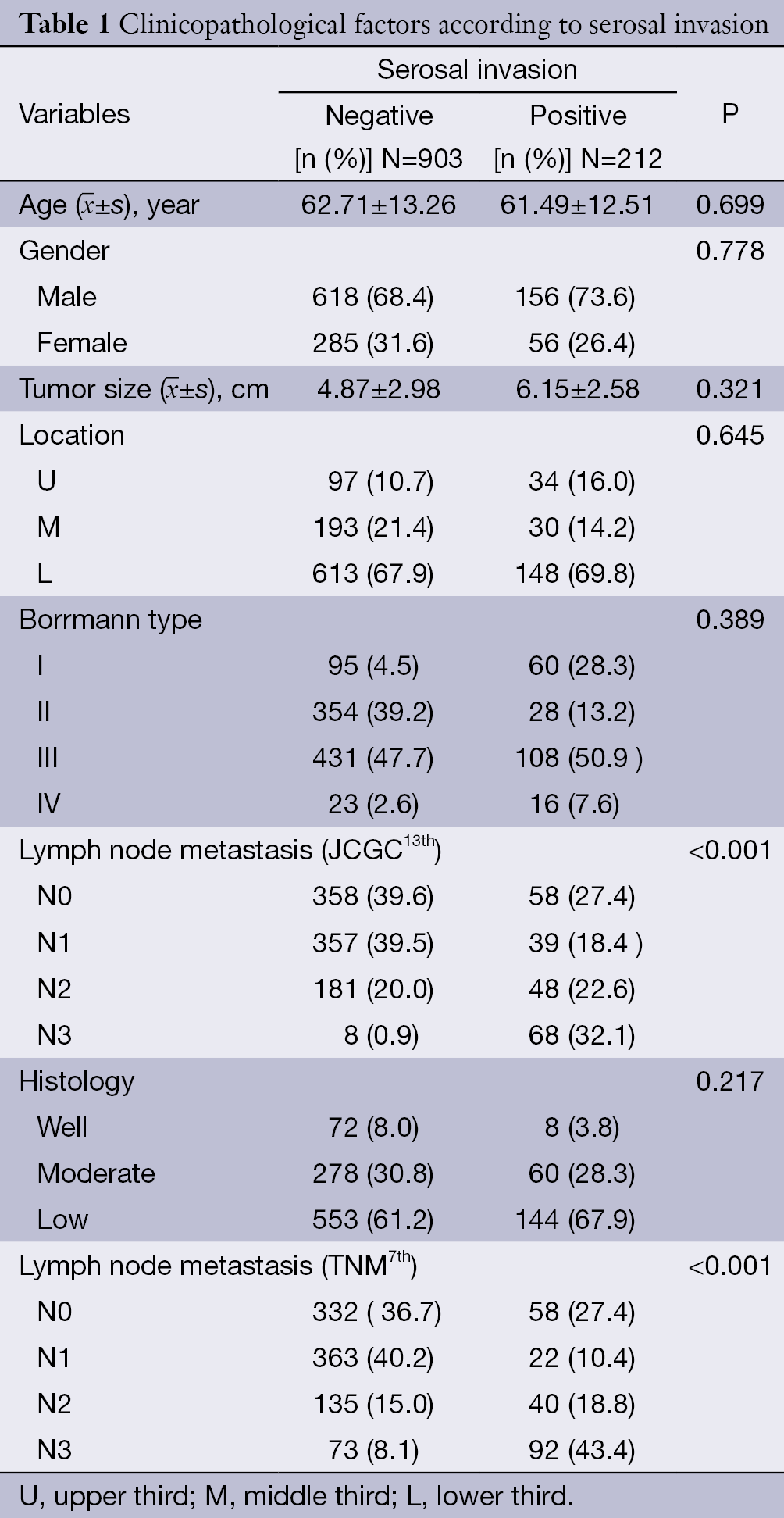
Full table
The TNM N-stages were equally divided within the T subgroups similar to the JCGC N-stages. As shown in Table 2, 416 patients (37.3%) were classified as pN0. According to the JCGC 13th edition, the N+ cases were subclassified as follows: 394 (35.3%) pN1, 229 (20.5%) pN2, and 76 (6.8%) pN3. According to the TNM 7th edition, the same cases were divided as follows: 372 (33.4%) pN1, 162 (14.5%) pN2, and 165 (14.8%) pN3.

Full table
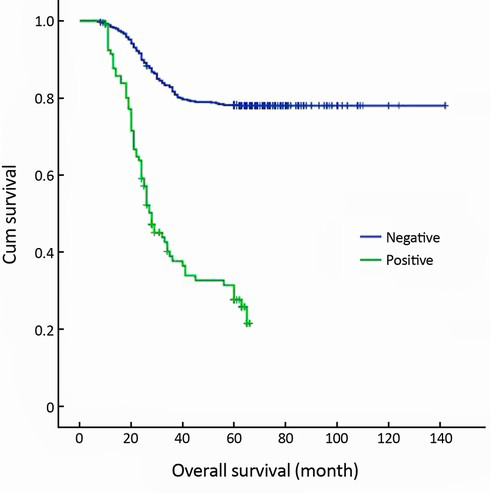
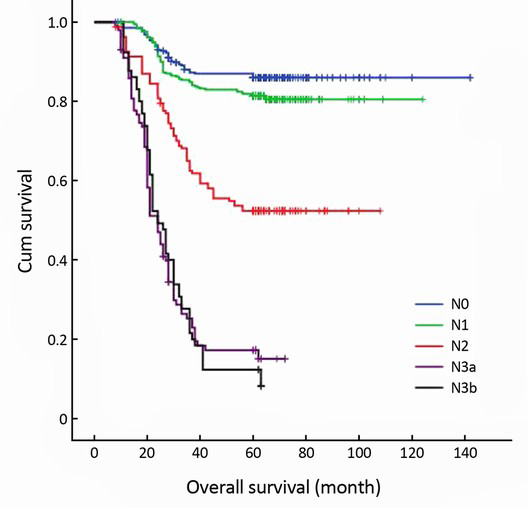
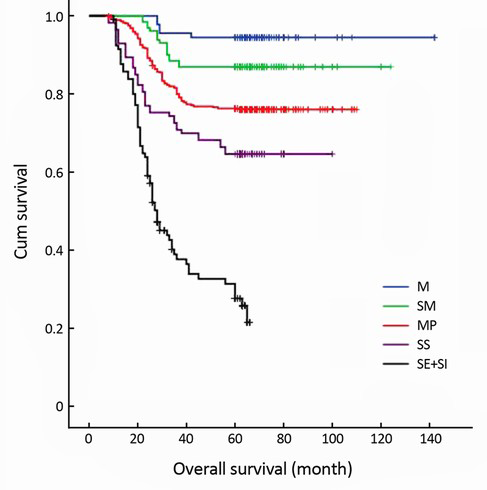
The 5-year survival rate of the serosal invasion-negative patients (78.2%) was significantly higher than that of the serosal invasion-positive patients (31.1%) (P<0.001) (Figure 1). According to the T-stages, the 5-year survival rates were as follows: mucosa (M)-stage 94.4%, submucosa (SM)-stage 86.9%, muscularis propria (MP)-stage 76.3%, subserosa (SS)-stage 64.6%, and serosa and adjacent structures (SE + SI)-stage 31.1% (Figure 2). According to the JCGC (13th ed.) system, the 5-year survival rates were as follows: N0-stage 86.5%, N1-stage 74.9%, and N2-stage 47.2%, and N3-stage 11.8% (P<0.001) (Figure 3). The TNM (7th ed.) system revealed that the 5-year survival rates were as follows: N0-stage 86.5%, N1-stage 80.9%, N2-stage 53.1%, N3a-stage 20.0%, and N3b-stage 9.2% (P<0.001) (Figure 4).
Univariate survival analysis indicated that the statistically significant prognostic factors affecting 5-year survival rate were tumor size (P=0.024), depth of invasion (P<0.001), the 13th JCGC PN stage (P<0.001), and the 7th TNM PN stage (P<0.001) (Table 3).

Full table
Multivariate Cox regression survival analysis revealed that depth of invasion (P=0.013), the 13th JCGC PN stage (P<0.001), and the 7th TNM PN stage (P<0.001) were meaningful independent prognostic factors for the serosal invasion-negative gastric cancer patients (Table 4).

Full table
Discussion
Cancer staging systems are the most important tools for treatment planning in oncology and for assessing patient prognosis. The JCGC system defines N stage by the site of lymph node metastasis relative to the primary tumor (10). The UICC (5th ed., 1997) system is based on the number of metastatic lymph nodes (12). Before the 5th edition was published, N classification of gastric cancer was based on the anatomic locations of metastatic lymph nodes (13). Cancer staging systems should be simple, reproducible, and possess prognostic relevance. The 13th JCGC N-classification system, which is frequently used worldwide, is based on the extent of lymphatic metastasis and anatomic location, but this system is complicated and not always applicable. Many studies have reported that the UICC N staging system, which is based on the number of metastatic lymph nodes, is superior to that of the JCGC in terms of feasibility, objectivity, reproducibility and accuracy of prognostic prediction (14,15). Thus, the new 14th JCGC and 7th TNM staging systems are both based on the number metastatic lymph nodes and are considered to be better prognostic determinants than the former Japanese system (9,16).
T-stage and N-stage have been proven to be the most important factors influencing survival in gastric cancer patients, and they are included in the JCGC and UICC systems (9,16). N stage is the most important survival predictor for gastric cancer. To date, three main N-stage classifications have shown utility in predicting prognosis of gastric cancer patients worldwide, including classifications based on the number of positive nodes, on the location of positive nodes, and on the ratio between metastatic and examined nodes (16-18).
In this study, we found 394 (35.3%) pN1, 229 (20.5%) pN2, and 76 (6.8%) pN3 cases according to the JCGC 13th ed. According to the TNM 7th ed., the same cases were divided as follows: 372 (33.4%) pN1, 162 (14.5%) pN2, and 165 (14.8%) pN3. The 13th JCGC revealed the following 5-year survival rates: N0-stage 86.5%, N1-stage 74.9%, N2-stage 47.2%, and N3-stage 11.8% (P<0.001). The 7th TNM system indicated the following 5-year survival rates: N0-stage 86.5%, N1-stage 80.9%, N2-stage 53.1%, N3a-stage 20.0%, and N3b-stage 9.2% (P<0.001). Therefore, our results suggest that both the 13th JCGC N staging system and the 7th TNM N staging system can accurately estimate prognosis of gastric cancer patients but that the 7th TNM N staging system is simpler and easier to use.
Many studies have reported that the 5-year survival rate of gastric cancer patients with serosal invasion is very poor (4,5,7). When gastric carcinomas have invaded the serosa or surrounding organs and tissues, curative resection may be difficult, and it is not associated with a good prognosis (19-21). In this study, out of all of the gastric cancer patients who underwent curative gastric resection with D2 lymphadenectomy, the 5-year survival rate for the serosal invasion-negative patients (78.2%) was significantly higher than that of the serosal invasion-positive patients (31.1%) (P<0.001). The 5-year survival rates were as follows: M-stage 94.4%, SM-stage 86.9%, MP-stage 76.3%, SS-stage 64.6%, and SE + SI-stage 31.1%. Univariate survival analysis revealed that the depth of invasion was a significant prognostic factor affecting the 5-year survival rate (P<0.001).
Conclusions
In conclusion, the prognosis of gastric cancer patients with serosal invasion is very poor. Both the 13th JCGC N staging system and the 7th TNM N staging system are able to accurately estimate the prognosis of gastric cancer patients, but the 7th TNM system is simpler and easier to use.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lee IS, Park YS, Ryu MH, et al. Impact of extranodal extension on prognosis in lymph node-positive gastric cancer. Br J Surg 2014;101:1576-84. [PubMed]
- Deng J, Liang H, Sun D, et al. Suitability of 7th UICC N stage for predicting the overall survival of gastric cancer patients after curative resection in China. Ann Surg Oncol 2010;17:1259-66. [PubMed]
- Chae S, Lee A, Lee JH. The effectiveness of the new (7th) UICC N classification in the prognosis evaluation of gastric cancer patients: a comparative study between the 5th/6th and 7th UICC N classification. Gastric Cancer 2011;14:166-71. [PubMed]
- Mita K, Ito H, Fukumoto M, et al. Surgical outcomes and survival after extended multiorgan resection for T4 gastric cancer. Am J Surg 2012;203:107-11. [PubMed]
- Carboni F, Lepiane P, Santoro R, et al. Extended multiorgan resection for T4 gastric carcinoma: 25-year experience. J Surg Oncol 2005;90:95-100. [PubMed]
- Fujiwara Y, Okada K, Hanada H, et al. The clinical importance of a transcription reverse-transcription concerted (TRC) diagnosis using peritoneal lavage fluids in gastric cancer with clinical serosal invasion: a prospective, multicenter study. Surgery 2014;155:417-23. [PubMed]
- Kunisaki C, Akiyama H, Nomura M, et al. Surgical outcomes in patients with T4 gastric carcinoma. J Am Coll Surg 2006;202:223-30. [PubMed]
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989;8:98-101. [PubMed]
- Fokas E, Engenhart-Cabillic R, Daniilidis K, et al. Metastasis: the seed and soil theory gains identity. Cancer Metastasis Rev 2007;26:705-15. [PubMed]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer 1998;1:10-24. [PubMed]
- Fisseler-Eckhoff A. New TNM classification of malignant lung tumors 2009 from a pathology perspective. Pathologe 2009;30 Suppl 2:193-9. [PubMed]
- Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997;80:1803-4.
- Hermanek P, Scheibe O, Spiessl B, et al. TNM classification of malignant tumors: the new 1987 edition. Radiobiol Radiother (Berl) 1987;28:845-6. [PubMed]
- Kawaguchi T, Komatsu S, Ichikawa D, et al. Comparison of prognostic compatibility between seventh AJCC/TNM of the esophagus and 14th JCGC staging systems in Siewert type II adenocarcinoma. Anticancer Res 2013;33:3461-5. [PubMed]
- Celen O, Yildirim E, Gülben K, et al. Prediction of survival in gastric carcinoma related to lymph node grading by the new American Joint Committee on Cancer/Union International Contre le Cancer System or the Japanese system. Eur J Surg Suppl 2003;33-9. [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [PubMed]
- Zhou Y, Zhang J, Cao S, et al. The evaluation of metastatic lymph node ratio staging system in gastric cancer. Gastric Cancer 2013;16:309-17. [PubMed]
- Dong P. Radical gastrectomy for D2 distal gastric cancer. Chin J Cancer Res 2013;25:468-70. [PubMed]
- Ozer I, Bostanci EB, Orug T, et al. Surgical outcomes and survival after multiorgan resection for locally advanced gastric cancer. Am J Surg 2009;198:25-30. [PubMed]
- Cheng CT, Tsai CY, Hsu JT, et al. Aggressive surgical approach for patients with T4 gastric carcinoma: promise or myth? Ann Surg Oncol 2011;18:1606-14. [PubMed]
- Dhar DK, Kubota H, Tachibana M, et al. Prognosis of T4 gastric carcinoma patients: an appraisal of aggressive surgical treatment. J Surg Oncol 2001;76:278-82. [PubMed]