Familial nasopharyngeal carcinomas possess distinguished clinical characteristics in southern China
Introduction
Nasopharyngeal carcinoma (NPC) has unique epidemiological and biological characteristics. While age-standardized incidence rates are generally low across most of the world (<1/100,000), making it a rare cancer among Caucasians from North America and Europe, the rates are relatively high among the Chinese in southern China and Southeast Asia (1,2). In fact, the incidence rate per 100,000 is as high as over 40 among men in the Cantonese-speaking population in Guangdong province, which is the highest in the world (3,4). Familial clustering of NPC has been widely documented in both high- and low-risk regions (5-11). A typical example is the Ye-Liang family in Guangdong province, in which 15 of 109 family members across four generations had NPC (12). The geographic and familial clustering of this particular malignancy suggests that a heritable factor may play an important role in its etiology. It also suggested that the clinical characteristics of NPC may differ between the familial and sporadic types. Previous reports on familial clustering of NPC mainly focus on differences in epidemiological and general clinical characteristics between the familial and sporadic types (13,14), and little is known about the clinical characteristics of familial NPC. Therefore, in the present study, we compared clinical characteristics between familial NPCs and sporadic ones.
Materials and methods
Patient population
We randomly selected cases from NPC patients hospitalized at the Sun Yat-Sen University Cancer Center between January 1991 and December 2001. The enrollment criteria were pathological confirmation of newly diagnosed NPC without distant organ metastasis; only first course of radical radiotherapy (RT) completed; pre-treatment nasopharyngeal CT scanning images available; and residence within Guangdong province. In total, 7,944 NPC patients from Guangdong province were treated at Sun Yat-sen Cancer Center during the study period. Of these, 1,504 patients with combination treatment were excluded, leaving 6,440 patients with RT alone. A total of 1,073 cases were randomly selected from among these 6,440 NPC cases, and every patient’s medical record was reviewed. We further excluded 79 metastasis cases, 41 recurrence cases, and 20 cases with no clear information from NPC-related family history records. Finally, 933 NPC patients who received RT were recruited. Each patient was followed up via medical record examination and telephonic communication, from the date of pathological diagnosis to the date of death, loss to follow-up, or December 31, 2007, whichever occurred first. All patients were staged according to the 1992 Staging System followed in China (15).
Definition of familial NPCs
The family history of three generations (e.g., from one ascendant generation to one descendant generation) was retrospectively evaluated from the patients’ medical records. The NPC patients were divided into three groups according to the number of affected individuals among their relatives in these three generations: sporadic NPC (no other affected individuals in the family), low-frequency familial NPC (one additional NPC patient over the three generations), and high-frequency NPC (two or more additional affected individuals over the three generations).
Radiotherapy
Of the 993 patients, 215 received split RT while 672 received continuous conventional RT. The primary tumor was irradiated at a dose of 66-70 Gy. Additional (boost) RT of 8-12 Gy was administered for residual tumors and destructed skull bases. The neck received 50-70 Gy depending on lymph node involvement: 50 Gy was administered in case of node negativity and 60-70 Gy in case of node positivity. A daily fraction of 2.0 Gy and 5 fractions per week were delivered using cobalt-60 or linear accelerator. The standard RT technique involved opposing lateral facio cervical fields to cover the nasopharynx and upper cervical lymphatic drainage region, with 1 lower anterior cervical field to cover the lower cervical region. After 36-40 Gy was administered, opposing lateral preauricular fields were used to cover the nasopharyngeal region. When the nasal cavity or post-styloid area had been invaded, an anterior facial or postauricular field was added as a supplementary field.
Outcome analysis
Possible differences in the clinical characteristics of familial and sporadic NPC cases were investigated using the Pearson chi-square test (sex and family history of NPC), while analysis of variance was used for continuous variables (age). The Kruskal-Wallis test was used for ranked data [tumor stage, node stage, clinical stage, and titers of IgA antibodies against Epstein-Barr virus (EBV) capsid antigen (VCA/IgA) and early antigen (EA/IgA)]. Age was compared between any two groups using the least significant difference (LSD) test. Overall survival (OS), locoregional recurrence-free survival (LRFS), distant metastasis-free survival (DMFS) and disease-free survival (DFS) in the three NPC groups were calculated using Kaplan-Meier analysis. Differences between the potential prognostic subgroups were tested for statistical significance using the log-rank test, and P<0.05 was considered statistically significant. Demographic, clinical, and treatment covariates with P<0.05 in the univariate analysis were included in the multivariate analysis conducted using Cox proportional hazard model to test the significance of independent prognostic risk factors. Finally, to assess the potential effects of some covariates on the association between familial history and NPC survival, stratified analysis was individually performed by age, clinical stage, radiation technique, radiation dose, and diagnostic time. All statistical analyses were conducted using SPSS 11.0 (SPSS Inc., Chicago, IL, USA).
Results
Frequency and general clinical characteristics of familial NPCs
The male to female ratio in the 993 NPC patients was 3.3 to 1 (763 men and 230 women). A history of familial NPC was found in 131 (13.2%) patients, and low-frequency familial NPC was more common (83.8%) than high-frequency familial NPC (16.2%) among these patients. Table 1 shows the general characteristics of the examined cases. The average age at diagnosis was 46 years in both the sporadic NPC and low-frequency familial NPC groups, with no significant differences. However, at 39 years, the average patient age in the high-frequency familial NPC group was significantly lower than that in the sporadic NPC (P=0.014) and low-frequency familial NPC groups (P=0.026). No significant differences were found among the three groups in gender ratio, pathological classification, anatomical location of the primary tumor, size of the largest cervical node, clinical stage, or titers of VCA/IgA and EA/IgA (Table 1).

Full table
Effect of family history on NPC survival
The median follow-up period was 76 months (range, 5-209 months). The 5-year OS rates (67%, 70%, and 61%; P=0.515) (Figure 1A), DMFS (76%, 77% and 64%; P=0.591) (Figure 1B), and DFS (54%, 64%, and 55%; P=0.128) (Figure 1C) did not differ significantly among the sporadic, low-frequency, and high-frequency familial NPC groups, respectively.

The 5-year LRFS for all patients was 73%, and the LRFS tended to increase in the order sporadic NPCs (70%), low-frequency familial NPCs (83%), and high-frequency familial NPCs (87%). Figure 1D showed the recurrence-free survival curves among the three groups, which showed significant difference (P=0.009).
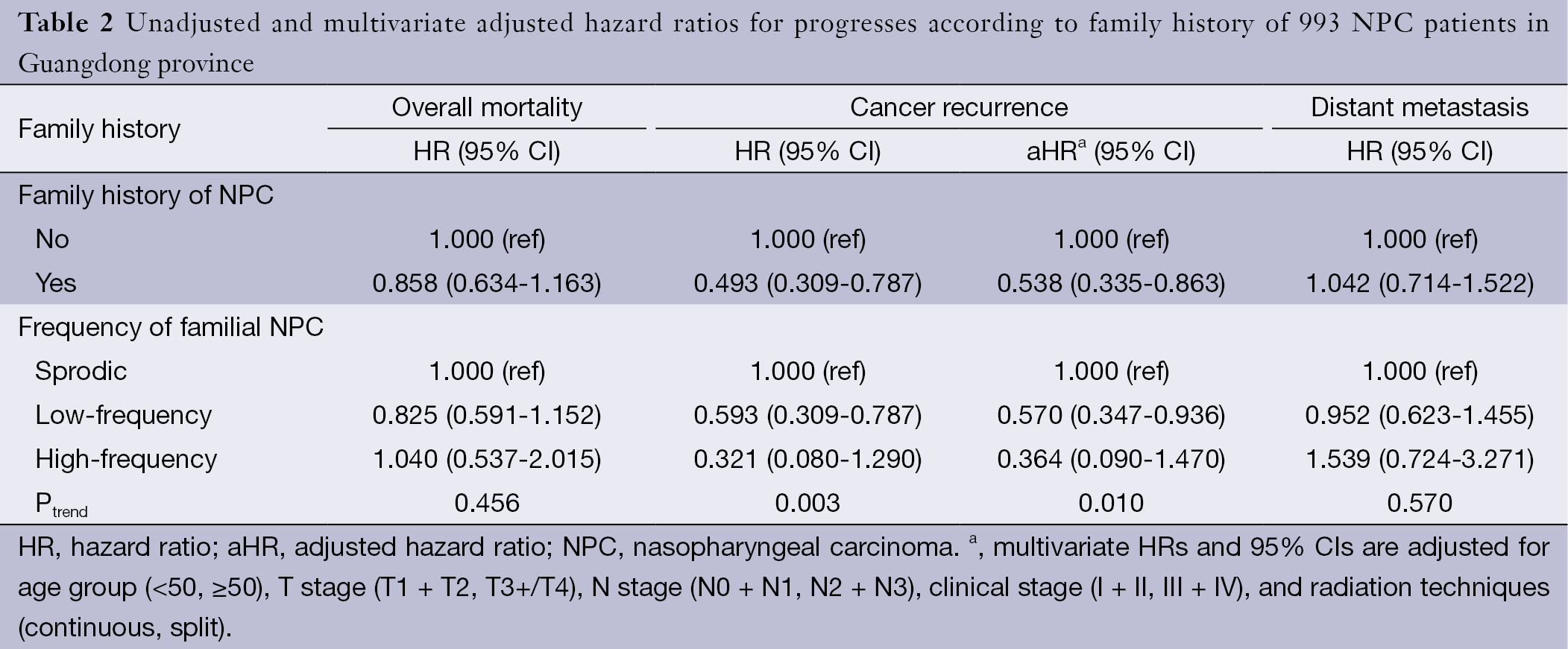
Multivariate analysis was conducted to determine the influence of family history on survival after adjustments for other prognostic factors. Consistent with the results of the univariate analysis, family history was found to be an independent favorable prognostic factor for LRFS, with an adjusted hazard ratio (aHR) of 0.538 [95% confidence interval (95% CI), 0.335-0.863]. Compared with sporadic NPCs, low-frequency familial NPCs had an aHR of 0.570 (95% CI, 0.347-0.936) and high-frequency familial NPCs had an aHR of 0.364 (95% CI, 0.090-1.470) (Table 2). There was a trend for reduction of locoregional recurrence in the order sporadic NPCs, low-frequency familial NPCs and high-frequency familial NPCs, and the P value for trend was 0.010. Compared with sporadic cases, low-frequency familial NPCs had HRs for overall mortality and distant metastasis of 0.825 (95% CI, 0.591-1.152) and 0.952 (95% CI, 0.623-1.455), respectively; high-frequency familial NPCs also had no effects on the overall mortality and distant metastasis, with HRs 1.040 (95% CI, 0.537-2.015) and 1.539 (0.724-3.271) respectively. Clinical stage showed an association with an increased risk of death, locoregional recurrence and distant metastasis in the entire patient population.
Full table
We examined whether the effect of family history on recurrence varied with the age at diagnosis. Among patients diagnosed at an age younger than 50 years, the HR for cancer recurrence was 0.324 (95% CI, 0.151-0.691). For those diagnosed at age 50 years or older, the aHR was 0.696 (95% CI, 0.382-1.268). Further, the three NPC groups showed differences in recurrence rates depending on clinical stage. Among familial NPC patients with advanced stage (stage III and IV), the reduced recurrence unchanged: the aHR was 0.480 (95% CI, 0.273-0.844).
Next, we examined the association between the family history of NPC patients and recurrence across depending on other diagnostic and treatment-related factors (i.e., radiation technique, radiation dose, and period of diagnosis). The favorable effect of family history on the reduction of recurrence was confined to patients diagnosed in the late period [1996-2001] (HR, 0.404; 95% CI, 0.202-0.808) and the ones received continuous radiation (HR, 0.527; 95% CI, 0.315-0.883). However, radiation dose had not changed the relationship between family history and cancer recurrence (Table 3).

Full table
Discussion
Several studies have confirmed that the incidence of NPC is significantly higher among relatives of the proband than among the general population, and the incidence is higher in high-risk regions (>5%) than in the intermediate-risk and low-risk regions (5-9,12,14). In Taiwan, Ung et al. found no significant difference between familial and sporadic NPC cases in terms of age, ethnicity, disease histology, disease stage, or family history of other malignancies (13). With regard to age, the findings have been inconsistent (12,13,16). In Guangdong province, which is a very high-risk area, Jia et al. found that the average age of patients with familial NPC decreased from 44.48 years to 40.43 years as the number of NPC cases per family increased from one to four (11). Consistent with this finding, our results showed that the median age of the high-frequency familial NPC patients was significantly lower than that of low-frequency familial and sporadic NPC patients, although other factors did not differ significantly among the three groups. We assume the reason for these results is that all groups of patients were from Guangdong province, the area with the highest risk of NPC in the world, and have the same clinical characteristics, which are however different from those of patients from low-risk areas. Although the etiology of NPC is considered multi-factorial, that is, a combination of genetic susceptibility and environmental factors involving EBV infection (3,4,17,18), we believe that genetic factors may play a more important role in NPC etiology in high-frequency familial NPCs in high risk areas, resulting in an earlier age of onset (19,20).
We also compared differences in prognosis between the sporadic and familial NPC cases in the present study. As the number of NPC cases among the relatives of the probands increased, the LRFS tended to improve. Additionally, we performed a multivariate analysis on the prognostic risk factors of NPC and found that only family history of NPC had a significant effect on LRFS. This result suggested that familial NPC was a distinguished subset of NPC with RT-sensitive characteristics.In the present study, familial NPC was not found to have a significant effect on DMFS, DFS, or OS, which contradicts the findings of Ouyang et al. (21). This may be because all patients in this cohort only underwent RT, while in the study of Ouyang et al., almost all advanced NPC patients were administered comprehensive treatment with RT combined with chemotherapy. Besides recurrence, the main cause of treatment failure for NPC is distant metastasis. Some studies have shown that concurrent chemoradiotherapy can reinforce local control and control the micrometastasis (22,23). Familial NPC patients who are sensitive to RT may also respond to radiotherapy or chemotherapy, and better treatment results may be obtained in such cases than those in sporadic NPC cases. Using stratification analysis, we showed that the low recurrence rates in familial NPCs were mainly limited to young (<50 years) patients with advanced clinical disease stages (stage III and IV). This finding may explain the high proportion of young and late-stage patients among familial NPC patients. Another explanation may be that the differences in biological characteristics characteristic between familial and sporadic NPCs are more apparent in advanced-stage patients. We also performed stratification analysis by diagnostic time and treatment factors (i.e., the year of diagnosis, radiation technique, and radiation dose) and found that LRFS among familial NPCs cases was mainly improved in patients who received continuous treatment in the late years [1996-2001] of the study period. These findings suggested that primary familial NPCs may be more sensitive to RT than sporadic cases and that continues RT may promote tumor control in the case of familial NPC.
Our study has some limitations. First, the sample size was relatively small, which restricted interaction analysis between family history and other factors related to NPC progression. Second, all patients included in the study had received RT alone, because of which evaluation the effects of combined treatment on the progression of familial NPCs could not be evaluated. Prospective studies that also examine the effects of combination therapy on survival rates in familial NPC patients are necessary to confirm our findings and to shed light on the underlying mechanisms.
In conclusion, this study shows that high-frequency familial NPC had early age of onset and sensitivity to radiotherapy, which suggests genetic factors may play an important role in the etiology of high-frequency familial NPC.
Acknowledgements
We are deeply indebted to the medical staff of the Departments of Nasopharyngeal Carcinoma, Radiation Oncology and Information Technology, Sun Yat-Sen University Cancer Center. We thank Zhiqiang Li, Zhijie Xiao, Zhiwei Liu and Wei Yi for helping with collection of medical information and follow-up data. This work was supported by the National High Technology Research and Development Program of China (No. 2006AA02Z4B4).
Disclosure: The authors declare no conflict of interest.
References
- Parkin DM, Ferlay J, Storm H, et al. eds. Cancer Incidence in Five Continents. Lyon: IARC Science Publication, 2005. I to VIII.
- Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 2011;30:114-9. [PubMed]
- Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell 2004;5:423-8. [PubMed]
- Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 2006;15:1765-77. [PubMed]
- Yu MC, Ho JH, Lai SH, et al. Cantonese-style salted fish as a cause of nasopharyngeal carcinoma: Report of a case-control study in Hong Kong. Cancer Res 1986;46:956-61. [PubMed]
- Chen DL, Huang TB. A case-control study of risk factors of nasopharyngeal carcinoma. Cancer Lett 1997;117:17-22. [PubMed]
- Yu MC, Garabrant DH, Huang TB, et al. Occupational and other non-dietary risk factors for nasopharyngeal carcinoma in Guangzhou, China. Int J Cancer 1990;45:1033-9. [PubMed]
- Yuan JM, Wang XL, Xiang YB, et al. Non-dietary risk factors for nasopharyngeal carcinoma in Shanghai, China. Int J Cancer 2000;85:364-9. [PubMed]
- Coffin CM, Rich SS, Dehner LP. Familial aggregation of nasopharyngeal carcinoma and other malignancies. A clinicopathologic description. Cancer 1991;68:1323-8. [PubMed]
- Yu KJ, Hsu WL, Chiang CJ, et al. Cancer patterns in nasopharyngeal carcinoma multiplex families in Taiwan. Int J Cancer 2009;124:1622-5. [PubMed]
- Jia WH, Feng BJ, Xu ZL, et al. Familial risk and clustering of nasopharyngeal carcinoma in Guangdong, China. Cancer 2004;101:363-9. [PubMed]
- Zhang F, Zhang J. Clinical hereditary characteristics in nasopharyngeal carcinoma through Ye-Liang’s family cluster. Chin Med J (Engl) 1999;112:185-87. [PubMed]
- Ung A, Chen CJ, Levine PH, et al. Familial and sporadic cases of nasopharyngeal carcinoma in Taiwan. Anticancer Res 1999;19:661-5. [PubMed]
- Cao SM, Guo X, Li NW, et al. Clinical analysis of 1,142 hospitalized cantonese patients with nasopharyngeal carcinoma. Ai Zheng 2006;25:204-8. [PubMed]
- Min H, Hong M, Ma J, et al. A new staging system for nasopharyngeal carcinoma in China. Int J Radiat Oncol Biol Phys 1994;30:1037-42. [PubMed]
- Ng WT, Choi CW, Lee MC, et al. Familial nasopharyngeal carcinoma in Hong Kong: Epidemiology and implication in screening. Fam Cancer 2009;8:103-8. [PubMed]
- Cao SM, Liu Z, Jia WH, et al. Fluctuations of Epstein-Barrepstein-barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. PLoS One 2011;6:e19100. [PubMed]
- Chen CJ, Liang KY, Chang YS, et al. Multiple risk factors of nasopharyngeal carcinoma: Epstein-Barr virus, malarial infection, cigarette smoking and familial tendency. Anticancer Res 1990;10:547-53. [PubMed]
- Bei JX, Li Y, Jia WH, et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet 2010;42:599-603. [PubMed]
- Feng BJ, Huang W, Shugart YY, et al. Genome-wide scan for familial nasopharyngeal carcinoma reveals evidence of linkage to chromosome 4. Nat Genet 2002;31:395-9. [PubMed]
- Ouyang PY, Su Z, Mao YP, et al. Prognostic impact of family history in southern Chinese patients with undifferentiated nasopharyngeal carcinoma. Br J Cancer 2013;109:788-94. [PubMed]
- Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma:positive effect on overall and progression-free survival. J Clin Oncol 2003;21:631-7. [PubMed]
- Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol 2002;20:2038-44. [PubMed]
