A lobe-specific lymphadenectomy protocol for solitary pulmonary nodules in non-small cell lung cancer
Introduction
Surgery is still an essential treatment for non-small cell lung cancer (NSCLC), especially for patients staged clinically as I, II, and operable IIIA, which can be treated with radical resection. A radical operation is a combination of pulmonary resection with dissection of the intrapulmonary and mediastinal lymph nodes, using systematic lymph node dissection (SND), systematic lymph node sampling (LS), or selective lymph node dissection (SD). Currently, most guidelines recommend SND (1). For example, in the prospective American College of Surgeons Oncology Group (ACOSOG) Z0030 trial, SND identified 3.8% more patients with N2 disease than did LS (2). Since a pN2 patient would benefit from postoperative therapy, the main theoretical advantage of SND is more precise staging, so that patients do not miss adjuvant therapy. The validity of SD remains controversial.
A solitary pulmonary nodule (SPN) is defined as a single, spherical, well-circumscribed, radiographic opacity ≤3 cm in diameter surrounded by aerated lung without atelectasis, hilar enlargement, or pleural effusion (3). A patient with an SPN that is highly suspicious of NSCLC is more likely to be considered to have early stage disease. Many early-stage patients are detected at screening. It is not clear whether these patients can achieve the same overall survival as those without SND.
Here, we define non-regional mediastinal metastasis (NRM) as either upper lobe cancer with inferior mediastinal lymph node involvement or lower lobe tumors with superior mediastinal metastasis. We also hypothesized that in a subset of patients without NRM a lobe-specific SD will have the same curative effect as SND. This retrospective study investigated the sentinel station that affects metastasis distribution, especially that indicating NRM, to establish a lobe-specific lymphadenectomy protocol for SPNs in NSCLC.
Materials and methods
The study was approved by the institutional review board of Guangdong General Hospital, Guangzhou, China. Informed consent was obtained from each patient.
Criteria
The study enrolled 401 patients who had pathological diagnoses of NSCLC and underwent radical resection with a systematic lymphadenectomy between March 2004 and June 2011 in our hospital. All of the patients were diagnosed with an SPN by computer tomography scan preoperatively, those who with a enlarged lymph node more than 1.5 cm in lobar or interlobar were considered as hilar enlargement and excluded. Information about the primary tumor location, lymph node metastasis, and pathological diagnosis was collected (Table 1). The Mountain-Dresler lymph node map was used in this study (4).
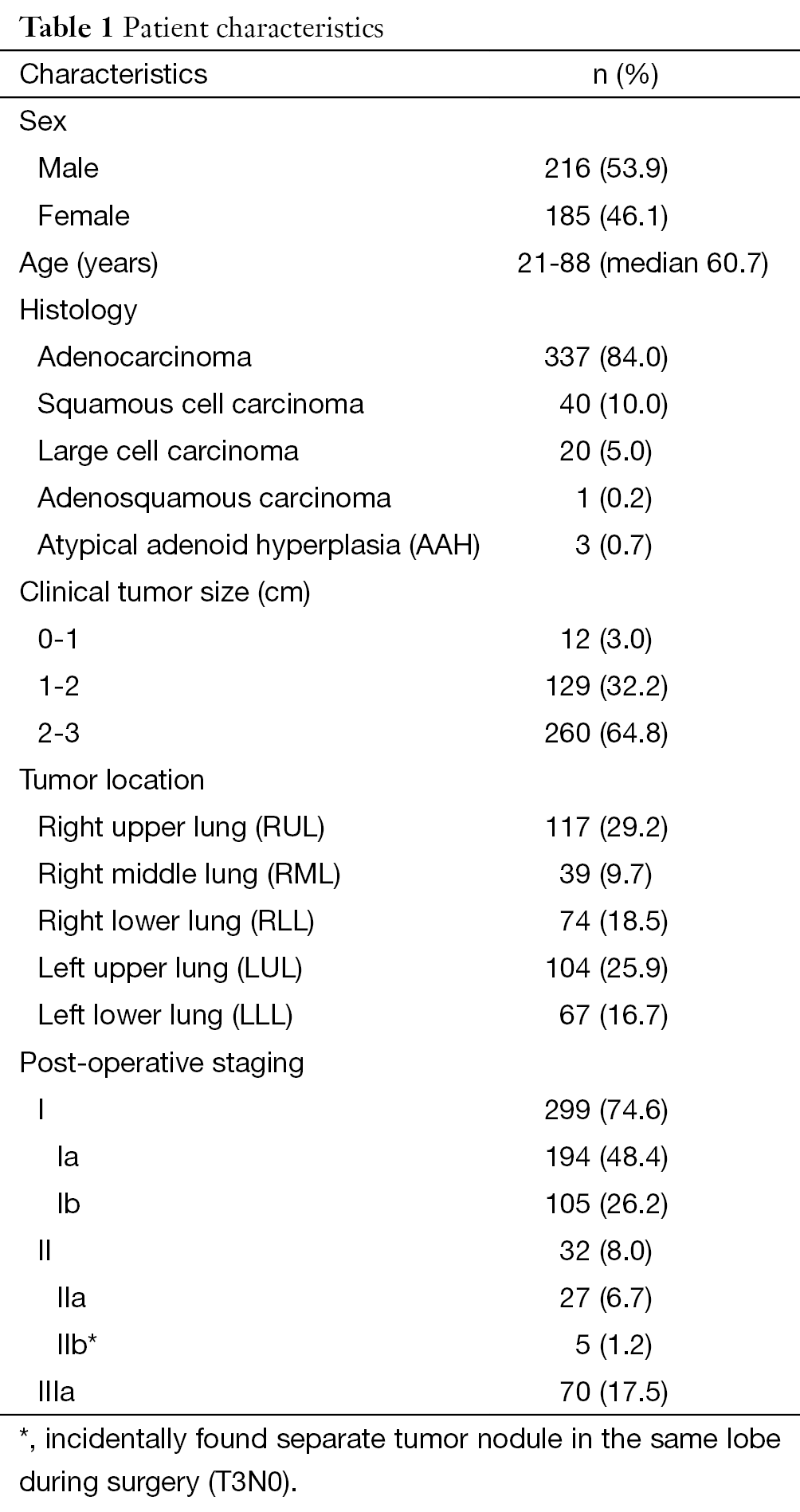
Full table
Surgery procedure
The average number of nodes that harvested was 20.96±9.066 and all patients underwent the radical operations introduced by Naruke: a pulmonary resection (lobectomy, bilobectomy, or pneumonectomy) was performed after a thoracotomy or video-assisted thoracoscopic surgery (VATS) (5). An intraoperative pathological diagnosis was mandatory if there was no preoperative pathological diagnosis. For right-side tumors, the superior [including the highest mediastinal (#1), upper paratracheal (#2), prevascular and retrotracheal (#3), and lower paratracheal (#4)] and inferior [including the subcarinal (#7), para-esophageal (#8), and pulmonary ligament (#9)] mediastinal nodes were removed; for left-side tumors, the aortic nodes [A-P window (#5) and para-aortic (#6)], #4, and inferior mediastinal nodes were dissected. The intrapulmonary lymph nodes were also dissected, including the hilar (#10), interlobar (#11), lobar (#12), segmental (#13), and subsegmental (#14) nodes.
Definition of regional node
For upper-lobe lung cancer, the superior mediastinal or aortic nodes are defined as regional nodes and inferior mediastinal as non-regional nodes. Likewise, inferior mediastinal nodes are regarded as regional nodes as for lower-lobe tumors.
Statistical analysis
The chi-square or Fisher’s exact test when needed was used to compare frequencies. Stepwise logistic regression was used to identify the key factors that indicated NRM. A two-sided P values ≤0.05 were considered significant. All statistical analyses were performed using the SPSS ver. 13.0 (SPSS, Chicago, IL, USA).
Results
Overview of lymph node metastases
No significant difference was found as regard to N1 [right upper lung (RUL) 7/117, 6.0%; right middle lung (RML) 0/39; right lower lung (RLL) 5/74, 6.8%; left upper lung (LUL) 8/104, 7.7%; left lower lung (LLL) 7/67, 10.4%, P=0.301] or N2 (RUL 19/117, 16.2%; RML 8/39, 20.5%; RLL 13/74, 17.6%; LUL 15/104, 14.4%; LLL 15/67, 22.4%; P=0.709) metastases.
Selection of lobe-specific factors that indicate NRM
As shown in Tables 2,3, multivariate analysis was performed after a univariate analytic process to indentified factors that might affected the occurrences of NRM. Results indicate that #2,4, #10,11, and #10,11 as well as #7 was the key lymph node station for RUL, LUL, and lower lobes: #2,4 [odds ratio (OR)=28.000, 95% confidence interval (CI): 2.917-268.790, P=0.004] for RUL, #10,11 (OR=31.667, 95% CI: 2.502-400.833, P=0.008) for LUL, #10,11 (OR=19.540, 95% CI: 4.217-90.541, P<0.001) and #7 (OR=7.395, 95% CI: 1.586-34.484, P=0.011) for lower lobes, respectively.
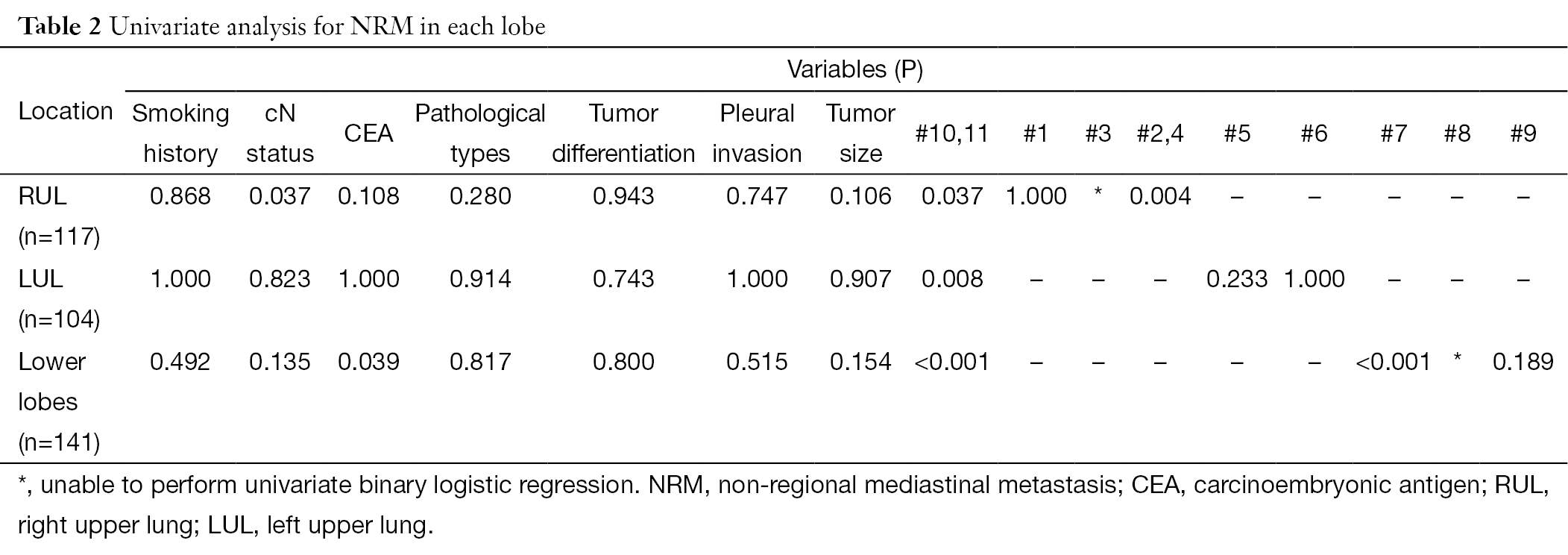
Full table
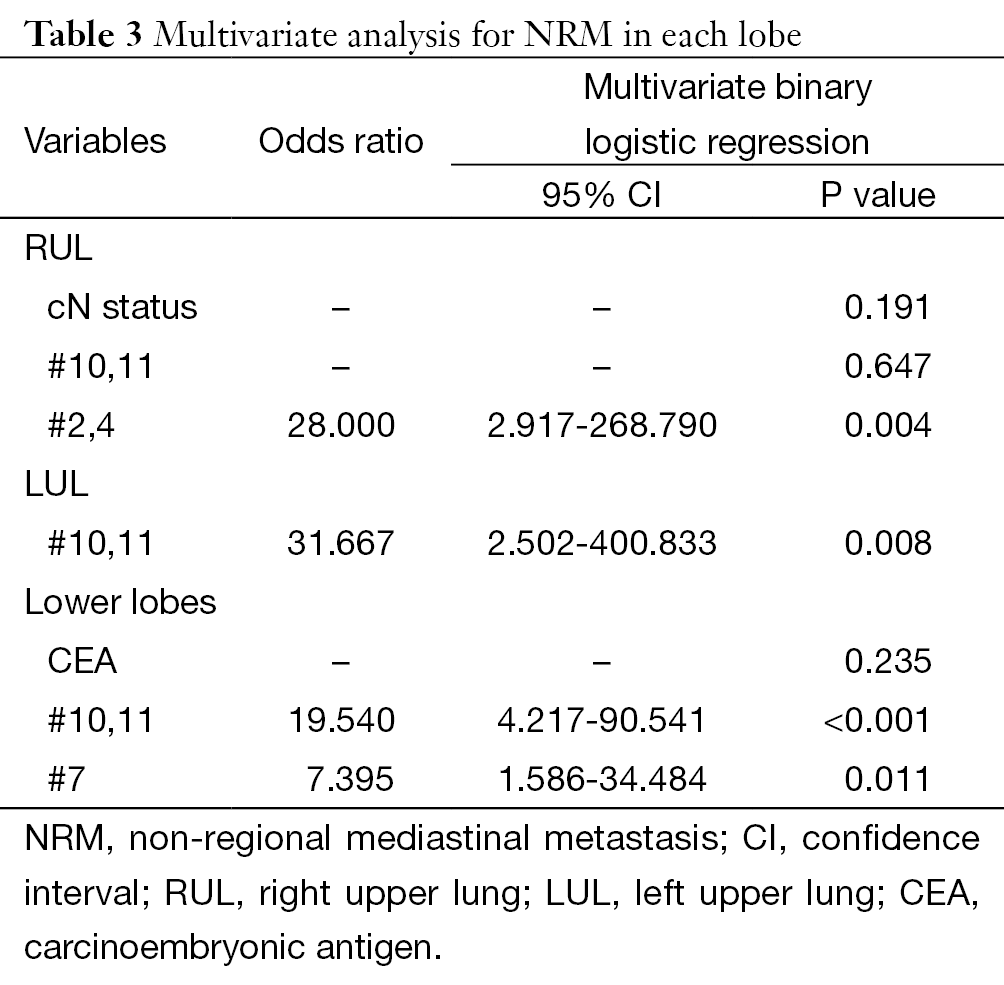
Full table
NRM without primary regional mediastinal involvement
Six patients (1.5%) had NRM without primary regional mediastinal involvement: RUL 1/117, 0.9%; RLL 2/74, 2.7%; LUL 2/104, 1.9%; LLL 1/67, 1.5%; P=0.814. In detail, five of them were larger than 2 cm and the pathological grade distribution is as follows: two patients with grade 1, three patients with grade 2 and one patient with grade 3.
Single involved skip N2 cases
A skip N2 refers to a patient who was pN1 (–) N2 (+). In our series, 23 patients (5.7%) had skip N2; the prevalence was as follows: RUL 8/117, 6.8%; RML 3/39, 7.7%; RLL 5/74, 6.8%; LUL 4/104, 3.8%; LLL 3/67, 4.5%; P=0.792. With a single skip N2, called a minimal skip N2, only one station has metastasis; 4.5% of the patients (18/401) had this unique characteristic (Table 4).
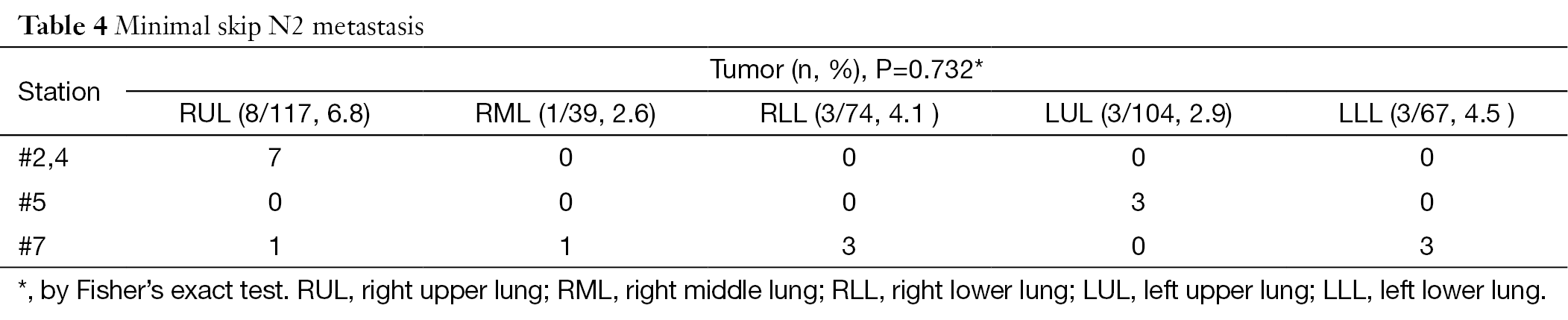
Full table
Stage migrations under SD procedure
Totally, two cases (2/401, 0.5%; one in RUL and one in LUL) would have stage migrations if a SD procedure performed (protocol in Table 5). Besides, both of these tumors were larger than 2 cm preoperatively.
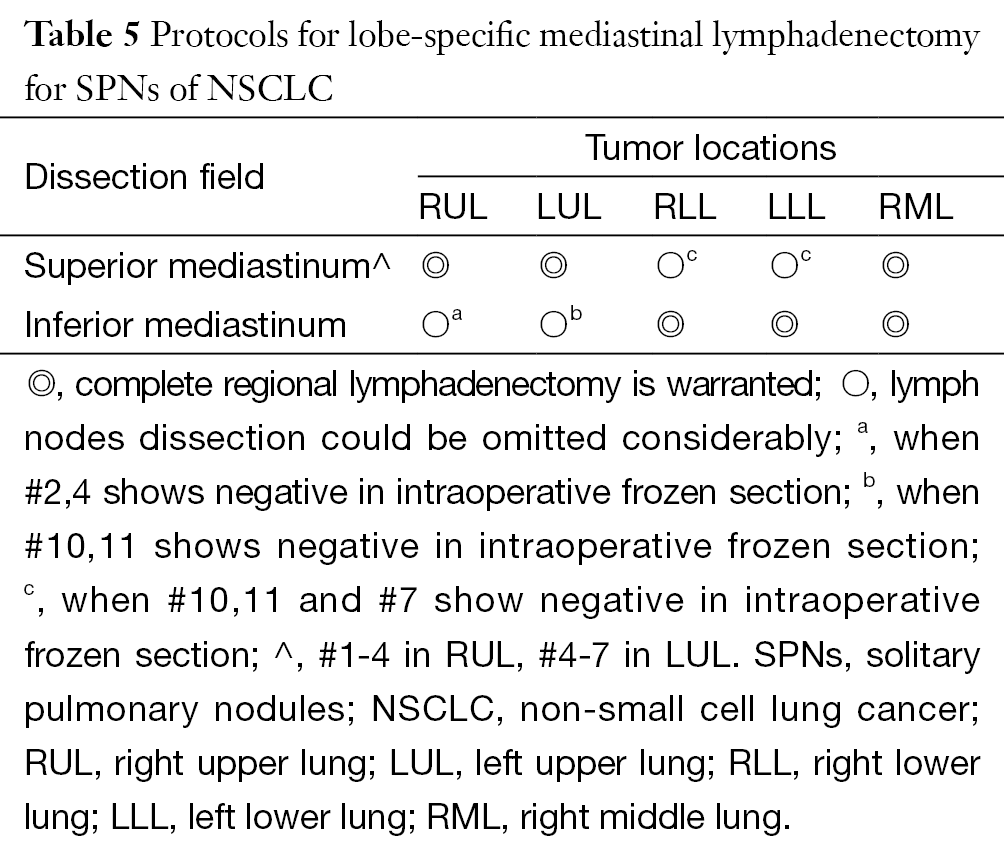
Full table
Discussion
Theoretically, SND refers to a systematic en bloc mediastinal lymph node dissection (6). Our previous clinical trial demonstrated the superiority of SND vs. LS (7). However, the definition of LS varies and the details of nodes sampled were inconsistent (8). The Z0030 trial used the most thorough definition of LS. In addition, this trial provided level I evidence that there is no significant difference in mortality and morbidity between SND and LS, which differs from the retrospective cohort study by Okada et al. (6,9). The 5-year survival rates also did not differ between the two groups in the Z0030 trial, indicating that a LS procedure in T1 or T2, N0 or non-hilar N1 NSCLC equals a SND (2). In other words, there is an alternative to SND in a specific patient group. Yet the LS introduced in the Z0030 requires precise intraoperative staging and this “extensive LS” cannot be generalized to patients staged radiographically or those with higher-stage tumors.
In addition, elderly patients, who make up a large proportion of newly diagnosed cases, are more vulnerable to an invasive operation than the young. Aoki et al. reported that the 5-year survival according to the type of lymph node dissection was not significantly different for stage I NSCLC in octogenarians (10). Chida et al. reported that a pulmonary resection with an SND was associated with higher mortality and postoperative cardiac complications in octogenarians with lung cancer (11). Okasaka et al. noted that not performing a radical lymphadenectomy is an acceptable surgical treatment for elderly patients based on a retrospective study (12). However, the prospective Z0030 trial did not include data on this group. In general, SPNs account for 8-51% of incidental or screening-detected pulmonary lesions and much of this is malignant (6-28% for 5-10 mm and 64-82% for 20-30 mm) (13). Furthermore, the majority of cases are at an early stage (299/401, 74.6% in our series). Hence, a less-invasive radical operation is still warranted for the increasing numbers of these clinically early-stage senior citizens.
Recently, lobe-specific lymphadenectomy or a complete mediastinal lymph node dissection in a reduced area was introduced (14). Ishiguro et al. found that patients undergoing SD had a significantly shorter operating time, less blood loss, and shorter hospital stay than those undergoing SND, suggesting that SD is less invasive (15). In Japan, SD is frequently performed for patients with a poor physical status and earlier diseases (15).
In theory, a lobe-specific mode and the extent of nodal spread are fundamental to lobe-specific SD (16,17). In patients with early-stage NSCLC, the cancer cells are most likely to metastasis to level 3 or 4 lymph nodes in RUL tumors, 3 or 7 in RML tumor, 7 in RLL tumors, 5 or 6 in LUL tumors, and 7 in LLL tumors (18). Okada et al. reported that among patients with skip N2 metastases with an upper-lobe lesion, none had positive subcarinal nodes (17). Only one of 13 (7.7%) patients with lower-lobe lesions showed nodal spread to the superior mediastinum. Aokage et al. suggested that dissection for subcarinal nodes is dispensable in cN0 upper-lobe squamous cancer (19). Likewise, Asamura et al. found that the most common site of metastasis for pN2 tumors located in the right upper lobe was the superior mediastinal station, whereas metastases to the subcarinal station were seen in only 12-13% of cases (16). Indeed, they proposed that subcarinal lymphadenectomy is not always necessary for tumors located there.
Many researchers have reported their experiences with SD. Watanabe et al. proposed an SD based on a segment-specific pattern; however, a lobe-specific SD is preferred (20). The report by Ichinose omitting subcarinal lymphadenectomy was for clinically stage I patients with a tumor in the right upper lobe or left upper segment, and a superior mediastinum or aortic region lymphadenectomy was omitted for lower lesions (14). The overall and 5-year locoregional recurrence-free survival rates were 78.5% and 76.6%, respectively, illustrating that SD obtains acceptable locoregional control. Okada et al. suggested that a lower mediastinal lymphadenectomy was dispensable if the hilar and superior mediastinal nodes were tumor-free in upper-lobe tumors (17). For lower tumors, an upper mediastinal lymphadenectomy was dispensable when the hilar and inferior mediastinal nodes were tumor-free. Ishiguro et al. performed a propensity score stratified cohort study comparing SD (a strategy resembling that in Okada et al.) vs. SND, and revealed that SD did not adversely affect the overall survival in each quartile of propensity score (9,15). Because a randomized control trial would be difficult to conduct, this article did offer the most convincing evidence.
Among the tumors with a single involved N2 station, the most common site of lymph node metastases was level 4R for RUL tumors, levels 5/6 for LUL tumors, and level 7 for middle and lower-lobe tumor, whereas Ilic found that the distribution of skip N2 metastases was roughly equal among the different mediastinal lymph node stations regardless of primary tumor location (21,22). In our study, lobe-specific patterns might still exist in these cases (Table 4). Because an isolated N2 in skip N2 patients has a better prognosis, it is critical that these patients receive sequential adjuvant therapy instead of being down-staged under an SD procedure (23).
In this study, we found that key sentinel lymph nodes indicating NRM status for RUL, LUL and lower lobes. Based on previous studies, we propose a lobe-specific SD protocol that is slightly different from those of Okada et al. and Ishiguro et al. (Table 5) (9,15).
Note that it is dangerous to perform an SD without selecting candidates rationally. First, SND did not increase mortality or morbidity in the vast majority of patients with NSCLC; in addition, patients with occult metastases (OMs) in lymph nodes might obtain a survival benefit from SND and OMs were found even in early peripheral lung cancer (24). Further study of OMs to non-regional nodes in patients without NRM microscopically is warranted, to determine whether OMs have a lobe-specific drainage tendency.
In conclusion, a lobe-specific lymphadenectomy is feasible in practice for patients with NSCLC, especially for SPNs <2 cm. We recommend that each key station be subject to routine intraoperative frozen section. The protocol (Table 5) should be generalized only to those cases identified as SPN initially. If precise staging and a comprehensive evaluation are unavailable, then a standard SND remains mandatory for all resectable cases, as our previous work suggested (25).
Acknowledgements
None.
Footnote
Conflicts of Interest: Part of the content had been shown as a mini oral in 15th IASLC World Conference on Lung Cancer, Sydney [Abstract No.1763, J Thorac Oncol. 2013;8: S216]. None of this manuscript had been submitted as abstract for the EACTS annual meeting. The authors have no conflicts of interest to declare.
References
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [PubMed]
- Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:108S-30S.
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [PubMed]
- Naruke T, Goya T, Tsuchiya R, et al. The importance of surgery to non-small cell carcinoma of lung with mediastinal lymph node metastasis. Ann Thorac Surg 1988;46:603-10. [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [PubMed]
- Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [PubMed]
- Whitson BA, Groth SS, Maddaus MA. Surgical assessment and intraoperative management of mediastinal lymph nodes in non-small cell lung cancer. Ann Thorac Surg 2007;84:1059-65. [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Selective mediastinal lymphadenectomy for clinico-surgical stage I non-small cell lung cancer. Ann Thorac Surg 2006;81:1028-32. [PubMed]
- Aoki T, Tsuchida M, Watanabe T, et al. Surgical strategy for clinical stage I non-small cell lung cancer in octogenarians. Eur J Cardiothorac Surg 2003;23:446-50. [PubMed]
- Chida M, Minowa M, Karube Y, et al. Worsened long-term outcomes and postoperative complications in octogenarians with lung cancer following mediastinal lymph-node dissection. Interact Cardiovasc Thorac Surg 2009;8:89-92. [PubMed]
- Okasaka T, Usami N, Taniguchi T, et al. Can non-performance of radical systematic mediastinal lymphadenectomy be justified in elderly lung cancer patients? An evaluation using propensity-based survival analysis. Eur J Cardiothorac Surg 2010;38:27-33. [PubMed]
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S-107S.
- Ichinose J, Kohno T, Fujimori S, et al. Locoregional control of thoracoscopic lobectomy with selective lymphadenectomy for lung cancer. Ann Thorac Surg 2010;90:235-9. [PubMed]
- Ishiguro F, Matsuo K, Fukui T, et al. Effect of selective lymph node dissection based on patterns of lobe-specific lymph node metastases on patient outcome in patients with resectable non-small cell lung cancer: a large-scale retrospective cohort study applying a propensity score. J Thorac Cardiovasc Surg 2010;139:1001-6. [PubMed]
- Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 1999;117:1102-11. [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Proposal for reasonable mediastinal lymphadenectomy in bronchogenic carcinomas: role of subcarinal nodes in selective dissection. J Thorac Cardiovasc Surg 1998;116:949-53. [PubMed]
- Naruke T, Tsuchiya R, Kondo H, et al. Lymph node sampling in lung cancer: how should it be done? Eur J Cardiothorac Surg 1999;16 Suppl 1:S17-24. [PubMed]
- Aokage K, Yoshida J, Ishii G, et al. Subcarinal lymph node in upper lobe non-small cell lung cancer patients: is selective lymph node dissection valid? Lung Cancer 2010;70:163-7. [PubMed]
- Watanabe S, Asamura H, Suzuki K, et al. The new strategy of selective nodal dissection for lung cancer based on segment-specific patterns of nodal spread. Interact Cardiovasc Thorac Surg 2005;4:106-9. [PubMed]
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12.
- Ilic N, Petricevic A, Arar D, et al. Skip mediastinal nodal metastases in the IIIa/N2 non-small cell lung cancer. J Thorac Oncol 2007;2:1018-21. [PubMed]
- Riquet M, Assouad J, Bagan P, et al. Skip mediastinal lymph node metastasis and lung cancer: a particular N2 subgroup with a better prognosis. Ann Thorac Surg 2005;79:225-33. [PubMed]
- Rusch VW, Hawes D, Decker PA, et al. Occult metastases in lymph nodes predict survival in resectable non-small-cell lung cancer: report of the ACOSOG Z0040 trial. J Clin Oncol 2011;29:4313-9. [PubMed]
- Zhong W, Yang X, Bai J, et al. Complete mediastinal lymphadenectomy: the core component of the multidisciplinary therapy in resectable non-small cell lung cancer. Eur J Cardiothorac Surg 2008;34:187-95. [PubMed]
