Transcatheter embolization therapy in liver cancer: an update of clinical evidences
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world and the third most common cause of cancer-related death (1). With improved surveillance of patients with chronic liver disease and advances in imaging, more patients are diagnosed with early-stage HCC (2-4). For the treatment of early stage HCC, curative therapies including liver transplantation, hepatic resection, and radiofrequency ablation (RFA) are recommended. Liver transplantation is the treatment option especially for patients with decompensated cirrhosis, but potential recipients outnumber donors. Hepatic resection is widely used as the main choice of treatment for resectable HCC. However, the risk of postoperative hepatic dysfunction often precludes surgery (5).
Intrahepatic cholangiocarcinoma (IHC) is the second most common primary hepatic neoplasm after HCC (6), with the highest incidence in Asia. At the time of diagnosis, patients with IHC usually present with advanced stage disease and only 30% among them are candidates for surgical treatment (7). Intravenous regimens including gemcitabine and various combinations of 5-fluorouracil (FU) with cisplatin provide low response rate (8).
Liver metastases can be found in 40% to 70% of patients with colorectal cancer (CRC) (9). Surgical resection is usually the standard treatment modality. However, resection can only be performed in a minority of patients due to the presence of multifocal tumors or limited hepatic reserve at the time of diagnosis (10). In the past, 5-FU and leucovorin (LV) constituted the foundation of most chemotherapy regimens. Recent years have seen important results in the treatment of advanced CRC, particularly in the use of new chemotherapy approaches and their combination with targeted therapies (bevacizumab, cetuximab and panitumumab). Modern regimens such as combined 5-FU/LV with oxaliplatin or camptothecin (CPT)-11 and monoclonal antibodies have achieved response rates of approximately 80%, and median survival of patients with non-resectable liver metastases has increased to 20-26 months. Nevertheless, the new systemic chemotherapeutic regimens have been associated with skin reactions, high costs and impaired liver functions. A further goal is therefore how to successfully achieve local control and increase the proportion of patients able to undergo liver resection, reduce recurrences, and prolong survival and quality of life of patients who remain unsuitable for resection.
Gastroenteropancreatic (GEP) neuroendocrine neoplasms, also called GEP neuroendocrine tumours (NETs), were previously regarded as rare, but in fact are increasing in incidence (11). Liver metastases represent the most crucial prognostic factor, irrespective of the primary NET site. In historical series, 5-year survival is 13-54% compared with 75-99% for patients without hepatic metastases (12). Despite various complex management strategies for neuroendocrine liver metastases, surgery is the only treatment that offers potential for cure.
Percutaneous ablations, including percutaneous ethanol injection (PEI) and RFA, represent the recommended curative modalities for patients with early-stage liver cancer who are not candidates for surgical resection or liver transplantation. Conventional transarterial chemoembolization (TACE) is the gold standard for the treatment of patients with HCC who cannot receive curative therapies and radioembolization is an interesting alternative therapy for HCC patients who are poor candidates for TACE. Chemoembolization might offer long-term survival rates comparable to those of hepatic resection and RFA for small single-nodule HCC if underlying liver function was similar among the patients receiving each treatment (13,14).
Choice of minimally invasive treatment for HCC
PEI and RFA are widely used in clinical practice. With PEI, the distribution of ethanol may be blocked by the intratumoral fibrotic septa and/or the tumor capsule, resulting in a heterogeneous distribution. As a result, curative capacity of PEI, particularly in tumors greater than 2 cm in diameter is limited, and frequently requires multiple injections over multiple sessions. In contrast, RFA results in coagulative necrosis of both the tumor and a rim of surrounding parenchymal tissue producing a margin of ablated non-tumoral tissue, which might eliminate small-undetected satellites. RFA has been shown to be as effective as hepatic resection in the treatment of small single-nodule HCC (15,16). However, RFA of lesions located close to major organs or the liver capsule is often contraindicated (17). Giorgio et al. (18) compared the 5-year survival of patients with a single HCC ≤3 cm, who were randomly assigned to receive either PEI or RFA. No differences were observed in terms of overall survival (OS) or local recurrence rate. Oeda et al. (19) evaluated the association of treatment method with OS in 98 patients treated with PEI and 92 subjects who received with RFA. The 5-year survival rate in the PEI group was 40%, whereas it was 51% in the RFA group (P=0.04). When stratifying patients according to tumor stage, a significant advantage in survival was observed for RFA in individuals with stage II disease (5-year survival: 48 vs. 28% with PEI, P=0.03). However, RFA resulted in more severe complications and was more expensive than PEI. A recent meta-analysis of about 8,500 patients, with a 10-year perspective, showed that in patients with very early HCC and Child-Pugh class A, RFA provides similar life-expectancy and quality adjusted life-year at a lower cost compared with resection (20). While RFA is usually considered a front-line treatment choice in patients eligible for percutaneous techniques, with low cost and low complication rate PEI should be considered with suitable candidates with small HCC, particularly for HCC at difficult-to-treat location for RFA (21,22).
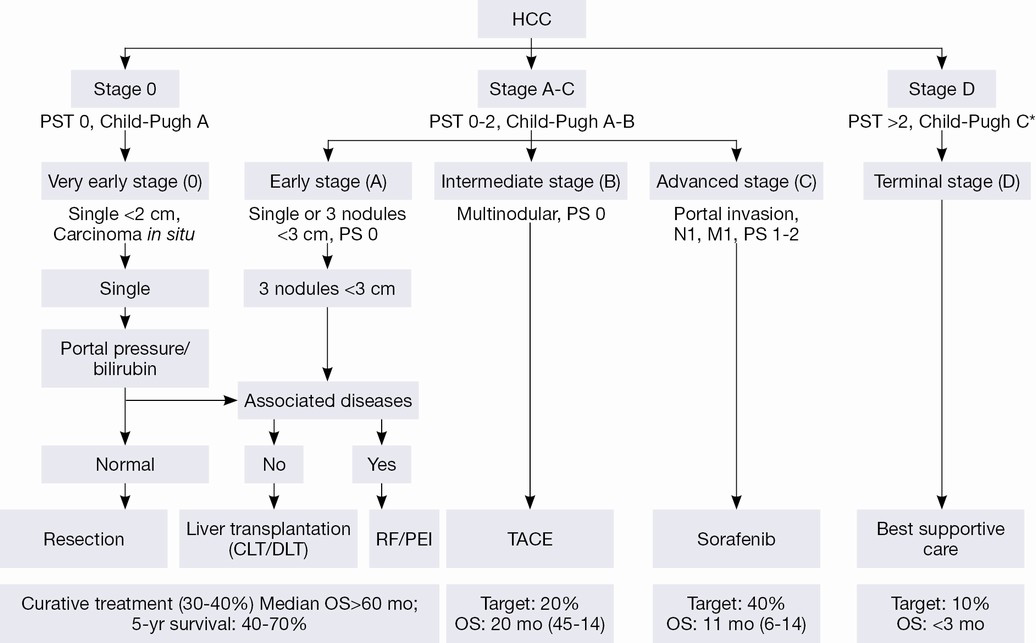
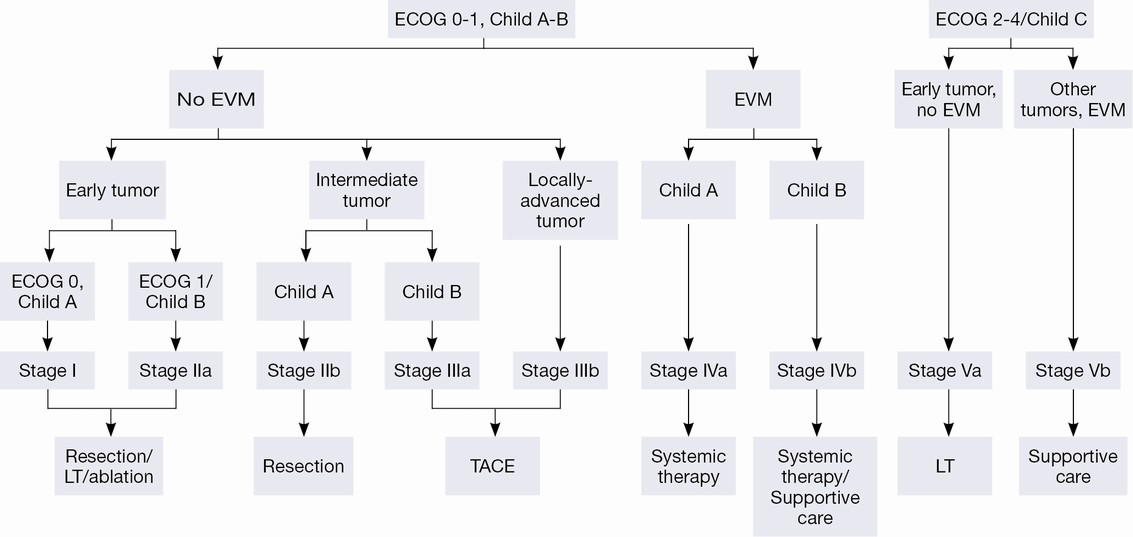
The Barcelona Clinic Liver Cancer (BCLC) algorithm (23) is widely used for the management of HCC in Europe and the USA (Figure 1). The European Association for the Study of Liver Disease (EASL) and the American Association for the Study of Liver Diseases (AASLD) guidelines approved the BCLC classification system as a favorable staging system for prognosis allocation and treatment schedule which were validated from cohort studies and randomized controlled trials (RCTs). However, this classification also has limitations, such as absence of consideration of nodule location and etiology of cirrhosis (non-cirrhotic patients are not manageable with this classification). It does not consider treatment sequences or combination therapies which could lead to indications for selected patients with specific approaches that are not recommended to date. This comes from a too heterogeneous population, notably in the intermediate stage (BCLC stage B) in respect to tumor burden and liver function. In clinical practice, guidelines do not systematically reflect the best therapeutic approach for each patient. In selected patients treatment allocation should be determined on an individualized rather than a guideline-based medicine by a multidisciplinary board. In Asia, resection of tumors in advanced stages and in patients with less than perfect liver function is more aggressively pursued. Consensus-Based Clinical Practice Guidelines Proposed by the Japan Society of Hepatology (JSH) 2010 Updated Version is shown in Figure 2 (2). A recent staging and treatment allocation system issued by The Hong Kong Liver Cancer (HKLC) identified subsets of BCLC intermediate- and advanced-stage patients for more aggressive treatments than those were recommended by the BCLC system (Figure 3) (24). Very recently, a retrospective and single-center study (Johns Hopkins Hospital, Baltimore) on 968 North American patients showed that HKLC staging outperformed BCLC staging as a prognostic classification system in patients treated with intra-arterial therapy (presented at the Society of Interventional Radiology Congress, Feb 2015 by Sohn S & Geschwind JH). However, this HKLC staging system will require extra validation both in Asia and elsewhere, and it should also be tested in patients with liver disease other than hepatitis B (25).

Recently, Yang et al. (26) compared the treatment effects of hepatic resection, RFA, and conventional TACE on long-term survival. It was found that 5-year OS with conventional TACE (c-TACE) was similar to that with hepatic resection and RFA in patients with single-nodule HCC of 3 cm or smaller without vascular invasion when the underlying liver status was balanced among the patients receiving each treatment. In addition, most of the patients initially treated with c-TACE achieved a complete response, which was one of the independent prognostic factors of survival, although some should receive repeated treatments. However, when c-TACE is used as an initial treatment, special care should be taken to obtain a complete response, and surveillance for tumor recurrence should be undertaken. These results are consistent with those of cohort studies demonstrating that TACE provided OS similar to hepatic resection in early-stage HCC (27,28).
Procedure for transarterial chemoembolization (TACE)
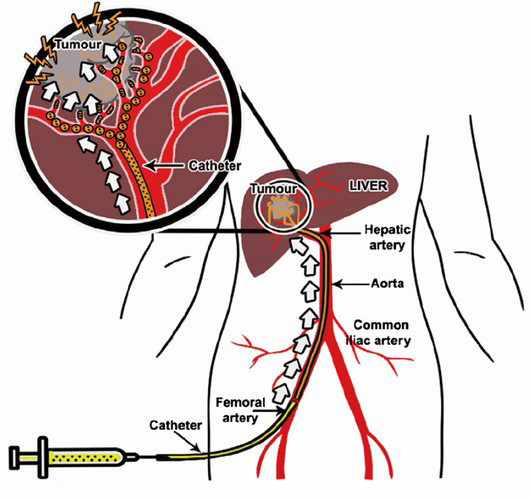
TACE with use of anticancer drugs followed with gelatin sponge (Gelfoam®) was introduced by Yamada et al. in the late 1970s (29,30). The liver has a unique dual blood supply from both the portal vein and the hepatic artery. The normal parenchyma of the liver receives two-thirds of its necessary blood supply from the portal vein and receives the remaining one-third from the hepatic artery. Hepatic tumors receive their blood supply mainly from the hepatic artery. TACE is able to offer highly concentrated doses of chemotherapeutic agents to the tumor tissues, while the surrounding normal hepatic parenchyma is preserved. The embolic agent(s) causes ischemia and necrosis of the tumor, and slows anticancer drug washout (Figure 4). On the other hand, the blood supply to the normal liver tissue is maintained by the dominant blood supply from the portal vein system.
Chemoembolization is the infusion of a mixture of chemotherapeutic agents with or without iodized oil, associated with embolisation (32). According to the guidelines published by AASLD (33) and EASL (23), c-TACE is recommended as first-line therapy for patients who are not candidates for surgery, transplantation or ablation, i.e., HCC who do not have vascular invasion or extrahepatic spread.
TACE should be distinguished from three other interventional procedures: (I) transarterial oily chemoembolization (TOCE, or “chemo-lipiodolization”) where the anticancer agent is mixed with Lipiodol® without any other embolizing agent; (II) bland transarterial embolization (TAE) where no anticancer drug is given; (III) transarterial chemotherapy (TAC) where the anticancer drug is infused without Lipiodol or embolization particles.
TACE for liver tumors involves the following steps:
Prior to TACE, a thorough angiography is performed to locate all the feeding arteries of a tumor including any possible extrahepatic arteries that may feed the tumor. Once the arterial anatomy is clearly understood, a catheter is advanced superselectively into the feeding artery of the tumor. A 4F hydrophilic cobra catheter used with a hydrophilic guide-wire suffices for about half of cases. Use of a standard lumen catheter allows rapid injection of the viscous chemoembolic emulsion and is less likely to clog with particles. However, the catheter should not be used in vessels less than twice its diameter, as the catheter will cause a partial occlusion of the vessel lumen, resulting in a pseudo-stasis. Withdrawal of the catheter then results in a reflow to the tumor. Small vessels and branches which cannot be accessed with a standard angiographic catheter can usually be catheterized with micro-catheters with a catheter diameter in between 2.0 and 2.4 French and 0.018- or 0.025-inch glide wires (34). The recent Surefire® Infusion System (Surefire Medical, Westminster, USA) is an 0.027-inch lumen microcatheter with the an expandable tip at the distal end. This device is intended for use in angiographic procedures to increase targeted delivery, to minimize reflux and to dynamically collapse in forward flow. It is designed to deliver radiopaque contrast media and therapeutic agents (chemotherapeutic agents and solid and liquid embolic agents) to selected sites during TACE procedure (35,36).
When the catheter is positioned for treatment, it is important to perform an arteriography to confirm the anatomy before injecting any chemotherapy agents. This superselective injection may reveal findings not depicted in the celiac or superior mesenteric artery injection, such as cystic, right gastric or falciform arteries arising from the target hepatic artery, or guide-wire induced spasm in the target artery. The end point of the TACE procedure is visualization of the complete blockage of the tumor-feeding branch. It is essential to check for extrahepatic collateral arterial supply to the tumor lesion. An extra-hepatic collateral artery supplying a tumor is more frequent for subcapsular location or exophytic tumor. CT findings of a peripherally located portion of viable tumor on a follow-up CT scan should induce investigation of such arteries because of a close contact between the liver and the diaphragm, the blood supply to the diaphragm can reach the liver by direct adherence. Thus, the right inferior phrenic artery is the most common collateral pathway. Modification of TACE in patients with hepatic arteriovenous shunt (AV shunt) can be performed by either embolization with gelfoam or using balloon occlusion of the hepatic vein draining the shunt (37).
The most common sole-agent anticancer drug used in published TACE studies has been doxorubicin (36%), followed by cisplatin (31%), epirubicin (12%), mitoxantrone (8%), mitomycin C (8%), and SMANCS (5%) (38). The administered dose of the anticancer agent should depend on the size of the tumor, the position of the catheter, the patient’s liver function, and the response to previous courses of TACE, if any. It is worth mentioning that RCTs failed to show significant differences in survival between doxorubicin and other drugs such as cisplatin or epirubicin, and till now, there is no evidence of the superiority of any single chemotherapeutic agent over other drugs or for mono-drug chemotherapy versus combination chemotherapy (38).
TACE is not recommended in early stages as a first option. At very early stage the HCC is not highly vascularized and its main blood supply comes from the portal vein, but as the HCC grows its blood supply increasingly comes from the hepatic artery, notably when the lesions are histologically well/moderately differentiated (39).
Liver functional reserve is the key to an optimal selection of candidates. Conventional TACE should be contraindicated in patients with decompensated cirrhosis. A panel of experts has recommended a series of absolute and relative contraindications for TACE that include hepatic encephalopathy, reduced or absent portal vein flow, biliary obstruction and large/massive tumors (40). TACE is generally contraindicated in patients with branch or main portal vein thrombosis (PVT), since occlusion of arterial blood flow by may induce liver failure, although superselective TACE may not be harmful in selected patients with segmental PVT. Super-selective TACE, i.e., the catheter is selectively placed in a medium-small branch of hepatic artery, can be used in a patient with compromised liver function. There are recent uncontrolled trials and cohort studies that suggest a treatment benefit in selected patients with preserved liver function (41,42). A recent meta-analysis including 8 studies with 1,601 patients, concluded that TACE in patients with PVT improved the 6-months and 1-year survival compared with conservative treatment (43). If the patient has a diffuse or massive HCC or an HCC involving the major portal veins, TACE procedure cannot be safely performed.
TACE can cause a number of complications resulting from underlying factors of the patient or inadvertent techniques. Post-embolization syndrome that consists of transient abdominal pain and fever is common. It is not a complication of TACE per se. 60-80% of the patients after liver TACE experience this syndrome. It is usually self-limiting within 3-4 days (44).
A transient decline in liver function is common but acute liver decompensation (ascites, encephalopathy or jaundice) is reported in only 0.1-3% of procedures. Biliary and gastrointestinal complications have been reported in 2-10% and 1-5% of patients, respectively. Other complications include liver abscesses in patients with incompetent ampulla, vascular injury from repeated intraarterial chemotherapy, and tumor rupture. The most serious complication is treatment-induced liver failure. TACE benefits should be balanced with the risk of this liver failure, thus the best candidates are patients with preserved liver function and asymptomatic multinodular tumors without vascular invasion or extrahepatic spread. Compromised liver function, main portal vein obstruction, biliary tract obstruction, a previous history of bile duct surgery, over dose of embolic agents, hepatic artery occlusion due to repeated TACE and nonselective TACE increase the chance for complications. The presence of these factors should be identified prior to TACE procedure, and an adjustment of the cytotoxic drug dosage, and a more selective procedure should be performed. The most morphologic contraindications for TACE also include hepatofugal flow or portosystemic anastomosis. Patients with Child-Pugh C and some with B, patients with a BCLC stage D, and patients with clinical symptoms of end-stage cancer should be excluded since the ischemic insult can lead to severe and even fatal adverse events.
Complete responses are rarely seen after a single session of conventional TACE and repeated sessions can be scheduled at fixed pre-planned intervals or depending on the observed response (40). Most of the recurrent tumors are supplied by feeders from the adjacent segmental arteries (45). Patients are thus evaluated every 3-8 weeks and additional TACE sessions are performed if contrast-enhanced areas revealing tumor activity are observed in cross-sectional imaging. Depending on the arterial anatomy, two to four procedures are required to treat the entire liver. Thereafter, response is assessed by repeated imaging studies and follow-up of tumor biomarkers.
Clinical evidence for transarterial embolization (TACE)
The most reliable way to confirm a survival benefit is large RCTs; however, initial small RCTs had failed to show a survival benefit of TACE treatment for HCC patients. In 2002, two RCTs from and Spain and Hong Kong investigated the survival benefits of conventional TACE compared to the best conservative treatment (46,47). These RCTs were followed by cumulative meta-analyses (48,49), showing that c-TACE significantly reduced the overall 2-year mortality rate compared to control patients who received conservative treatments. In 2003, Llovet et al. (49) reported a meta-analysis, constructed from 7 RCTs including 545 HCC patients, comparing c-TACE or bland transarterial embolisation (TAE) vs. conservative management or other therapies (systemic chemotherapy or tamoxifen). Most patients had cirrhosis, with Okuda stages I-II, and lacked evidence of PVT. Doxorubicin was used in one study and cisplatin in three; Gelfoam® was used as the embolic agent in all the trials. Mean number of treatment ranged between 1 and 5 sessions. Survival benefits were identified in two studies. The two-year survival rate in the treated group was 41% (range, 19-63%) vs. 27% (range, 11-50%) in the control group (P=0.017). The significant survival benefit was for c-TACE with doxorubicin or cisplatin, but not for bland TAE alone. In 2007, Marelli et al. (38) also found similar results in a meta-analysis. In a recent Asian prospective cooperative study including 99 HCC patients, however, conventional TACE was associated with median OS of 3.1 years with 2-year OS of 75% (95% CI: 65.2-82.8%) (50). O’Suilleabhain et al. (51) evaluated the long-term survival of TACE in patients with unresectable HCC and suggested that a cure for unresectable HCC may be possible with TACE, although this is rare. TACE after radical excision of HHC can also destroy remnant cancer cells, decrease recurrence rate, and increase survival rate. A possible survival advantage has also been reported in patients treated with TACE before resection of HCC when compared with resection alone (52).
TACE has shown to be effective in the treatment of CRC metastases for unresectable patients. In one article, nineteen trials were reviewed. In these studies, TACE has been applied in 324 patients with CRC metastases with conventional method or its variants (53), with response rates varying from 25-100%. In a prospective study, 463 patients with unresectable liver metastases of CRC that did not respond to systemic chemotherapy were repeatedly treated with TACE in 4-week intervals. The anticancer drug was mixed with Lipiodol® and consisted of mitomycin C alone, mitomycin C with gemcitabine, or mitomycin C with irinotecan. Embolization was performed with starch microspheres. Partial response was achieved in 68 patients (14.7%), stable disease in 223 patients (48.2%), and progressive disease in 172 patients (37.1%). The 1-year survival rate after TACE was 62% and the 2-year survival rate was 28% (54).
In addition to HCC and liver CRC metastases, TACE is also performed for cholangiocarcinoma (55), and hepatic metastases from neuroendocrine tumors (56), breast cancer (57), and other tumors including sarcoma (58), pancreas (59), and gastric cancer (60). There are also a few series supporting the use of TACE as a palliative option for metastatic neuroendocrine liver metastases. In a retrospective analysis, the combination of mitomycin C with gemcitabine was found more effective in controlling local tumor growth than mitomycin C alone, with an improved 5-year survival of 46.7% vs. 11.1% with monotherapy (61). Liapi et al. retrospectively evaluated tumor response in 26 patients with decreased tumor size after treatment but with partial response in only 27% (WHO criteria) and 23% (RECIST criteria). Mean OS was 78 months (62). In cholangiocarcinoma, a single-center study with 115 patients confirmed excellent tumor response rates (57.4% with stable disease). The safety profile and tolerability was also good for the entire cohort with only 15 patients showing adverse effects. Finally the mean OS was 20.8 months with a 3-year survival of 10% (63). The data on the utility of TACE in cholangiocarcinoma is growing, especially in view of TACE ability to elicit a strong tumor response and disease control, As a result, and because the patients are living longer, there is a strong interest in designing studies—notably with DEBs—that would combine TACE with systemic therapies (such as gemcitabine and cisplatin).
Till now, several important issues remain to be clarified including what is the best chemotherapeutic drug, what is the best embolization agent and what is the most appropriate retreatment schedule. Centers differ in the characteristics of the patients treated, the choice of the embolizing agent used, the choice and/or dose of the anticancer agents used, the anticancer/Lipiodol® mixture preparation, embolization end-points, and the schedule and/or interval of retreatment. In the next sections, we will discuss some of the commonly used materials in conventional TACE and discuss some examples used clinically. Then, drug-eluting beads (DEBs) in TACE and radioembolization agents will be discussed. Results from the relevant key RCTs will also be highlighted.
Embolic agents
Transcatheter vascular occlusion can be achieved by using embolization agents such as gelatin sponge, starch microspheres, polyvinyl alcohol (PVA) beads, or collagen particles. Some embolization agents such as PVA polymer are not biodegradable. To allow repeated transcatheter therapy, biodegradable agents, such as gelatin sponge and starch microspheres are used. In general, small embolization agents (less than 100 µm) that embolize end-branches of the hepatic artery are favored as these agents can prevent the development of collateral arterial flow to a tumor. However, embolic agents too small in size such as gelatin powder that are able to reach far smaller vessels can damage extratumoral liver tissue, including biliary duct system.
An early study on an in vivo rat model revealed that a mean particle diameter of at least 40 µm is required for embolization. Microparticles less than 40 µm in diameter can distribute to non-targeted organs, such as the lungs (64). On the other hand, particle size much larger than 1,000 µm can induce catheter clogging. Embolization agents with following size ranges are currently available from various vendors: 40-120, 100-300, 500-700, 700-900 and 900-1,200 µm. The diameter of occluded arteries generally correlates well with the embolic particle size. In addition, slower infusion of more diluted suspension provides a more distal arterial occlusion (65). The elasticity and shape of the particles also play a role; embolization particles with irregular surfaces tend to lodge in larger diameter vessels compared with regularly surfaced particles, and particles with a high degree of elasticity are more likely to reach small vessels (66). One of the common issues during intra-arterial embolization procedure is particle reflux, which could lead to embolization of untargeted areas within an organ or even other vital organs. Generally, large particles occlude more proximal vascular areas more quickly, which increases the risk of reflux and nontarget embolization (67). If the total number of particles injected exceed the target area that can maximally fill, reflux is likely to occur. A reduction in injection rate can reduce the risk of reflux and non-targeted embolisation. The use of calibrated particles (PVA or acrylic copolymer gelatin particles) is increasing worldwide since they can be chosen by size according to the target vessel (68).
Gelatin sponge
Gelatin sponge is one of the most commonly used embolization agents. It is a hemostatic agent composed of purified porcine-derived gelatin, and marketed as Gelfoam®. To prepare the embolisation particles, the gelatin sheets are cut into small pieces, and softened in fluid. Particle sizes are typically in the range of 0.5-2 mm. The vessel occlusion is temporarily, and recanalization occurs within a few days to weeks. Temporary embolization facilitates repeated intra-arterial treatment. As Gelfoam® particle size tends to be of millimeters in size, they are likely to clump in the larger artery and may not penetrate into the targeted small vessels. Gelatin sponge is also available as a powder, and can reach smaller vessels to achieve more distal embolisation. However, as gelatin powder can get much deeper into tissues, it can be more likely to lead to nontargeted embolisation than the Gelfoam® particles.
Polyvinyl alcohol (PVA)
PVA particles cause permanent or semi-permanent vessel occlusion. PVA has a good safety profile. However, because PVA particles can be quite varied in size and shape, the particles tend to clump up occasionally, which can cause catheter clogging. Several vendors developed PVA-based microspheres specifically for TACE, such as PVA (Cook, Bloomington, USA), Contour SE® particles (Boston Scientific, Natick, USA) and Bead Block® (BTG, Surrey UK). DC/LC Bead® (BTG, Surrey UK) is microsphere that consist of PVA with a hydrogel core. The size range of these products varies from 100-1,200 µm (69). PVA could also be used to occlude collaterals that form after repeated embolization with other agents. A comparative study showed little difference in patient survival between TACE performed using gelatin sponge particles and TACE using PVA particles (70).
Embosphere®
To overcome the issue generated with irregular particle size and shape, spherical particles have been developed. Embosphere® is a spherical embolic agent marketed by Merit Medical (Rockland, MA, USA). It is polymeric microsphere made of trisacryl cross-linked with gelatin. It is also a permanent agent, and comes in calibrated size ranges. Due to the lack of aggregation, the smooth and hydrophilic surface, and its deformability, Embosphere® can penetrate deeper and embolise smaller vessels than PVA particles (71). However, it is not yet clear where Embosphere® or PVA is the most clinically effective embolization agent.
Embozene®
Embozene® (CeloNova BioSciences Inc., Atlanta, GA, USA) is a recently developed long acting embolizing agent, composed of a hydrogel core of polymethylmethacrylate and an exterior shell of a proprietary flexible polymer of polyphosphazene: Polyzene®-F, which is shown to be anti-inflammatory and bacterial-resistant (72). Embozene® microspheres are the only microspheres offering tightly calibrated sizes, namely 100, 250, 400, 500, 700, and 900 µm, with each size calibrated to have 95% of the particles within 50 µm of the nominal size. However, it remains to be demonstrated whether Embozene® microsphere, with such a tight controlled particle size, would bring additional clinical benefits for embolisation.
Degradable starch microsphere
Some studies suggested the post-embolization syndromes can be less pronounced using temporary embolizing agents (73). As discussed above, gelatin sponge can maintain occlusion up to several weeks. For shorter duration, Degradable Starch Microsphere (Spherex®, Magle Life Science, Lund, Sweden; EmboCept®, Pharmacept, Berlin, Germany) provides transient occlusion of small arteries. Spherex® consists of sterilized starch microspheres suspended in saline solution. The TACE procedure involves the co-injection of the anticancer drug with Spherex® (74). More recently, EmboCept S® (PharmaCept, Berlin, Germany) has been marketed (in vitro degradation half-life =35 min, only size available is 50 µm). In the blood stream, the starch microspheres are degraded by serum-amylases and the blood flow is restored within 60-80 minutes. Favorable response suggests that TACE using mixture with Spherex®, Lipiodol® and anticancer drug could be a suitable palliative measure in patients who might not tolerate long acting embolic agents (74). Poly (ethylene glycol) methacrylate (PEGMA) hydrolyzable microspheres (ResMic®, Occlugel, Jouy-en-Josas, France) is another calibrated and resorbable embolic agent (75).
Lipiodol®
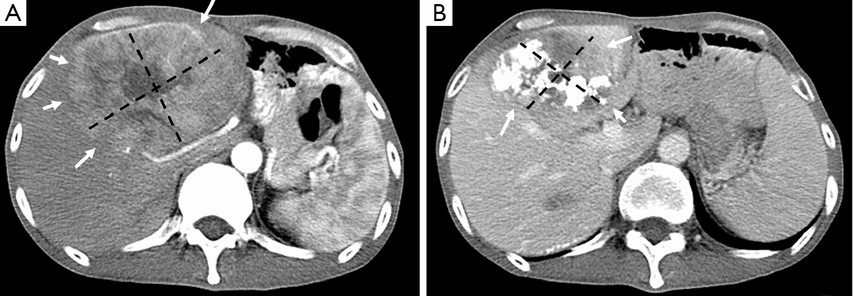
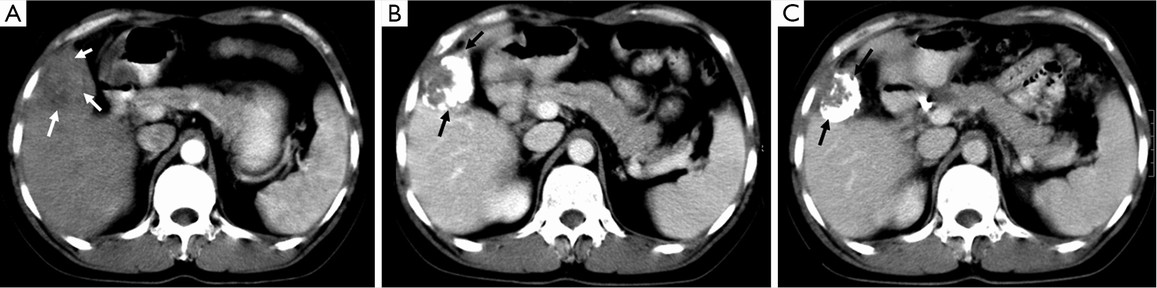
Lipiodol® (Lipiodol® Ultra Fluid, Guerbet, Roissy, France), also known as ethiodized oil, is an oily contrast medium with an iodine content of 38 percent by weight. Its iodine concentration is 480 mg/mL. The viscosity of Lipiodol at 37 °C is approximately 25 mPa.s and its density is 1.28. It consists of a mixture of di-iodinated ethyl esters of fatty acids from poppy seed (Papaver somniferum L.) oil (31). Basically, Lipiodol® combines four characteristics that explain its wide use in TACE procedures: (I) it is opaque to X-rays; (II) it can be used for drug delivery purposes, with substantial versatility regarding the therapy that can be delivered (including immune or gene therapies); (III) it has tumor-seeking properties; (IV) it induces a transient and plastic embolization of tumor microvessels (Figure 5) (76-79). It is not designed to achieve complete and permanent arterial occlusion, as it is eventually washout from the target organ/area. When selectively injected into the hepatic artery, Lipiodol® selectively remains more in tumor nodules for several weeks to over a year due to a siphoning effect from hypervascularization of the tumor vessels and the absence of Kupffer cells inside tumor (Figures 6,7). Non-clinical studies with fluorescent tracer have shown that, in the case of exclusive arterial embolization, the drop in the peribiliary plexus blood pressure would allow portal perfusion of the liver tumor. Conversely, because of its oily nature, Lipiodol® distributes in both the tumor artery branches and the peritumor portal venules, thus allowing transient dual embolization (79).
Lipiodol® is used as a vehicle to carry and localize the anticancer drug inside the tumor. Broad-spectrum of anticancer drugs are used in conjunction with Lipiodol®. When the solubility of the anticancer drug in Lipiodol® is low, the so-called “lipiodolization” technique is used. In brief, the cytotoxic drug is first dissolved in saline. Then the drug dissolved in saline and Lipiodol® are vigorously mixed, and shaken to form an homogeneous mixture. It is recommended to start by pushing the syringe with the anticancer drug first into the Lipiodol® syringe. The mixture is to be prepared at the time of use and must be used promptly after preparation (within 3 hours). If necessary during the procedure, the mixture can be re-homogeneized. When the Lipiodol® and drug mixture is injected into a tumor supplying vessels, the anticancer drug is slowly released from Lipiodol® and remains in high concentrations within the tumor for a prolonged period.
Generally, embolic agent is applied immediately after the injection of the Lipiodol® formulation into the hepatic artery. Further embolization procedures may be necessary if blood supply to the tumor has been unexpectedly developed via various extrahepatic collateral pathways. Studies have shown that Gelfoam® embolization facilitates the slow release of doxorubicin from Lipiodol®, hence further increasing the drug concentration inside the tumor by preventing washout of the mixture (80). Recent studies have tried to develop new formulations. A Lipiodol®–pirarubicin mixture may be more effective and more stable in vitro than the classical doxorubicin-Lipiodol® mixture (81). A novel lipophilic platinum complex (SM-11355), which is a derivative of cisplatin, developed for Lipiodol® suspension, has been shown in clinical studies to lead to a lower plasma platinum concentration but a longer half-life, reflecting the sustained release properties of this formulation (82).
Patients with heterogeneous Lipiodol® uptake on CT scan have higher tendency of recurrences during the follow-up period than those with homogeneous uptake. The degree of Lipiodol® labeling has been found to be an independent prognostic factor (83,84). While Lipiodol® has been widely adopted in TACE protocols, it may also mask assessment of residual vascularity on CT imaging following therapy, thereby requiring routine follow-up with contrast enhanced MRI.
Drug-eluting beads (DEBs)
DEB is a relatively novel drug delivery embolization system, comprising biocompatible, nonresorbable PVA polymeric microspheres doped with sulfonyl groups resulting in a static charge leading to reversible ionic binding with polar molecules such as doxorubicin (Figure 8). These beads allow for fixed dosing and the ability to release the anticancer agents in a sustained and controlled manner. Significant reductions of peak plasma concentrations have been observed with DEBs when compared with conventional chemoembolization in a limited number of patients (86,87). Two particles are commercially available, i.e., DC/LC-Beads® (Biocompatibles, UK) and HepaSphere® (BioSphere Medical, Inc., USA) that can be loaded with doxorubicin for the treatment of HCC.
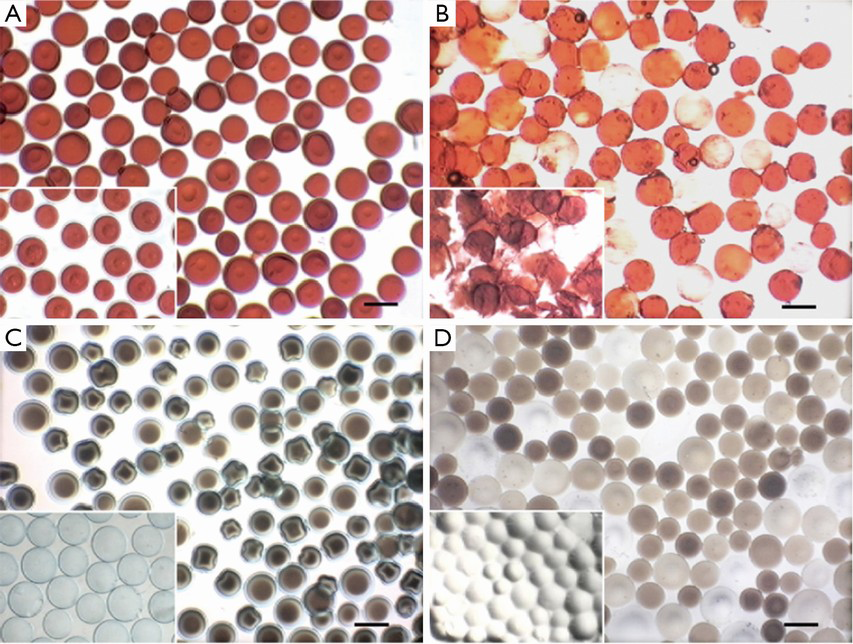
DC/LC-Beads®
The DC/LC Bead® has undergone clinical investigations (88,89). The product is indicated for the treatment of treating a variety of malignant hypervascularised tumours, including HCC. It is a PVA based microspherical embolization agent, prepared from N-acrylamidoacetaldehyde derivatized PVA copolymerized with 2-acrylamido-2-methylpropane sulfonate. The presence of the anionic sulphonate group enables the sequestering of positively charged drugs, such as doxorubicin, epirubicin or irinotecan, by Coulomb charge interactions. The drug is slowly but incompletely released from the beads in the targeted site (85). The transcatheter drug delivery is simplified as the drug (e.g., doxorubicin) and the embolic particles (the sulfonate modified PVA bead) are administered at the same time.
The sizes of the bead are available in different size ranges: 100-300, 300-500, 500-700, and 700-900 µm, with drug loadings varying from 5 to 45 mg/mL hydrated beads (90). Patients could receive three or four chemoembolization treatments within 6 months. It has been demonstrated that DC Bead® spheres could be loaded with doxorubicin to a recommended level of 25 mg/mL hydrated beads, whereas other commercial embolic microspheres such as Contour SE®, Embosphere®, and Bead Block® were shown not to load doxorubicin to the same extent or release it in the same fashion (91). In vitro study showed doxorubicin does not release from the beads when the elution medium was pure water, while when the elution medium contained ions and phosphate-buffered saline solution, reproducible and sustained release profiles were demonstrated (89). With a drug load of 25 mg/mL bead, the rate of drug release from the 700-900 µm beads was slower than that from the 100-300 µm beads, with a half-life of 1,730 and 150 hours, respectively (91). These half-life data translate to a less than 1% and 20% of drug released over 24 hours from the total available drug loaded to the 700-900 and 100-300 µm beads, respectively. In a subsequent study (85), it was shown that the loading and release of doxorubicin followed a dose-response relationship. Using the 500-700 µm beads, it was found that the half-life increased from 381 to 3,658 hours as the concentration of doxorubicin load increased from 6.25 to 37.5 mg/mL. For a fixed drug load of 37.5 mg/mL, the half live was only weakly dependent on bead size, with a minimum of 1,505 hours for the 100-300 µm beads. One study on a rabbit liver VX-2 tumor model confirmed a high level of doxorubicin in the tumor over the entire period of study of 14 days and associated widespread necrosis of the tumor tissue (86). The in vitro elution data of doxorubicin have been shown to correlate well with the areas under the curve of 15 patients treated with DC/LC Bead® loaded with doxorubicin in the PRECISION V clinical study (92). This covered all doses used in the study: 6.25, 12.5, 18.75, 25, and 37.5 mg/mL in 24 hours.
The size of the DC/LC Bead® used is usually selected based on the anatomy of the feeding vessels. It is recommended to choose smaller (100-300 or 300-500 µm) particles first, followed by larger (500-700 µm) particles. Other groups used small (40-120 µm) particles until stasis in the target vessel was achieved. In the case of diffuse tumors, lobar or segmental embolization is performed, and if hepatic vein shunting is identified, larger particles are used to minimize the risk of non-targeted pulmonary embolization. While the DEB relies on passive release/diffusion of drug from the carrier, a delivery system with the ability to actively release the drug payload (e.g., via heat/magnetic triggered release) would enhance the flexibility of the dosing regimen and potentially improve the efficacy of the treatment.
HepaSphere®
HepaSphere® (Merit Medical, Rockland, MA, USA) is biocompatible, hydrophilic (absorbent), nonresorbable, and expandable microsphere. HepaSphere® is conformable and swells upon exposure to aqueous solution. It was made with sodium acrylate and vinyl alcohol copolymer. The particle size is precisely calibrated in the dry state. The dry microsphere absorbs fluid and swells within several minutes when exposed to aqueous-based media. The swollen particle is soft, deformable, and easily delivered through the majority of the currently available microcatheters.
In vitro doxorubicin release has been investigated for DC-Beads® and Hepasheres®. While doxorubicin-loaded DC Beads® maintained their spherical shape throughout the release, Hepaspheres showed less homogeneous drug loading and, after release, some fractured microspheres were found. Interestingly, incomplete doxorubicin release was observed in saline over 1 week for both DEBs (27±2% for DC Beads® and 18±7% for Hepaspheres®; P<0.013). This effect was attributed to strong doxorubicin-bead ionic interactions. With irinotecan, drug release was found to be faster, an effect which may be explained to weaker interactions (92).
The dry HepaSphere® DEBs are supplied in a range of sizes, namely, 50-100, 100-150 and 150-200 µm. In vitro studies demonstrated that particle diameters in ionic contrast media are approximately 2 and 3.5 times larger than the original diameters in the dry state and 4 times larger in human serum. The polymer contained within HepaSphere® is anionic, which allows the sequestering of cationic drug molecule, such as doxorubicin or epirubicin by Coulomb charge attraction (as in the case of DC Bead®). This enables cationic chemotherapeutic agent to be carried within the microsphere. Moreover, these particles, because of the slightly larger expansion in human serum, are able to mold to the morphology of the vessel lumen.
HepaSphere® has been evaluated in an initial clinical study which comprised of 50 patients in four centers (93). The microspheres were either loaded with doxorubicin (mean dose 43.6±8.7 mg) or with epirubicin (mean dose 41.7±14.6 mg). It has been shown that that TACE using HepaSphere® is feasible, well-tolerated, and is associated with good tumor response. Repeated TACE procedures can be carried out without difficulties. The objective response rate of the initial HepaSphere® study was comparable to that of DC Bead® obtained in initial clinical studies (93). However, it is currently unclear of the clinical benefit of using HepaSphere® over DC Bead®, other DEBs or conventional TACE.
Irinotecan-eluting beads
5-FU has been the standard treatment for CRC metastases for more than 40 years. Irinotecan, a topoisomerase inhibitor, has recently been developed as a chemotherapy agent for the treatment of CRC metastases. With the combined use of 5-FU and irinotecan the survival rate of CRC patients has been shown to improve significantly comparing with those given 5-FU alone. Based on the interest of DC Bead®, the same vendor (BTG, Surrey, UK) has developed irinotecan-eluting bead for the treatment of liver CRC metastases (94). The system consists in combining embolisation beads (DEBIRI®) with irinotecan hydrochloride solution. It has been shown that the DEB of sizes ranging from 100-900 µm can load irinotecan up to a maximum capacity of 50 mg/mL of beads. The in vitro release profile of irinotecan was shown to be sustained and dependent on the presence of ions in the elution medium, drug loading, and bead size (95). Irinotecan-eluting bead is currently undergoing several RCTs in the treatment of liver metastases of CRC (96,97).
Conventional TACE (c-TACE) versus DEB-TACE for hepatocellular carcinoma management: a comparison
In a small and non-comparative study, TACE performed using DEBs loaded with doxorubicin has been shown to reduce the drug-related side effects while maintaining the same therapeutic efficacy (87). A prospective randomized comparison of chemoembolization with doxorubicin-eluting DC/LC Beads® and arterial bland embolization with Bead Block® PVA microspheres (BTG, UK) for HCC concluded that there is an additional benefit from the addition of doxorubicin (98). In this study, there was a complete response in 26.8% of patients in the DEB group and 14% in the arterial bland embolisation group at 6 months. Time to progression was longer for the DEB group than in the group with bland embolisation (42.4±9.5 vs. 36.2±9.0 weeks, P=0.008). The prospective randomized PRECISION V phase II study compared TACE doxorubicin loaded DC Beads® to conventional TACE procedure (intra-arterial injection of doxorubicin emulsified in Lipiodol® followed by particle embolization with Gelfoam® or PVA particles). The primary endpoint was tumor response according to the amended EASL criteria (99). This study included 212 HCC patients with large or multinodular HCC. At six months, both groups had similar tumor response rate (complete response in the DC/LC Beads® group: 27%, in the conventional TACE group: 22%, objective response rate: 57% and 44% respectively and disease control rate: 63% vs. 53%, P=0.11). Treatment-related serious adverse events within 30 days of the procedure were similar. However, secondary safety outcomes, including incidence and severity of adverse events, liver function parameters, and cardiac function, were significantly better in the DC Beads® group (100). A sub-analysis of this trial showed that liver toxicity and cardiac toxicity were significantly lower in DC/LC Beads® group (101). Subsequently, a RCT compared TACE doxorubicin loaded DC Beads® to conventional TACE followed by selective embolization with gelatin sponge particles, in 67 patients with unresectable HCC. The one-month complete response rates were 51.5 and 70.6% after DEB-TACE and conventional TACE respectively. No difference between groups was found with respect to time to recurrence, local recurrence, radiological progression and survival. The increase in alanine aminotransferase was higher in the conventional TACE than in the DEB-TACE at 24 hours (102). A recent randomized clinical trial (PRECISION Italia study) compared the clinical efficacy and safety of DC-Beads® and conventional TACE in 177 patients. The 1- and 2-year survival rates were similar: 86.2% and 56.8% after DC/LC-Bead®-based TACE and 83.5% and 55.4% after conventional TACE (P=0.949). There were no differences in terms of adverse events incidence and severity, except less post-procedural pain with DEBs (103). Two recent meta-analyses (both concerning 7 RCTs and around 700 patients) comparing DEB-TACE with conventional TACE concluded that both techniques lead to similar clinical response and tolerance (104,105).
In a retrospective study of patients treated for a well-differentiated metastatic neuroendocrine tumors or HCC, the occurrence of biloma and parenchymal infarct was significantly associated with DEB-TACE, irrespectively of the tumor type (106). Similar results were subsequently reported in patients treated for neuroendocrine liver metastases (107).
In a recent retrospective study of 164 patients receiving 374 TACE, multivariate analysis revealed that DEBs of size >300 µm induced more non-tumoral liver necrosis compared to Lipiodol®-based TACE or DEBs <300 µm, and pretreatment bile duct dilatation and PVT were predictive of liver necrosis (108). As with conventional TACE, DEB-TACE is generally well tolerated and not surprisingly the spectrum of adverse events is similar to conventional TACE. It has a more favourable pharmacokinetic profile than conventional TACE that translated into less doxorubicin-related systemic adverse events in one RCT (100). With DEBs launching, physicians hoped to standardize TACE procedure in comparison with conventional practice (109), aimed at defining standards for an appropriate and consistent use DC/LC-Beads®. These general guidelines are related to pretreatment imaging, peri-procedure medication, loading dose of doxorubicin, planned dose of doxorubicin, choice of beads size, beads dilution, catheter positioning, injection rate and embolization end-point. However, given the many patient- and tumor-related variables that play a role in the decision-making process and given the complexity of HCC, individual patient and tumor characteristics may require a different approach with DEBs which often require a customized/non-standardized approach.
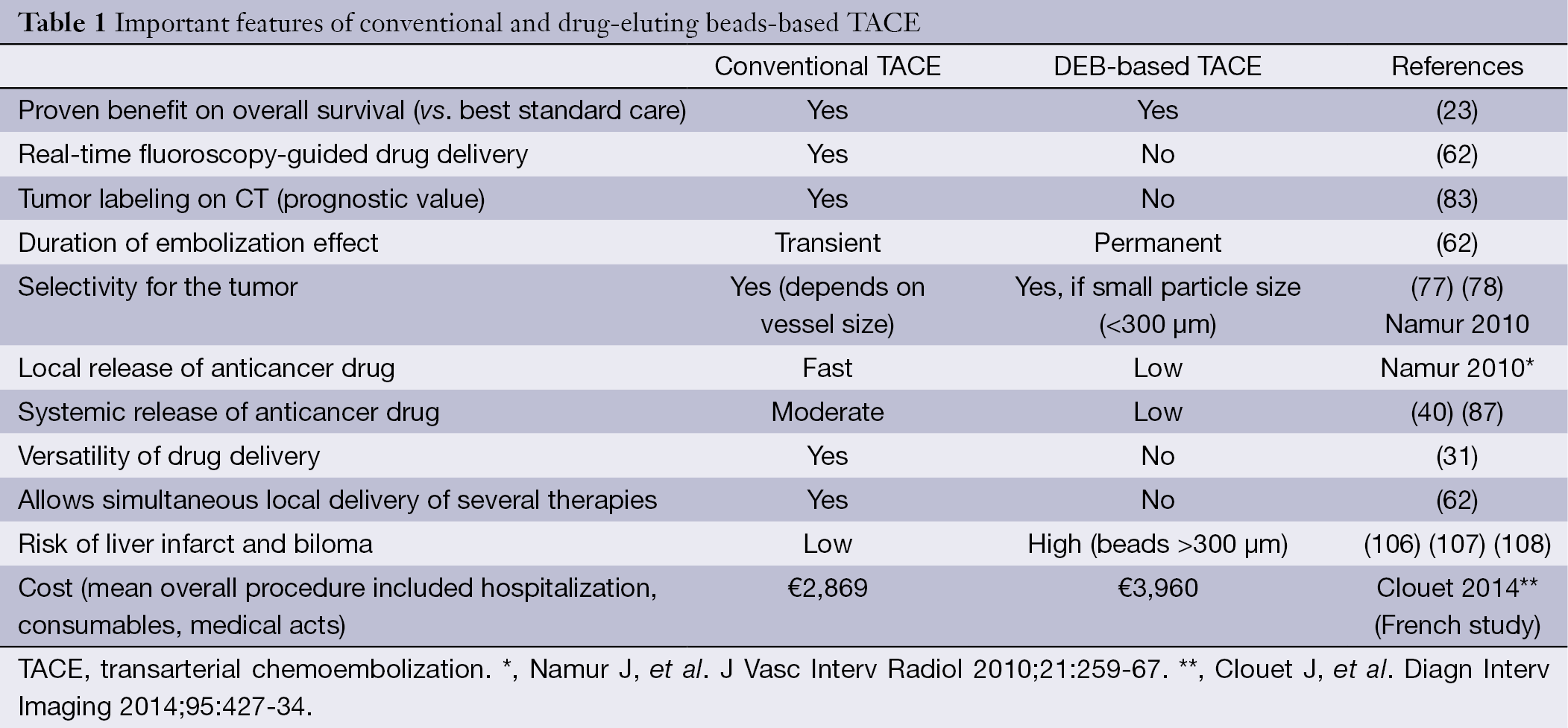
The most important features of conventional and drug-eluting beads-based TACE are summarized in Table 1.
Full table
Transarterial radioembolization (TARE)
External beam irradiation has historically played a limited role in the treatment of HCC due to the radiosensitive nature of normal hepatic tissue. Radiation exposure limit in the liver is rather 70 Gy in non-cirrhotic liver and 50 Gy in cirrothic liver. Liver exposure to greater radiation doses may result in a clinico-pathological syndrome characterized by ascites, anicteric hepatomegaly, and elevated liver enzymes, developing weeks to months following therapy. Given these limitations, minimally invasive TARE has emerged. Radioembolization is defined as the injection of micron-sized embolic particles loaded with a radioisotope by using percutaneous transarterial techniques. Yttrium-90 (90Y) is commonly used for this purpose. 90Y is a pure beta emitter that decays to stable zirconium. Its physical half-life is 64.2 hours. The emissions generated have a mean tissue penetration of 2.5 mm, with a maximum reach of 11 mm. This limited tissue penetration allows for local high dose radiation with less risk of radiation induced hepatic necrosis than may be seen with external beam therapy. Two types of microspheres are commercially available, i.e., SIR-Spheres® (Sirtex Medical Limited, Australia) and TheraSphere® (Biocompatibles, UK). These two devices are different in a number of important respects. TheraSphere® has higher specific activity (2,500 Bq) and lower number of spheres (1.2 million microspheres/3 GBq). Conversely, SIR-Sphere® has lower specific activity (50 Bq), and greater number of spheres (approximately 40-80 million spheres/3 GBq).
TheraSphere® was approved in 1999 by the Food and Drug Administration under humanitarian device exemption for the treatment of unresectable HCC in patients who can have appropriately positioned hepatic arterial catheters. TheraSphere® is composed of non-biodegradable glass microspheres ranging from 20 to 30 µm in diameter, in which 90Y is an integral constituent of the glass. One gigabecquerel (27 mCi) of 90Y per kilogram of tissue provides a dose of 50 Gy. The microspheres are supplied in 0.5 mL of sterile, pyrogen-free water contained in a 0.3-mL V-bottom vial secured within a 12-mm clear acrylic shield. The specific activity is 2,500 Bq at the time of calibration.
SIR-Sphere® was granted premarketing approval in 2002 from the Food and Drug Administration for the treatment of colorectal metastases in conjunction with intrahepatic floxuridine, an analog of 5-FU. SIR-Sphere® consists of biodegradable resin-based microspheres containing 90Y. The average size of a sphere is 35 µm (range, 20-60 µm) in diameter. Each vial contains 3 GBq of 90Y in a 5 mL vial. Each vial contains 40-80 million spheres. The activity per microsphere is 50 Bq at the time of calibration.
Rhenium-188 radioconjugate can be available through the use of a Rhenium-188 generator. The half-life of Rhenium-188 is 16.9 hours. The isotope delivers high-energy beta emission (2.1 MeV max) and a low energy gamma emission (155 keV) permitting SPECT/PET imaging for dosimetry step and follow-up post-TARE. Usually, this radioconjugate is in the form of Rhenium-188 4-hexadecyl 1, 2, 9, 9-tetramethyl-4, 7 diaza-1, 10-decaethaniol labeled with Lipiodol® (110). Dosimetry is based on the safe and tolerable dose to organs at risk including the liver, lungs and bone.
In contrast with the larger than 100 microns particles used in TACE to occlude tumor feeding vessels, much smaller particles (25-35 microns) are used in TARE to reach the tumor microvasculature. Clinical experience with TARE has shown a low incidence of post-embolisation syndrome, supporting its minimally embolic effect (110-123). Gulec et al. (117) retrospectively analyzed the data from a heterogeneous cohort of 40 patients with primary and metastatic liver malignancies who underwent treatments using 90Y resin microspheres (SIR-Sphere®). The average administered activity was 1.2 GBq and tumor absorbed doses ranged from 40.1 to 494.8 Gy. The authors concluded that doses up to 100 Gy to the uninvolved liver were tolerated by this procedure without the development of veno-occlusive disease or liver failure. The authors further noted that lowest tumor dose necessary to generate a detectable response was 40 Gy.
Broadly equivalent survivals after TACE and TARE have been reported in retrospective analyses of single institutions. A comparative analysis was reported including 463 patients treated with either TACE or TARE (118). Fatigue and fever were more common following TARE; while abdominal pain, diarrhea and aminotransferases elevations were more frequent following TACE. Response rate was in favor of TARE over TACE (49% vs. 36%, P=0.052). Overall, although TARE time to progression was significantly better than TACE (13.3 vs. 8.4 months, P=0.0232), median 5-year survival was not significantly different. In the largest comparative study, all-type adverse events, response rate and time to progression were better in TARE than in conventional TACE but OS was no different (119).
Most patients currently treated by TARE are poor candidates to TACE because of a high tumor burden, presence of vascular invasion or lack of response to previous TACE. Radioembolization is one of the more technically challenging transcatheter embolisation procedures because of the risk of non-target embolisation. Two absolute contraindications exist for the use of 90Y microsphere treatment in any patient (116,123). The first is a pretreatment 99mTc macro-aggregated albumin (MAA) scan demonstrating significant hepato-pulmonary shunting (>20%) that would result in >30 Gy being delivered to the lungs with a single infusion or as much as 50 Gy for multiple infusions. The second is the inability to prevent deposition of microspheres to the gastrointestinal tract with modern catheterization techniques. Patients can only be considered for TARE is the degree of arterio-venous shunting to the lung is limited (usually less than 20%) and there is no possibility that microspheres may reach the gastrointestinal tract.
Evidence supporting the use of TARE in the treatment of HCC patients comes from consistent, large cohort series involving patients with more advanced HCC, not suitable for other locoregional therapies or who have failed to TACE. Radioembolization can be used in HCC patients who progressed to TACE and for those in the advanced stage because of portal vein invasion.
Many clinical studies (total of 25 in USA and Europe) with TheraSphere® and SIR-Sphere® are on-going to evaluate feasibility, efficacy and tolerance in primary and secondary liver cancer (HCC, ICH, mCRC and NET) management. Ongoing trials will also answer the question of whether radioembolization is any better than sorafenib in prolonging the survival of poor TACE candidates. Altogether treatment with intra-arterial therapies (TACE+TARE) procedures of all primary and secondary liver cancer lesions is estimated higher than half a million in the world per year based on market studies of intra-arterial devices/products.
Response assessment of TACE in HCC patients
The range of patients treated by TACE in clinical practice largely exceeds the boundaries of the intermediate stage and reported survivals widely range from 8-26% at five years (13). Among 4,966 Japanese patients without vascular invasion, extrahepatic metastases or prior treatment that received superselective conventional TACE, median survival was 3.3 years (124). However, when median survival is reported by tumor stage, it ranges from 16 to 45 months in the early stage, from 15.6 to 18.2 months in intermediate stage, and from 6.8 to 13.6 months in the advanced stage (13).
Radiologic parameters by CT and MRI may be useful in biological characterization of tumors and predictive efficacy for HCC treated with chemoembolization. OS was significantly longer for patients with completely encapsulated HCC versus patients with incompletely or nonencapsulated tumors (125). Kim et al. (126) reported that gross vascular invasion, bile duct invasion, irregular tumor margin, peripheral ragged enhancement, and satellite nodules on CT or MRI were associated with less favorable response after chemoembolization. After adjusting tumor size, tumor number, and alpha-fetoprotein (AFP) level, these CT and MRI scores were independently associated with OS. MRI-specific parameters such as signal intensity on T2- or T1-weighted images, fat signal, or hyperintensity on diffusion-weighted images did not have prognostic value. Kawamura et al. (127) reported that the arterial- and portal-phase dynamic CT images obtained preoperatively were classified into four enhancement patterns: Type-1 and Type-2 are homogeneous enhancement patterns without or with increased arterial blood flow, respectively; Type-3, heterogeneous enhancement pattern with septum-like structure; and Type-4, heterogeneous enhancement pattern with irregular ring-like structures. The percentages of poorly-differentiated HCC according to the enhancement pattern were 6% of Type-1 and -2, 13% of Type-3, and 73% of Type-4. Type-4 pattern was a significant and independent predictor of poorly-differentiated HCC while Type-3 pattern was a significant predictor of simple nodular type with extranodular growth or confluent multi-nodule.
Assessment of tumor response is of extreme importance in patients undergoing locoregional treatments of liver cancer. The Clinical Practice Guidelines jointly issued by the EASL and the European Organization for Research and Treatment of cancer (EORTC) state that assessment of response in HCC should be based on mRECIST criteria by performing contrast-enhanced CT or MRI 4 weeks after treatment. Conventional methods, such as classical Response Evaluation Criteria in Solid Tumors (RECIST) criteria, have no predictive value in HCC patients treated with TACE or TARE (128). These criteria only rely on tumor shrinkage as a measure of antitumor activity, an assumption that is only valid with cytotoxic drugs. TACE and TARE induce direct tumor necrosis and their anticancer activity is not predictive to a reduction in overall tumor load but rather to a reduction in viable tumor, as identified by contrast-enhanced radiologic imaging. Thus, a modification of the RECIST criteria, named modified RECIST (mRECIST), for HCC based on the fact that diameter of the target lesions with viable tumor, should guide all measurements. Treatment response after TACE is assessed with identification of intra-tumoral necrotic areas and reduction of tumor burden in dynamic studies in regular intervals utilizing cross sectional modalities, such as triphasic CT or MRI. In addition, specific modifications of the original criteria regarding assessment of vascular invasion, lymph nodes, ascites, pleural effusion and new lesions have been introduced (129). Tumor response measured by EASL or mRECIST after TACE has been shown to correlate with survival outcomes (130,131).
Pre-procedural AFP has not been demonstrated to be a prognostic marker of post procedural clinical response. In patients with high AFP before treatment, subsequent decrease after treatment is indicative of response; however, this is not reliable, and monitoring of AFP should not substitute dynamic imaging studies. Immediate post procedural elevations in tumor markers may be reflective of cellular lysis, not disease progression, and should not be used to assess response in the acute setting.
Patients that show no tumor response shortly after TACE is completed have a worse prognosis. If complete tumor necrosis is not achieved after the first session of TACE, a second attempt is warranted because feeding arteries may have been missed. However, patients that do not respond to two consecutive sessions of TACE should be considered for alternative therapies (13).
Recently Wang et al. (132) showed evidence of an association between intraprocedural tumor perfusion reduction during chemoembolization and transplant-free survival and suggests the utility of transcatheter intraarterial perfusion magnetic resonance (MR) imaging measured tumor perfusion reduction as an intraprocedural imaging biomarker during chemoembolization. Loffroy and colleagues (133) proposed the use of intraprocedural C-arm dual phase- cone-beam computed tomography immediately after TACE with doxorubicin-eluting beads to predict HCC tumor response at 1-month MR imaging follow-up. They reported a significant relationship between tumor enhancement seen at DP-CBCT after TACE and objective MR imaging response at 1-month follow-up, suggesting that DP-CBCT can be used to predict tumor response after TACE. Sahani et al. suggested that perfusion MRI may be a more sensitive biomarker in predicting early response than RECIST and mRECIST (134,135). Other functional imaging methods, such as 18F-fluorodeoxyglucose PET, contrast-enhanced ultrasound have been used to assess post-treatment evaluation (136-141). However, Xu et al. recently suggested that contrast enhanced ultrasound may occasionally miss small residual tumorous nodule (142).
Current and future developments
Combined therapies
There are several theoretical reasons to combine TACE and other recommended therapies such as RFA or sorafenib. RFA is an excellent therapeutic approach of small (<3 cm) lesions. As the size of lesions increases, its local efficacy is reduced, due to a maximum volume of ablation in the range of 4 cm, and in heat loss due to perfusion mediated tissue cooling. It has been demonstrated in animal model that performing TACE before RFA increase volume of ablation (143), thus making this approach of interest in large tumors (144). TACE may also allow down-staging of 3-5 cm lesions to permit subsequent RFA treatment. A RCT in 189 patients with HCC <7 cm showed that patients assigned to conventional TACE+RFA had better OS and recurrence-free survival than patients on RFA only (145). A recent meta-analysis compares the effectiveness of combination of RFA and TACE with that of RFA alone in HCC patients (7 trials comprising 571 patients). The combination of RFA and TACE was associated with a significantly higher OS rates and recurrence-free survival rate compared with RFA alone (146).
Combining TACE and sorafenib has also a strong theoretical rationale. Tumor hypoxia intentionally caused by TACE can induce upregulation of circulating vascular endothelial growth factor (VEGF), which is essential for HCC growth, invasion, and metastasis. Recent studies have reported a significant association between VEGF upregulation after TACE and poor prognosis (147,148). Sorafenib is an oral multitargeted receptor tyrosine kinase inhibitor with, notably, VEGFR-2/3 inhibitory properties. Sorafenib (Nexavar®, Bayer and Onyx Pharmaceuticals Inc., USA) was approved from the United States Food and Drugs Administration (FDA), the European Medicines Agency (EMA), Chinese Health Authorities, etc. for the treatment of advanced HCC (149,150). The addition of sorafenib to TACE compared to TACE alone in patients with advanced or intermediate unresectable HCC and good liver function is feasible with a rate of adverse events predictable and manageable with dose reduction (149,150). In the SPACE trial, the safety and efficacy of sorafenib vs. placebo associated with DEB-TACE (DEBDOX®) was investigated in 304 patients with intermediate-stage HCC. Addition of sorafenib to DEB-TACE improved time-to radiological progression (TTP). Median TTP was 169 and 166 days in the sorafenib and placebo groups respectively (HR 0.797, 95% CI: 0.588-1.080, P=0.07). TTP at the 25th and 75th percentiles (preplanned) was 112/88 and 285/224 days in the sorafenib and placebo groups, respectively (151). Several clinical trials are currently evaluating this combined effect on the outcome of patients with unresectable HCC [e.g., on the site ClinicalTrials.gov, studies number NCT01833299; NCT01906216 (the SELECT trial); NCT01829035; etc.]
The risk exists of early rebound with VEGF release leading to tumor relapse. Several important questions remain open, such as the best sequential timing of targeted therapy and TACE to prevent such rebound effect, the best imaging technique to evaluate clinical response, the best targeted drug to use in combination with TACE, and the most reliable primary endpoints.
Immune therapy
Second generation immune therapy of tumors is attracting widespread attention, including for HCC (152,153). The liver is permanently exposed to food-derived dietary and microbial antigens from the gastro-intestinal tract, as well as antigens from apoptotic tumour cells, thus leading to liver being an inherent tolerogenic microenvironment (154). Local immune therapy is an interesting option for the treatment of HCC or liver metastases. Promising results on survival have been reported in patients with liver metastases from primary uveal melanoma after immunoembolization with granulocyte-macrophage colony-stimulating factor (GM-CSF) mixed with Lipiodol® (associated with Gelfoam®) and administered into the hepatic artery (155). Local administration of dendritic cells (DCs) stimulated with OK432, a streptococcus-derived anti-cancer immunotherapeutic agent, in the presence of interleukin (IL)-4 and GM-CSF, during TACE procedure in HCC patients has been found to be safe and prolonged recurrence-free survival of patients compared with the historical controls treated with transcatheter hepatic arterial embolization without DC transfer (156). Minimally invasive thermal ablation techniques (cryoablation or hyperthermic ablations) are associated with the local release of tumour antigens (157) which may lead to innovative techniques of immune therapy, possibly involving Lipiodol® as a drug-delivery system. However, many challenges remain as individual cancers have their own pattern of cancer antigen expressions, thus making the development of universally applicable therapy difficult. Indeed, safety issue is crucial. The involvement of large number of tumor-associated antigens is another challenging issue.
Conclusions
In patients diagnosed with HCC, a survival benefit has been observed in patients that meet the rigorous criteria for curative resection or transplantation (158). TACE has been proven to be useful in local tumor control, to prevent tumor progression, prolong patients’ life and control patient symptoms. TACE alone or combined with other minimally invasive procedures can also be used as a neoadjuvant therapy or as a bridging therapy to liver transplantation or resection. In the latter condition it prevents tumor progression and patient drop-out from the waiting list of liver transplantation. Multimodal treatment may be the best way to optimize TACE/TARE outcomes in HCC. So far, there is no significant evidence of the clinical superiority of DEB-TACE over conventional TACE in terms of clinical efficacy. TARE may be safe in advanced disease, including portal vein invasion and larger tumors. With introduction of sorafenib as standard treatment for advanced HCC, phase II and III studies are ongoing to explore safety and efficacy of RFA, TACE or TARE in combination with sorafenib or targeted drugs under clinical development. With these and other studies, the clinical indications and specific patients ideally suited for these palliative interventions will continue to be refined.
Acknowledgements
Disclosure: Jean-Marc Idée and Sébastien Ballet are employees of Guerbet (France). Guerbet markets contrast agents and specifically Lipiodol® mentioned in this review. Other authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011;29:339-64. [PubMed]
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417-22. [PubMed]
- Maurea S, Mainenti PP, Tambasco A, et al. Diagnostic accuracy of MR imaging to identify and characterize focal liver lesions: comparison between gadolinium and superparamagnetic iron oxide contrast media. Quant Imaging Med Surg 2014;4:181-9. [PubMed]
- Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996;111:1018-22. [PubMed]
- Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173-84. [PubMed]
- Scheuermann U, Kaths JM, Heise M, et al. Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma--a single-center experience. Eur J Surg Oncol 2013;39:593-600. [PubMed]
- Dodson RM, Weiss MJ, Cosgrove D, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg 2013;217:736-750.e4.
- Adam R, Lucidi V, Bismuth H. Hepatic colorectal metastases: methods of improving resectability. Surg Clin North Am 2004;84:659-71. [PubMed]
- Julianov A. Radiofrequency ablation or resection for small colorectal liver metastases - a plea for caution. Quant Imaging Med Surg 2013;3:63-6. [PubMed]
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1-18. [PubMed]
- Rindi G, D'Adda T, Froio E, et al. Prognostic factors in gastrointestinal endocrine tumors. Endocr Pathol 2007;18:145-9. [PubMed]
- Sangro B. Chemoembolization and radioembolization. Best Pract Res Clin Gastroenterol 2014;28:909-19. [PubMed]
- Yang HJ, Lee JH, Lee DH, et al. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology 2014;271:909-18. [PubMed]
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8. [PubMed]
- Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008;47:82-9. [PubMed]
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 2003;226:441-51. [PubMed]
- Giorgio A, Di Sarno A, De Stefano G, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res 2011;31:2291-5. [PubMed]
- Oeda S, Mizuta T, Isoda H, et al. Survival advantage of radiofrequency ablation for hepatocellular carcinoma: comparison with ethanol injection. Hepatogastroenterology 2013;60:1399-404. [PubMed]
- Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013;59:300-7. [PubMed]
- Forner A, Bruix J. Ablation for hepatocellular carcinoma: Is there need to have a winning technique? J Hepatol 2010;52:310-2. [PubMed]
- Pompili M, De Matthaeis N, Saviano A, et al. Single hepatocellular carcinoma smaller than 2 cm: are ethanol injection and radiofrequency ablation equally effective? Anticancer Res 2015;35:325-32. [PubMed]
- European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43.[PubMed]
- Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691-700.e3.
- Sherman M. Staging for hepatocellular carcinoma: complex and confusing. Gastroenterology 2014;146:1599-602. [PubMed]
- Yang HJ, Lee JH, Lee DH, et al. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology 2014;271:909-18. [PubMed]
- Hsu CY, Huang YH, Chiou YY, et al. Comparison of radiofrequency ablation and transarterial chemoembolization for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Liver Transpl 2011;17:556-66. [PubMed]
- Hsu KF, Chu CH, Chan DC, et al. Superselective transarterial chemoembolization vs hepatic resection for resectable early-stage hepatocellular carcinoma in patients with Child-Pugh class a liver function. Eur J Radiol 2012;81:466-71. [PubMed]
- Yamada R, Sato M, Kawabata M, et al. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 1983;148:397-401. [PubMed]
- Yamada R, Nakatsuka H, Nakamura K, et al. Hepatic artery embolization in 32 patients with unresectable hepatoma. Osaka City Med J 1980;26:81-96. [PubMed]
- Idée JM, Guiu B. Use of Lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: a review. Crit Rev Oncol Hematol 2013;88:530-49. [PubMed]
- Brown DB, Gould JE, Gervais DA, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2007;18:1469-78. [PubMed]
- Bruix J, Sherman M; Practice Guidelines Committee, et al. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [PubMed]
- Vogl TJ, Naguib NN, Nour-Eldin NE, et al. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol 2009;72:505-16. [PubMed]
- Rose SC, Kikolski SG, Chomas JE. Downstream hepatic arterial blood pressure changes caused by deployment of the surefire antireflux expandable tip. Cardiovasc Intervent Radiol 2013;36:1262-9. [PubMed]
- Morshedi MM, Bauman M, Rose SC, et al. Yttrium-90 resin microsphere radioembolization using an antireflux catheter: an alternative to traditional coil embolization for nontarget protection. Cardiovasc Intervent Radiol 2015;38:381-8. [PubMed]
- Lee JH, Won JH, Park SI, et al. Transcatheter arterial chemoembolization of hepatocellular carcinoma with hepatic arteriovenous shunt after temporary balloon occlusion of hepatic vein. J Vasc Interv Radiol 2007;18:377-82. [PubMed]
- Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol 2007;30:6-25. [PubMed]
- Yoshimitsu K. Transarterial chemoembolization using iodized oil for unresectable hepatocellular carcinoma: perspective from multistep hepatocarcinogenesis. Hepat Med 2014;6:89-94. [PubMed]
- Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011;37:212-20. [PubMed]
- Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 2011;258:627-34. [PubMed]
- Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol 2011;18:413-20. [PubMed]
- Xue TC, Xie XY, Zhang L, et al. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol 2013;13:60. [PubMed]
- Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol 2009;10:425-34. [PubMed]
- Park SH, Cho YK, Ahn YS, et al. Local recurrence of hepatocellular carcinoma after segmental transarterial chemoembolization: risk estimates based on multiple prognostic factors. Korean J Radiol 2007;8:111-9. [PubMed]
- Llovet JM, Sala M, Castells L, et al. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology 2000;31:54-8. [PubMed]
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71. [PubMed]
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002;224:47-54. [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42. [PubMed]
- Ikeda M, Arai Y, Park SJ, et al. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol 2013;24:490-500. [PubMed]
- O'Suilleabhain CB, Poon RT, Yong JL, et al. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg 2003;90:325-31. [PubMed]
- Gerunda GE, Neri D, Merenda R, et al. Role of transarterial chemoembolization before liver resection for hepatocarcinoma. Liver Transpl 2000;6:619-26. [PubMed]
- Tellez C, Benson AB 3rd, Lyster MT, et al. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer 1998;82:1250-9. [PubMed]
- Vogl TJ, Gruber T, Balzer JO, et al. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology 2009;250:281-9. [PubMed]
- Burger I, Hong K, Schulick R, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol 2005;16:353-61. [PubMed]
- Vogl TJ, Gruber T, Naguib NN, et al. Liver metastases of neuroendocrine tumors: treatment with hepatic transarterial chemotherapy using two therapeutic protocols. AJR Am J Roentgenol 2009;193:941-7. [PubMed]
- Giroux MF, Baum RA, Soulen MC. Chemoembolization of liver metastasis from breast carcinoma. J Vasc Interv Radiol 2004;15:289-91. [PubMed]
- Rajan DK, Soulen MC, Clark TW, et al. Sarcomas metastatic to the liver: response and survival after cisplatin, doxorubicin, mitomycin-C, Ethiodol, and polyvinyl alcohol chemoembolization. J Vasc Interv Radiol 2001;12:187-93. [PubMed]
- Azizi A, Naguib NN, Mbalisike E, et al. Liver metastases of pancreatic cancer: role of repetitive transarterial chemoembolization (TACE) on tumor response and survival. Pancreas 2011;40:1271-5. [PubMed]
- Vogl TJ, Gruber-Rouh T, Eichler K, et al. Repetitive transarterial chemoembolization (TACE) of liver metastases from gastric cancer: local control and survival results. Eur J Radiol 2013;82:258-63. [PubMed]
- Vogl TJ, Gruber T, Naguib NN, et al. Liver metastases of neuroendocrine tumors: treatment with hepatic transarterial chemotherapy using two therapeutic protocols. AJR Am J Roentgenol 2009;193:941-7. [PubMed]
- Liapi E, Geschwind JF, Vossen JA, et al. Functional MRI evaluation of tumor response in patients with neuroendocrine hepatic metastasis treated with transcatheter arterial chemoembolization. AJR Am J Roentgenol 2008;190:67-73. [PubMed]
- Vogl TJ, Naguib NN, Nour-Eldin NE, et al. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: Results and prognostic factors governing treatment success. Int J Cancer 2012;131:733-40. [PubMed]
- Bastian P, Bartkowski R, Köhler H, et al. Chemo-embolization of experimental liver metastases. Part I: distribution of biodegradable microspheres of different sizes in an animal model for the locoregional therapy. Eur J Pharm Biopharm 1998;46:243-54. [PubMed]
- Choe DH, Han MH, Kang GH, et al. An experimental study of embolic effect according to infusion rate and concentration of suspension in transarterial particulate embolization. Invest Radiol 1997;32:260-7. [PubMed]
- Siskin GP, Dowling K, Virmani R, et al. Pathologic evaluation of a spherical polyvinyl alcohol embolic agent in a porcine renal model. J Vasc Interv Radiol 2003;14:89-98. [PubMed]
- López-Benítez R, Richter GM, Kauczor HU, et al. Analysis of nontarget embolization mechanisms during embolization and chemoembolization procedures. Cardiovasc Intervent Radiol 2009;32:615-22. [PubMed]
- Bilbao JI, de Luis E, García de Jalón JA, et al. Comparative study of four different spherical embolic particles in an animal model: a morphologic and histologic evaluation. J Vasc Interv Radiol 2008;19:1625-38. [PubMed]
- Kettenbach J, Stadler A, Katzler IV, et al. Drug-loaded microspheres for the treatment of liver cancer: review of current results. Cardiovasc Intervent Radiol 2008;31:468-76. [PubMed]
- Brown DB, Pilgram TK, Darcy MD, et al. Hepatic arterial chemoembolization for hepatocellular carcinoma: comparison of survival rates with different embolic agents. J Vasc Interv Radiol 2005;16:1661-6. [PubMed]
- Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Intervent Radiol 2008;25:204-15. [PubMed]
- Bonomo G, Pedicini V, Monfardini L, et al. Bland embolization in patients with unresectable hepatocellular carcinoma using precise, tightly size-calibrated, anti-inflammatory microparticles: first clinical experience and one-year follow-up. Cardiovasc Intervent Radiol 2010;33:552-9. [PubMed]
- Vogl TJ, Zangos S, Eichler K, et al. Colorectal liver metastases: regional chemotherapy via transarterial chemoembolization (TACE) and hepatic chemoperfusion: an update. Eur Radiol 2007;17:1025-34. [PubMed]
- Kirchhoff TD, Bleck JS, Dettmer A, et al. Transarterial chemoembolization using degradable starch microspheres and iodized oil in the treatment of advanced hepatocellular carcinoma: evaluation of tumor response, toxicity, and survival. Hepatobiliary Pancreat Dis Int 2007;6:259-66. [PubMed]
- Louguet S, Verret V, Bédouet L, et al. Poly(ethylene glycol) methacrylate hydrolyzable microspheres for transient vascular embolization. Acta Biomater 2014;10:1194-205. [PubMed]
- Tam KY, Leung KC, Wang YX. Chemoembolization agents for cancer treatment. Eur J Pharm Sci 2011;44:1-10. [PubMed]
- de Baere T, Dufaux J, Roche A, et al. Circulatory alterations induced by intra-arterial injection of iodized oil and emulsions of iodized oil and doxorubicin: experimental study. Radiology 1995;194:165-70. [PubMed]
- de Baere T, Zhang X, Aubert B, et al. Quantification of tumor uptake of iodized oils and emulsions of iodized oils: experimental study. Radiology 1996;201:731-5. [PubMed]
- Kan Z, Madoff DC. Liver anatomy: microcirculation of the liver. Semin Intervent Radiol 2008;25:77-85. [PubMed]
- Nakamura H, Hashimoto T, Oi H, et al. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology 1989;170:783-6. [PubMed]
- Favoulet P, Cercueil JP, Faure P, et al. Increased cytotoxicity and stability of Lipiodol-pirarubicin emulsion compared to classical doxorubicin-Lipiodol: potential advantage for chemoembolization of unresectable hepatocellular carcinoma. Anticancer Drugs 2001;12:801-6. [PubMed]
- Okusaka T, Kasugai H, Ishii H, et al. A randomized phase II trial of intra-arterial chemotherapy using a novel lipophilic platinum derivative (SM-11355) in comparison with zinostatin stimalamer in patients with hepatocellular carcinoma. J Clin Oncol 2009;abstr 4583.
- Mondazzi L, Bottelli R, Brambilla G, et al. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology 1994;19:1115-23. [PubMed]
- Dumortier J, Chapuis F, Borson O, et al. Unresectable hepatocellular carcinoma: survival and prognostic factors after lipiodol chemoembolisation in 89 patients. Dig Liver Dis 2006;38:125-33. [PubMed]
- Jordan O, Denys A, De Baere T, et al. Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol 2010;21:1084-90. [PubMed]
- Hong K, Khwaja A, Liapi E, et al. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res 2006;12:2563-7. [PubMed]
- Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46:474-81. [PubMed]
- Malagari K. Drug-eluting particles in the treatment of HCC: chemoembolization with doxorubicin-loaded DC Bead. Expert Rev Anticancer Ther 2008;8:1643-50. [PubMed]
- Sadick M, Haas S, Loehr M, et al. Application of DC beads in hepatocellular carcinoma: clinical and radiological results of a drug delivery device for transcatheter superselective arterial embolization. Onkologie 2010;33:31-7. [PubMed]
- Lewis AL, Gonzalez MV, Leppard SW, et al. Doxorubicin eluting beads - 1: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med 2007;18:1691-9. [PubMed]
- Lewis AL, Gonzalez MV, Lloyd AW, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol 2006;17:335-42. [PubMed]
- Gonzalez MV, Tang Y, Phillips GJ, et al. Doxorubicin eluting beads-2: methods for evaluating drug elution and in-vitro: in-vivo correlation. J Mater Sci Mater Med 2008;19:767-75. [PubMed]
- Grosso M, Vignali C, Quaretti P, et al. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an Italian multicentre study. Cardiovasc Intervent Radiol 2008;31:1141-9. [PubMed]
- Tang Y, Taylor RR, Gonzalez MV, et al. Evaluation of irinotecan drug-eluting beads: a new drug-device combination product for the chemoembolization of hepatic metastases. J Control Release 2006;116:e55-6. [PubMed]
- Taylor RR, Tang Y, Gonzalez MV, et al. Irinotecan drug eluting beads for use in chemoembolization: in vitro and in vivo evaluation of drug release properties. Eur J Pharm Sci 2007;30:7-14. [PubMed]
- Kekez D, Badzek S, Prejac J, et al. Fluorouracil, leucovorin and irinotecan combined with intra-arterial hepatic infusion of drug-eluting beads preloaded with irinotecan in unresectable colorectal liver metastases: side effects and results of a concomitant treatment schedule. Clinical investigation. Tumori 2014;100:499-503. [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT01060423.
- Malagari K, Pomoni M, Kelekis A, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2010;33:541-51. [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [PubMed]
- Vogl TJ, Lee C, Lencioni R, et al. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: Results from the PRECISION V randomized trial. J Clin Oncol 2010;abstr e14660.
- Sacco R, Bargellini I, Bertini M, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 2011;22:1545-52. [PubMed]
- Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255-64. [PubMed]
- Gao S, Yang Z, Zheng Z, et al. Doxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology 2013;60:813-20. [PubMed]
- Han S, Zhang X, Zou L, et al. Does drug-eluting bead transcatheter arterial chemoembolization improve the management of patients with hepatocellular carcinoma? A meta-analysis. PLoS One 2014;9:e102686. [PubMed]
- Guiu B, Deschamps F, Aho S, et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol 2012;56:609-17. [PubMed]
- Bhagat N, Reyes DK, Lin M, et al. Phase II study of chemoembolization with drug-eluting beads in patients with hepatic neuroendocrine metastases: high incidence of biliary injury. Cardiovasc Intervent Radiol 2013;36:449-59. [PubMed]
- Joskin J, de Baere T, Auperin A, et al. Predisposing factors of liver necrosis after transcatheter arterial chemoembolization in liver metastases from neuroendocrine tumor. Cardiovasc Intervent Radiol 2015;38:372-80. [PubMed]
- Lencioni R, de Baere T, Burrel M, et al. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol 2012;35:980-5. [PubMed]
- Bernal P, Raoul JL, Vidmar G, et al. Intra-arterial rhenium-188 lipiodol in the treatment of inoperable hepatocellular carcinoma: results of an IAEA-sponsored multination study. Int J Radiat Oncol Biol Phys 2007;69:1448-55. [PubMed]
- Ibrahim SM, Lewandowski RJ, Sato KT, et al. Radioembolization for the treatment of unresectable hepatocellular carcinoma: a clinical review. World J Gastroenterol 2008;14:1664-9. [PubMed]
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64. [PubMed]
- Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47:71-81. [PubMed]
- Raoul JL, Guyader D, Bretagne JF, et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology 1997;26:1156-61. [PubMed]
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140:497-507.e2.
- Wang YX. Transcatheter embolization therapy in liver cancer. Recent Patents on Medical Imaging 2012;2:150-62.
- Gulec SA, Mesoloras G, Dezarn WA, et al. Safety and efficacy of Y-90 microsphere treatment in patients with primary and metastatic liver cancer: the tumor selectivity of the treatment as a function of tumor to liver flow ratio. J Transl Med 2007;5:15. [PubMed]
- Salem R, Riaz A, Lewandowski R, et al. Comparative effectiveness of radioembolization, the standard of care for hepatocellular carcinoma: is a randomized survival study feasible? J Clin Oncol 2010;28:4045A.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140:497-507.e2.
- Lepareur N, Ardisson V, Noiret N, et al. (188)Re-SSS/Lipiodol: Development of a Potential Treatment for HCC from Bench to Bedside. Int J Mol Imaging 2012;2012:278306.
- Raoul JL, Boucher E, Rolland Y, et al. Treatment of hepatocellular carcinoma with intra-arterial injection of radionuclides. Nat Rev Gastroenterol Hepatol 2010;7:41-9. [PubMed]
- Kumar A, Srivastava DN, Chau TT, et al. Inoperable hepatocellular carcinoma: transarterial 188Re HDD-labeled iodized oil for treatment--prospective multicenter clinical trial. Radiology 2007;243:509-19. [PubMed]
- Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868-78. [PubMed]
- Takayasu K, Arii S, Kudo M, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol 2012;56:886-92. [PubMed]
- Lu DS, Siripongsakun S, Kyong Lee J, et al. Complete tumor encapsulation on magnetic resonance imaging: a potentially useful imaging biomarker for better survival in solitary large hepatocellular carcinoma. Liver Transpl 2013;19:283-91. [PubMed]
- Kim BK, Kim KA, An C, et al. Prognostic role of magnetic resonance imaging vs. computed tomography for hepatocellular carcinoma undergoing chemoembolization. Liver Int 2014. [Epub ahead of print]. [PubMed]
- Kawamura Y, Ikeda K, Hirakawa M, et al. New classification of dynamic computed tomography images predictive of malignant characteristics of hepatocellular carcinoma. Hepatol Res 2010;40:1006-14. [PubMed]
- Forner A, Ayuso C, Varela M, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 2009;115:616-23. [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [PubMed]
- Gillmore R, Stuart S, Kirkwood A, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol 2011;55:1309-16. [PubMed]
- Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology 2012;262:708-18. [PubMed]
- Wang D, Gaba RC, Jin B, et al. Perfusion reduction at transcatheter intraarterial perfusion MR imaging: a promising intraprocedural biomarker to predict transplant-free survival during chemoembolization of hepatocellular carcinoma. Radiology 2014;272:587-97. [PubMed]
- Loffroy R, Favelier S, Cherblanc V, et al. C-arm dual-phase cone-beam CT: a revolutionary real-time imaging modality to assess drug-eluting beads TACE success in liver cancer patients. Quant Imaging Med Surg 2013;3:196-9. [PubMed]
- Sahani DV, Jiang T, Hayano K, et al. Magnetic resonance imaging biomarkers in hepatocellular carcinoma: association with response and circulating biomarkers after sunitinib therapy. J Hematol Oncol 2013;6:51. [PubMed]
- Yuan J, Chow SK, Yeung DK, et al. Quantitative evaluation of dual-flip-angle T1 mapping on DCE-MRI kinetic parameter estimation in head and neck. Quant Imaging Med Surg 2012;2:245-53. [PubMed]
- Kokabi N, Camacho JC, Xing M, et al. Apparent diffusion coefficient quantification as an early imaging biomarker of response and predictor of survival following yttrium-90 radioembolization for unresectable infiltrative hepatocellular carcinoma with portal vein thrombosis. Abdom Imaging 2014;39:969-78. [PubMed]
- Bozgeyik Z, Onur MR, Poyraz AK. The role of diffusion weighted magnetic resonance imaging in oncologic settings. Quant Imaging Med Surg 2013;3:269-78. [PubMed]
- Song MJ, Bae SH, Lee SW, et al. 18F-fluorodeoxyglucose PET/CT predicts tumour progression after transarterial chemoembolization in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 2013;40:865-73. [PubMed]
- de Bree R. Functional imaging to predict treatment response after (chemo) radiotherapy of head and neck squamous cell carcinoma. Quant Imaging Med Surg 2013;3:231-4. [PubMed]
- Kaneko OF, Willmann JK. Ultrasound for molecular imaging and therapy in cancer. Quant Imaging Med Surg 2012;2:87-97. [PubMed]
- Bahrami N, Swisher CL, Von Morze C, et al. Kinetic and perfusion modeling of hyperpolarized (13)C pyruvate and urea in cancer with arbitrary RF flip angles. Quant Imaging Med Surg 2014;4:24-32. [PubMed]
- Xu X, Luo L, Chen J, et al. Acoustic radiation force impulse elastography for efficacy evaluation after hepatocellular carcinoma radiofrequency ablation: a comparative study with contrast-enhanced ultrasound. Biomed Res Int 2014;2014:901642.
- Mostafa EM, Ganguli S, Faintuch S, et al. Optimal strategies for combining transcatheter arterial chemoembolization and radiofrequency ablation in rabbit VX2 hepatic tumors. J Vasc Interv Radiol 2008;19:1740-8. [PubMed]
- Zhu AX, Salem R. Combining transarterial chemoembolization with radiofrequency ablation for hepatocellular carcinoma: one step forward? J Clin Oncol 2013;31:406-8. [PubMed]
- Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-32. [PubMed]
- Liu Z, Gao F, Yang G, et al. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: an up-to-date meta-analysis. Tumour Biol 2014;35:7407-13. [PubMed]
- Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci 2008;99:2037-44. [PubMed]
- Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol 2008;103:914-21. [PubMed]
- Pawlik TM, Reyes DK, Cosgrove D, et al. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol 2011;29:3960-7. [PubMed]
- Cabrera R, Pannu DS, Caridi J, et al. The combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinoma. Aliment Pharmacol Ther 2011;34:205-13. [PubMed]
- Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): Phase II, randomized, double-blind SPACE trial. J Clin Oncol 2012:4;abstr LBA154.
- Strebel BM, Dufour JF. Combined approach to hepatocellular carcinoma: a new treatment concept for nonresectable disease. Expert Rev Anticancer Ther 2008;8:1743-9. [PubMed]
- Miamen AG, Dong H, Roberts LR. Immunotherapeutic approaches to hepatocellular carcinoma treatment. Liver Cancer 2012;1:226-37. [PubMed]
- Invernizzi P. Liver auto-immunology: the paradox of autoimmunity in a tolerogenic organ. J Autoimmun 2013;46:1-6. [PubMed]
- Yamamoto A, Chervoneva I, Sullivan KL, et al. High-dose immunoembolization: survival benefit in patients with hepatic metastases from uveal melanoma. Radiology 2009;252:290-8. [PubMed]
- Nakamoto Y, Mizukoshi E, Kitahara M, et al. Prolonged recurrence-free survival following OK432-stimulated dendritic cell transfer into hepatocellular carcinoma during transarterial embolization. Clin Exp Immunol 2011;163:165-77. [PubMed]
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014;14:199-208. [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [PubMed]
