Circulating cancer stem cells: the importance to select
Introduction
There is growing interest in the cancer field concerning two rather recently identified cell types, cancer stem cells and circulating tumor cells (CTCs), because of their fundamental biological and clinical implications. Both cancer stem cells and CTCs bear fascinating biological features that have major impact not only on the phenotype of the tumor, but also determine prognosis and therapy of individual cancers. The concept of cancer stem cells and their importance in tumor development, maintenance and metastasis has been extensively validated over the past decade in leukemia, breast, colon, brain, prostate and pancreas cancer (1-8). It is now well recognized that cancer stem cells are essential for tumorigenicity, metastasis and resistance to current therapeutic regimens, eventually leading to relapse of disease. Increasing evidence now also suggests that CTCs harbor a subset of cells that is essential for metastatic spread, hence there is great need to further understand and dissect this heterogeneous population of CTCs in the context of tumorigenicity and metastatic activity, respectively.
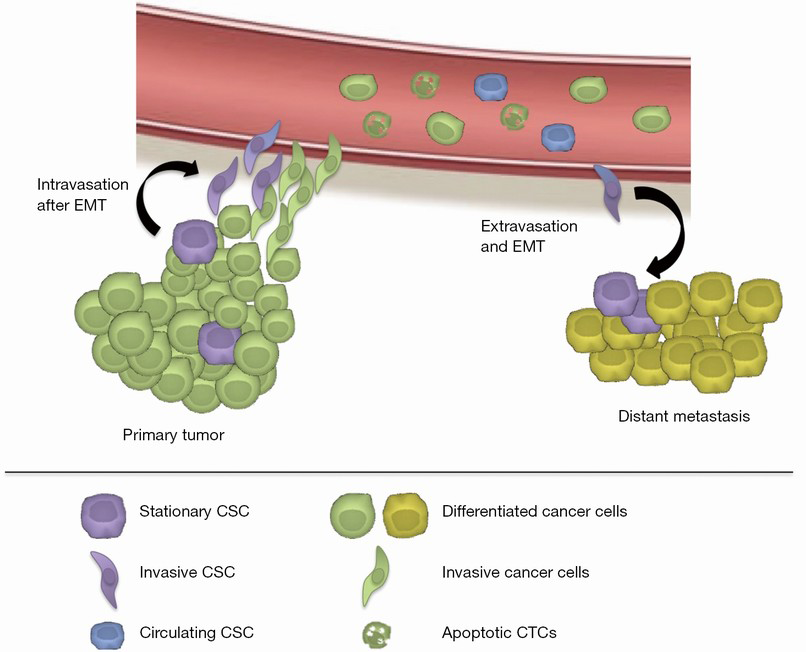
Tumor progression leading to metastasis is a complex multistage process that includes several fundamental biological processes. Metastatic cells have to successfully undergo epithelial-to-mesenchymal transition (EMT), detach from the primary tumor mass and invade the extracellular matrix, intravasate, survive in the circulating blood and disseminate into distant organs, extravasate, undergo reverse mesenchymal-epithelial transition (MET), colonize and eventually form micrometastases and, in some instances, outgrowth of clinically apparent secondary cancer lesions (9) (Figure 1). EMT is a process by which epithelial cells gain mesenchymal properties and is also involved in the efflux of CTCs from the primary tumor by means of invasion of the tumor microenvironment and evasion of cancer cells into blood stream (intravasation). Apparently, even a partial EMT process is capable of establishing such invasive phenotype giving rise to metastatic cancer cells (10). Also the reversible trait of this process, i.e., MET, implies that CTC features may be phenotypically dynamic, converting from one state to the other and vice versa.
Fortunately, the process of metastasis is very inefficient and only a small subset of CTCs is capable of successful metastasis and thus should bear cancer stem cell features including high invasiveness, therefore these cells are termed circulating cancer stem cells (11,12). To date, very little is known about these cells, therefore it is imperative to increase our research activities aiming for the prospective isolation and characterization of such circulating cancer stem cells. Current CTC enrichment and isolation techniques are designed for CTCs based on one or two common phenotypic characteristics amongst them and therefore may not necessarily be capable of capturing the entire heterogeneity of this dynamic population. Moreover, these common phenotypic characteristics of CTC cells are very few and may well vary between different cancer types, adding more complexity to the CTC isolation techniques. Thus, there is an urgent need for comprehensive studies of CTC heterogeneity and development of more robust CTC-detection methods that allow us to distinguish, select and study specific CTC sub-populations.
Stem cells and cancer
Stem cells are defined by their capacity to undergo unlimited cell division while retaining their stem cell identity (self-renewal) and to give rise to more specialized cells with limited proliferative capacity (differentiation) (Figure 2). Stem cells constitute a population of cells that maintain daily turnover in tissue homeostasis as well as the regenerative response upon tissue injury (13). Beside their key role in tissue homeostasis and regeneration, cells with stem cell features, thus termed cancer stem cells, have also been shown to promote cancer and possibly invasion into distant organ sites (metastasis). Evidence for the existence and functional relevance of cancer stem cells was first convincingly documented in leukemia and multiple myeloma. Based on these early studies, only a relatively small subset of cancer cells was capable of unlimited self-renewal and represented the source for disease relapse. Specifically, in murine myeloma cells derived from ascites and depleted for normal hematopoietic cells, only a small fraction of these cells (0.01%) was able to form clonal colonies in vitro (14). Most leukemia cells were unable to proliferate extensively and only a small subset of cells was consistently clonogenic. Such tumor cells with stem cell-like characteristics were first prospectively isolated and characterized by John Dick and his colleagues in 1994 (15). The investigators studied different classes of leukemia cells and identified human AML stem cells in patient samples as CD34+CD38– cells, which represented only a small but variable proportion of AML cells capable of reproducibly transferring AML from human patients to NOD/SCID mice. These data for the first time conclusively demonstrated that a small and prospectively identifiable subset of leukemia cells is capable to self-renew and transfer disease (3). In 2003, Al-Hajj et al. studied primary breast cancer samples and determined CD44+CD24dim/– cells as functional cancer stem cells. Thus, this study suggested that cancer stem cells exist in solid cancer as well (4). Subsequently, cancer stem cells were identified in other solid cancers, e.g., glioblastoma, colorectal cancer, prostate cancer, as well as pancreatic cancer (6,16-18). It is important to note, however, that cancer stem cells do not necessarily represent bona fide stem cells nor do they necessarily arise from tissue stem cells, but rather cancer stem cells have acquired certain traits of stem cells allowing them to indefinitely self-renew and give rise to their respective differentiated progenies. While cancer stem cells share several signaling pathways that are regularly operative in normal stem cells (10), they are obviously distinct from normal stem cells in terms of their in vivo tumorigenicity defined as the generation of malignant lesions upon transplantation into secondary hosts (19). Still, while it has been shown conclusively that cancer stem cells bear cell-intrinsic stemness features, they are also a product of their relationship with the tumor microenvironment affecting their aggressiveness, metastatic activity and drug resistance (20,21). Thus, in order to advance our understanding of cancer stem cell biology and to develop meaningful cancer stem cell-centered treatment strategies, these cells need to be studied in the context of their niche. Clinically it is of utmost importance that cancer stem cells have been proven to be highly resistant to current standard of care such as chemotherapy and radiotherapy, which makes them a probable cause of tumor recurrences after treatment (22). Consistently, primary tumors with a more prominent stem cell signature are associated with adverse outcome including higher rates of metastasis (23-25).
Cancer stem cell populations bear characteristic cell surface expression profiles, which allows for their prospective isolation from other cells in the tumor. Several of the most commonly used cancer stem cell markers are CD44, CD24, CD133, CD166, and ALDH1. ATP-Binding Cassette Transporters (ABCG2, ABCB5), EPCAM, CXCR4, Nestin and LRCs have also been utilized for the identification of cancer stem cells (26). As these can already be conveyed from this rather large and diverse panel of markers, the development of reliable cancer stem cells biomarker profiles for accurately and prospectively isolating viable cells at high purity represents a daunting task. While numerous cell surface proteins have each been positively evaluated in certain settings, the expression levels of many of these markers can drastically change based on environmental conditions (e.g., tumor digestion, cultivation in different conditions, xenografting), in response to treatment, and their expression is neither exclusively nor reproducibly linked to a functional cancer stem cell phenotype (2). Thus, alternative detection and isolation methods based on functional properties of cancer stem cells would not only avoid the use of such artifact-prone surface markers but should also provide novel insights into cancer stem cell biology. Towards this end, an intrinsic autofluorescent phenotype has been identified in cancer stem cells and was subsequently established as a novel and functionally relevant tool to isolate and characterize these cells down to single cell level (27). This distinct inherent cancer stem cell property represents a novel biological feature that is traceable in real time and provides unprecedented robustness and power for the identification and purification of cancer stem cells without the use of antibodies nor any kind of manipulation, thus drastically reducing experimental errors and artifacts. While surface marker panels are regularly validated for only certain cancer types, this novel marker has already been shown to reproducibility identify cancer stem cells across many tumor types including pancreatic, breast, lung, liver and colorectal cancer (27). Thus, it has now become possible to more accurately capture the dynamic complexity of cancer stem cells.
CTCs and circulating cancer stem cells
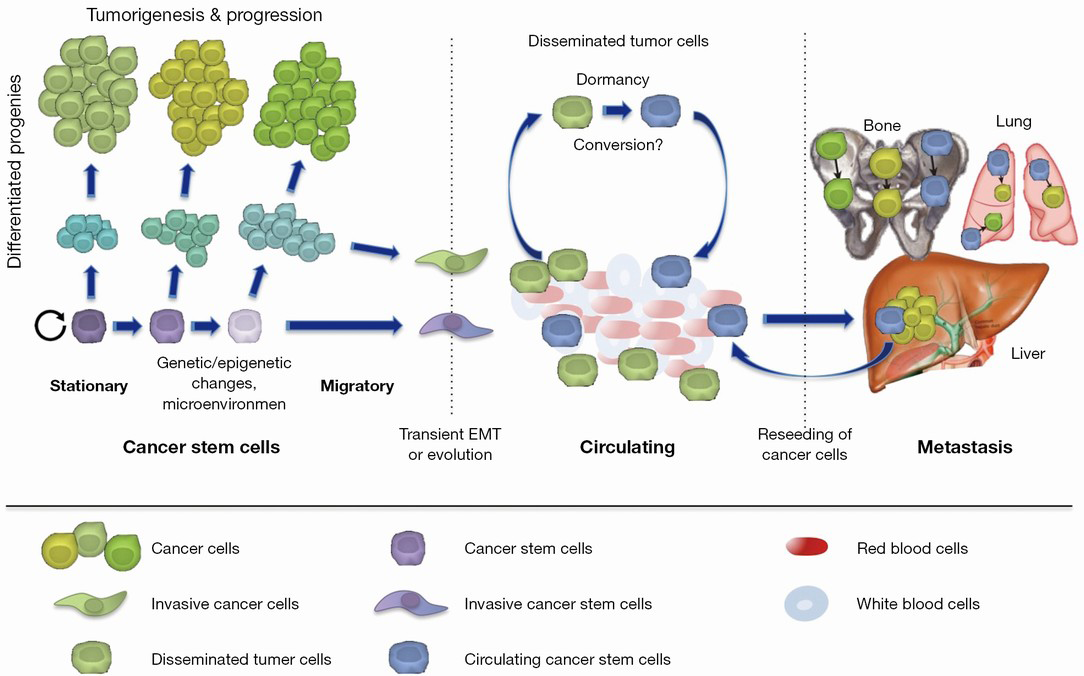
Increasing evidence suggests that a presumably small subset of CTCs also bears cancer stem cell characteristics based on their ability to give rise to tumors (28-30) and thus could be considered blood-born functional cancer stem cells or circulating cancer stem cells. These circulating cancer stem cells may represent cancer stem cells (31) with specific features allowing them to survive in the circulation and to give rise to metastatic lesions. Recent data also indicate an interesting link between cancer stem cells and CTCs that appears to show different functional states of the same pathogenically relevant subpopulation of cancer cells (32-36). Circulating cancer stem cells are likely to represent a small subset of CTCs as that only blood samples from patients with rather high numbers of CTCs were capable of giving rise to tumors in secondary recipients (37,38).
Most importantly, the origin of circulating cancer stem cells has not been established to date. Mostly two non-exclusive hypotheses have been put forward (Figure 2). First, circulating and thus metastatic cancer stem cells already arise in the primary tumor as cancer stem cells with additional features rendering them capable of evading the primary tumor, surviving in the blood stream and subsequently initiating metastatic spread (39). Second, circulating cancer stem cells may actually arise post hoc from disseminated tumor cells, e.g., out of a state of dormancy at a distant site after they already evaded from the primary tumor (40). Importantly, such disseminated tumor cells giving rise to later circulating cancer cells need to survive the hostile environment of the blood stream, evade immune surveillance and extravasate at a distant location, features that most certainly are not present in all CTCs that can be tracked in the blood stream. Of course, while both hypotheses are reasonable, none of them has been validated conclusively to date (41).
Consistent with the hypothesis that circulating cancer stem cells are already present in primary tumors, only the stem cell marker positive cells isolated from primary tumors are able to form distant metastases when transplanted into secondary hosts (39,42,43). Moreover, it has been clearly demonstrated that cancer stem cells in the primary tumor display heterogeneous characteristics, which coincided, at least in pancreatic ductal adenocarcinoma with the expression of distinct surface markers (39). As cancer stem cells also bear the functional plasticity for transitioning between mesenchymal-like and epithelial-like states, these cells are indeed most likely the most relevant source for metastasis at distant sites (44).
Compelling evidence exists that cancer cells are endowed with invasive characteristics through EMT, which is a complex process leading to loss of epithelial and gain of mesenchymal traits via cellular de-differentiation and subsequent increased motility via rearrangements of cellular contact junctions and eventually the loss of cell adhesion. During this process, cells partially or fully transition from their epithelial phenotype into a mesenchymal one (45). EMT naturally occurs during organogenesis and wound healing, but also plays a crucial role during tumor cell dissemination (46). This transition enables the tumor cells to acquire migratory and invasive abilities, which facilitates their evasion from the primary tumor site and penetration into the microenvironment and intravasation into the vasculature (47). EMT is induced by several transcription factors, such as SNAIL, TWIST, ZEB1, ZEB2, SLUG, BMI-1, and others (48). By disrupting epithelial adhesion and losing apical-basal polarity, carcinoma cells at the tumor invasive front acquire invasive capabilities allowing them to disseminate via the circulating blood (47). Importantly, EMT is thought to provide neoplastic epithelial cells not only with a mesenchymal and thus invasive phenotype, but may also induce stemness characteristics (33,49). Indeed, it has been shown that cells undergoing EMT acquire stem cell-like properties, which can be tracked in formerly differentiated epithelial cells by up-regulation of CD44 and down-regulation of CD24 as well as increased expression of other stem cell phenotypic markers (49,50). Thus, EMT may propagate or, in some instance, even generate de novo cells with exclusive tumorigenic and metastatic behavior (49). Actually, Mani et al. first demonstrated that EMT was sufficient to induce a population of cells with characteristics of stem cells bearing migratory and invasive capabilities (49). However, EMT is often transient and reversible. Re-establishment of micrometastasis in the distant sites requires a reversal process, termed mesenchymal-to-epithelial transition (MET), by which the cells re-gain their epithelial characteristics necessary for further colonization. Thus, the EMT-MET transition processes are considered as a driving force of metastasis that can occur in most if not any cancer cells (51).
On the other hand, however, it has also proposed that EMT is a dynamic process that occurs both in cancer stem cells and non-cancer stem cells, but actually only a subset, namely cancer stem cells are capable of giving rise to metastatic cancer stem cells via EMT. In this context, it is important to note that, by definition non-cancer stem cells cannot give rise to tumors in vivo, which suggests that their potential for de novo generation of cancer stem cells via EMT (or other mechanisms) is very limited (27). Still, more studies including in vivo cell fate tracking experiments are needed to conclusively demonstrate whether non-cancer stem cells are indeed capable of replenishing the cancer stem cells pool via EMT and therefore contributing to metastasis. Finally, while the cell autonomous signaling cascade initiating or reversing the EMT process has been studied extensively, very little is known about the exogenous triggers that control the fine balance between the EMT and MET during the metastatic cascade (52).
Moreover, fucosylation has also been implicated in the process of metastasis and is one of the most common glycosylation modifications, involving oligosaccharides on glycoproteins or glycolipids. Fucosylation is also one of the most important types of glycosylation in cancer. Hakomori et al. first reported the role of fucosylation in cancer in 1979 which compared the fucosylation levels of glycolipids in hepatoma cells and normal hepatocytes (53). Increased fucosylation has been associated with invasive and metastatic properties of cancer cells (54). A recent study by Desiderio et al. investigated the role of fucosylation in cancer stem cells and found that inhibition of fucosylation affected sphere formation and invasion ability of cancer stem cells, respectively (55). Moreover, inhibition of fucosylation was found to affect E-selectin binding and cell extravasation (56), features that are of crucial importance for the metastatic process (57). Thus, fucosylation may be a novel mechanism utilized by cancer stem cells to acquire invasive and metastatic features in order to generate metastatic cancer stem cells and seems amendable for therapeutic intervention.
Isolation and characterization of CTCs
The isolation of CTCs from the blood of patients with cancer bears great potential as a minimally invasive approach, but it certainly is technically challenging. Methods for the separation of CTCs vary greatly with respect to the underlying technology ranging from positive immunoselection (e.g., EPCAM-based enrichment), negative immunoselection (e.g., depletion of leucocytes by CD45 antibodies), size-based filtration, and density-based isolation (e.g., via centrifugation) and thus in terms of sensitivity and specificity. Various microfluidic-based devices have been developed over the past years to enrich CTCs in peripheral blood samples (58), but it is difficult to assess, which techniques captures most if not all CTCs as no gold standard for validation currently exists and devices are rarely compared head-to-head.
Cell surface proteins have been used as a target for antibody-based enrichment methods to attach CTCs to columns, chips or magnetic beads for their subsequent detection, isolation and characterization. For example, CellSearch® and IsoFluxTM both utilize magnetic beads targeted towards antigens expressed on the cell surface. Epithelial markers are expressed on carcinomas, but are downregulated/absent on mesenchymal leukocytes and therefore are frequently used to distinguish cancer cells from normal blood cells (59). Epithelial cell adhesion molecule (EPCAM) is the cell surface marker that is most frequently utilized for positive enrichment of CTCs, and members of the family of cytokeratins have become the “gold standard” for the validation of CTCs with an epithelial phenotype in patients with carcinoma (60,61). However, carcinoma cells can undergo EMT, which may result in reduced expression of epithelial markers, and thus EPCAM-based techniques may not efficiently capture CTCs with mesenchymal characteristic following EMT (62). The addition of markers for mesenchymal CTCs that are up-regulated during EMT, such as vimentin and N-cadherin, may be needed, but bear the caveat of potentially increasing the rate of false-positive findings. Tumor or tissue-specific markers for certain tumor types can also be utilized for the isolation of CTCs. Prostate-specific antigen (PSA), mammaglobin, HER2 and epidermal growth factor receptor (EGFR) provide high specificity (63-65). However, these markers may not cover the whole spectrum of CTCs due to their heterogeneity including undifferentiated cancer stem cells.
Alternatively, CTCs can be enriched by depleting blood from leukocytes using antibodies against CD45 or lineage cocktails, which are not expressed on cancer cells. This negative immunoselection method could avoid false-negative results or loss of CTCs due to phenotypic heterogeneity, but the isolated CTC are regularly still contaminated with large numbers of remaining blood cells resulting in rather low purity. Furthermore, CTCs can be isolated using immunodensity negative selection cocktail such as RosetteSepTM, which is a technique combining an antibody-mediated enrichment step with density gradient centrifugation. Importantly, this technique has been used for the isolation and generation of the first CTC-derived xenografts (CDX, see below) (38).
CTCs can also be positively or negatively enriched on the basis of physical properties such as size, density, deformability or electrical charges. Lymphocytes are around 8 µm in size, and have a very compact nucleus and minimal cytoplasm. CTCs are generally much larger, although this may depend of their level of differentiation. Thus, the vast majority of lymphocytes and neutrophils in blood sample can be removed by using filters or microchips with pores (66,67). Unfortunately, owing to the variable size and deformability of CTCs, this method is limited by capturing large and thus more differentiated cancer cells, whereas undifferentiated and invasive cells, respectively, may be captured with less efficiency resulting in low sensitivity and specificity. CTCs have also been enriched by centrifugation on a density gradient owing to the relatively distinct density of CTCs (68). Furthermore, CTC-iChip (69), a novel chip-based platform, separates nucleated cells from whole blood by using size-based separation, then aligns cells in a microfluidic channel using inertial focusing, and subsequently isolates CTCs by means of negative selection (leukocytes depletion) using microfluidic magnetophoresis. Thus, this innovative platform combines size-based filtration with an immunomagnetic depletion and therefore should significantly reduce contamination of the isolated CTCs with undesired hematopoietic cells as well as include CTCs that have undergone EMT and thus lost epithelial traits.
In summary, all listed techniques bear certain advantages and disadvantages and the selection of the most suitable method may also depend on the aim of the study (e.g., preferences for viability and purity) and the type of cancer studied. The use of epithelial markers for antibody-based technologies certainly misses out on CTCs that have undergone EMT. Moreover, circulating cancer stem cells also regularly express lower levels of epithelial markers (52). Thus, combinations of markers may overcome these limitations, but they need to be tested and validated in prospective studies including functional validation for the presence of circulating cancer stem cells. Currently, the limited availability of specific markers for CTCs combined with the inherent technical limitations of most, if not all CTC isolation platforms represent major challenges for further developing broadly applicable CTC isolation techniques.
Isolation and characterization of circulating cancer stem cells
While capturing rare CTCs from circulating blood is rapidly evolving, the prospective and reproducible identification and characterization of viable circulating cancer stem cells within the population of CTCs has remained technically challenging due to their low numbers, poorly defined identify and harsh isolation methods. Recently, Hodgkinson et al. showed that at least a subset of CTCs isolated patients with small cell lung cancer is capable of forming tumors in immunodeficient mice with preserved morphological and genetic characteristics (38). These results, while not prospectively identifying circulating cancer stem cells, are supporting the existence and presence of such cells with tumor-initiating capabilities within the blood. These findings clearly demonstrate that clinically relevant patient-derived circulating cancer stem cell models, also known as “liquid biopsies”, can be generated, although it is also important to note that the authors succeeded only with samples that contained very high numbers of CTCs suggesting that circulating cancer stem cells are indeed a very rare population. Importantly, those CDX also recapitulated drug responses recorded for the donor patients. Thus, such CDX models may now enable us to examine mechanisms of acquired drug resistance as blood samples can be collected before and after development of drug resistance.
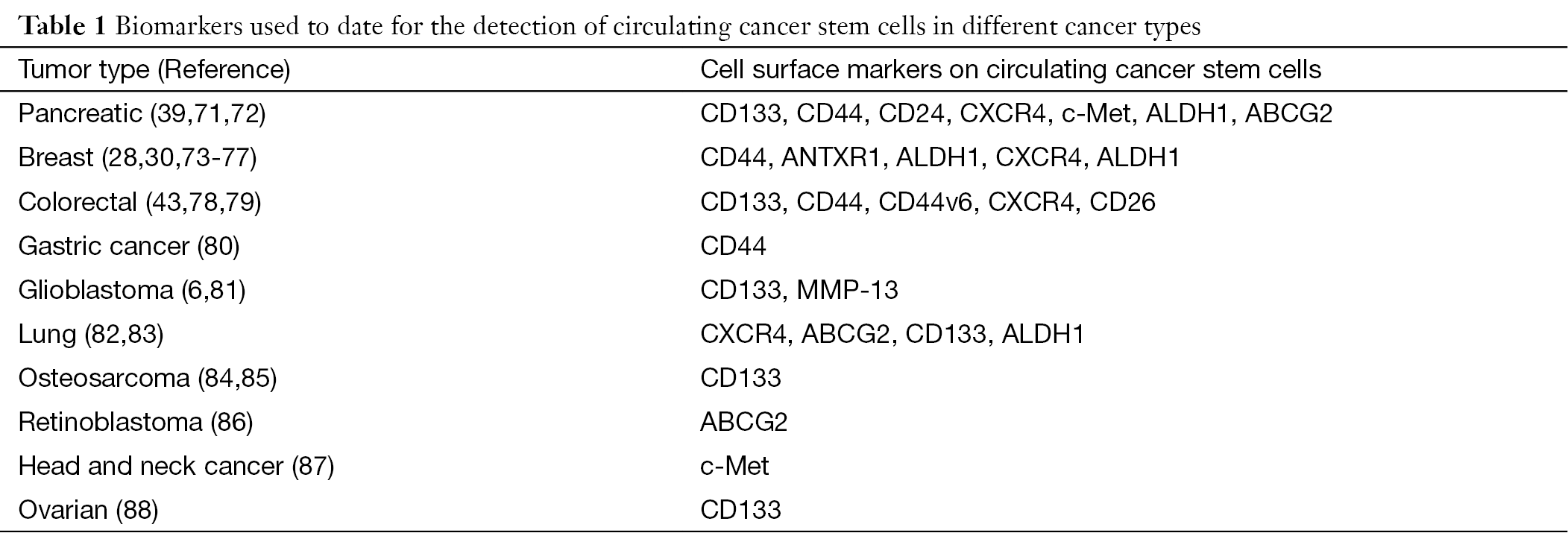
CTCs are believed to represent indicators of residual disease and thus pose an increased risk for disease relapse. However, as the subsets of circulating cancer stem cells is the main driver of tumor progression and metastatic spread, it may be even more important to track and eliminate such rare circulating cancer stem cells (70). Putative biomarkers for identifying circulating cancer stem cells have been proposed in recent studies (Table 1). For example, CTCs with stem cell-like characteristics have been found in primary and metastatic breast cancer. Aktas et al. found that detection of stem cell-like CTCs in peripheral blood of breast cancer patients was associated with therapy resistance (28). Most disseminated tumor cells in the bone marrow of breast cancer patients presented a CD44+/CD24−/low phenotype, which have been shown to be linked to a more aggressive phenotype including high metastatic activity (73-75). ALDH1 has also been shown to identify breast cancer stem cells in vivo and in vitro. Kasimir-Bauer et al. showed that 46% of CTC-positive primary breast cancer patients were also positive for ALDH1 (30). ANTXR1, a stem cell-enriching functional biomarker, has been associated with enhanced self-renewal capacity and metastatic ability of breast cancer stem cells (76). Finally, Krohn et al. showed that CXCR4 expression is also essential for invasiveness of breast cancer stem cells (77).
In pancreatic cancer, c-Met is considered a marker for (metastatic) cancer stem cells and is required for metastasis (71). Moreover, CD133+CXCR4+ cancer stem cells are mostly found in the invasive front of pancreatic cancers and have been shown to be essential for metastasis (39). Consistently, CD133+CXCR4+ cancer cells in colorectal cancer patients also have a higher metastatic capacity as compared to CD133+CXCR4– cancer cells (78). Todaro et al. reported that all colorectal cancer stem cells express CD44v6, which was required for their migratory activity and generation of metastatic tumors (79). Moreover, CD26+ colorectal cancer stem cells have been identified in colorectal cancer patients with liver metastasis, and they generated distant metastasis upon orthotopic transplantation into mice (43). CD44+ circulating cancer stem cells could be detected more frequently in gastric cancer patients with metastasis and served as a prognostic factor (80). CD133+ osteosarcoma cancer stem cells showed high tumorigenicity in vivo (84,85) and CD117+Stro-1+ osteosarcoma cancer stem cells have strong invasive and drug-resistant properties (85,89). In glioblastoma, both CD133+ and MMP-13+ cells showed stem cell properties (6,81). Furthermore, high expression of ABCG2 has been demonstrated in cancer stem cells of lung and pancreas cancer as well as retinoblastoma (72,82,86).
Thus, as already observed for cancer stem cells residing in primary tumors, a large panel of biomarkers has been used by now in various cancers to track blood-born or circulating cancer stem cells, but more stringent and large-scale studies are still needed to define the most suitable setup and marker panel for the prospective isolation of true and viable circulating cancer stem cells.
Full table
Conclusions
CTCs are rare events among millions of blood cells and they are a heterogeneous population of cells, including circulating cancer stem cells, bearing different phenotypic and functional characteristics. Hence the identification and characterization of CTCs requires highly sensitive and specific technologies, which, despite major advances over the past years, still has not been achieved to complete satisfaction due to their heterogeneity and dynamics (32,90). Circulating cancer stem cells are an even smaller sub-population of these CTCs indicating the gravity of today’s technical challenge for isolating and studying these cells. However, recent data already documented the potential for in-depth assessment of viable metastatic tumor cells from CTC populations by capturing single CTCs for next-generation sequencing analyses (91) as well as functionally their in vivo xenotransplantation into immunodeficient mice (37).
Developing new methods for efficient and reproducible isolation and subsequent comprehensive characterization of circulating cancers stem cells should provide the basis for eventually improving patient survival by specifically targeting these cells. Enumeration of CTCs bears prognostic value and is now commonly used in clinical settings for monitoring disease. An increasing number of studies is currently evaluating whether therapies directed by CTC numbers can improve the outcome of treatment and, whether reduced numbers or even eradication of CTCs in response to therapy is actually associated with improved long-term survival (92). Still, these gross CTC numbers may not provide the expected insights into tumor biology and treatment response. Similar to the regression of the bulk tumor that does not necessarily reflect successful targeting of the contained small subpopulation of cancer stem cells, a decrease in CTCs could be misinterpreted as evidence for treatment response while rare circulating cancer stem cells have remained unaffected. Therefore, detection and characterization of circulating cancer stem cells appears to be even more important for selecting and directing therapeutic strategies (93).
Thus, to further advance the CTC research field, we must acknowledge and address the issue of CTC heterogeneity similar to that found in primary tumors, but defining specific markers for such CTC subpopulation remains a challenging issue. Numerous studies have focused on epithelial markers for selection e.g., EPCAM and cytokeratins. Consequently, a varying fraction of CTCs undergoing EMT or bearing stemness features might have been overlooked. In addition, it is just now that we come to realize that most (circulating) cancer cells actually lack the ability to form new tumors and only rare circulating cancer stem cells will lead to metastatic disease. In light of these findings, the main goal of CTC research should shift towards the identification, characterization and subsequent elimination of circulating cancer stem cells, which may be challenging based on the enhanced drug resistance that has already been reported for cancer stem cells in primary tumors. Still, their detection and characterization should serve as a real-time “liquid biopsy” to continually improve prognosis and facilitate patient tailored therapy (Figure 3).
Acknowledgements
Funding: The research was supported by an ERC Advanced Investigator Grant (Pa-CSC 233460), the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 256974 (EPC-TM-NET) and n° 602783 (CAM-PaC), the Subdirección General de Evaluación y Fomento de la Investigación, Fondo de Investigación Sanitaria (PS09/02129 & PI12/02643) and the Programa Nacional de Internacionalización de la I+D, Subprogramma: FCCI 2009 [PLE2009-0105; both Ministerio de Economía y Competitividad (es), Spain], awarded to C.H.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006;66:9339-44. [PubMed]
- Hermann PC, Bhaskar S, Cioffi M, et al. Cancer stem cells in solid tumors. Semin Cancer Biol 2010;20:77-84. [PubMed]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730-7. [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [PubMed]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445:111-5. [PubMed]
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature 2004;432:396-401. [PubMed]
- Richardson GD, Robson CN, Lang SH, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci 2004;117:3539-45. [PubMed]
- Hermann PC, Mueller MT, Heeschen C. Pancreatic cancer stem cells--insights and perspectives. Expert Opin Biol Ther 2009;9:1271-8. [PubMed]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011;147:275-92. [PubMed]
- Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 2010;15:117-34. [PubMed]
- Powell AA, Talasaz AH, Zhang H, et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS One 2012;7:e33788. [PubMed]
- Khoja L, Shenjere P, Hodgson C, et al. Prevalence and heterogeneity of circulating tumour cells in metastatic cutaneous melanoma. Melanoma Res 2014;24:40-6. [PubMed]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science 2010;327:542-5. [PubMed]
- Park CH, Bergsagel DE, McCulloch EA. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst 1971;46:411-22. [PubMed]
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994;367:645-8. [PubMed]
- O'Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445:106-10. [PubMed]
- Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005;65:10946-51. [PubMed]
- Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res 2007;67:1030-7. [PubMed]
- Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: problems for therapy? J Pathol 2011;223:147-61. [PubMed]
- Sainz B Jr, Martín B, Tatari M, et al. ISG15 is a critical microenvironmental factor for pancreatic cancer stem cells. Cancer Res 2014;74:7309-20. [PubMed]
- Lonardo E, Frias-Aldeguer J, Hermann PC, et al. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle 2012;11:1282-90. [PubMed]
- Noman MZ, Messai Y, Carré T, et al. Microenvironmental hypoxia orchestrating the cell stroma cross talk, tumor progression and antitumor response. Crit Rev Immunol 2011;31:357-77. [PubMed]
- Dalerba P, Kalisky T, Sahoo D, et al. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat Biotechnol 2011;29:1120-7. [PubMed]
- Merlos-Suárez A, Barriga FM, Jung P, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 2011;8:511-24. [PubMed]
- Pece S, Tosoni D, Confalonieri S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010;140:62-73. [PubMed]
- Liao WT, Ye YP, Deng YJ, et al. Metastatic cancer stem cells: from the concept to therapeutics. Am J Stem Cells 2014;3:46-62. [PubMed]
- Miranda-Lorenzo I, Dorado J, Lonardo E, et al. Intracellular autofluorescence: a biomarker for epithelial cancer stem cells. Nat Methods 2014;11:1161-9. [PubMed]
- Aktas B, Tewes M, Fehm T, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 2009;11:R46. [PubMed]
- Theodoropoulos PA, Polioudaki H, Agelaki S, et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett 2010;288:99-106. [PubMed]
- Kasimir-Bauer S, Hoffmann O, Wallwiener D, et al. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res 2012;14:R15. [PubMed]
- Scatena R, Bottoni P, Giardina B. Circulating tumour cells and cancer stem cells: a role for proteomics in defining the interrelationships between function, phenotype and differentiation with potential clinical applications. Biochim Biophys Acta 2013;1835:129-43.
- Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer 2008;8:329-40. [PubMed]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009;9:265-73. [PubMed]
- Scatena R, Bottoni P, Pontoglio A, et al. Cancer stem cells: the development of new cancer therapeutics. Expert Opin Biol Ther 2011;11:875-92. [PubMed]
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559-64. [PubMed]
- Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest 2011;121:3804-9. [PubMed]
- Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 2013;31:539-44. [PubMed]
- Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 2014;20:897-903. [PubMed]
- Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313-23. [PubMed]
- Oskarsson T, Batlle E, Massagué J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell 2014;14:306-21. [PubMed]
- Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell 2009;139:1315-26. [PubMed]
- Malanchi I, Santamaria-Martínez A, Susanto E, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2011;481:85-9. [PubMed]
- Pang R, Law WL, Chu AC, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 2010;6:603-15. [PubMed]
- Liu S, Cong Y, Wang D, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports 2013;2:78-91. [PubMed]
- Thompson EW, Haviv I. The social aspects of EMT-MET plasticity. Nat Med 2011;17:1048-9. [PubMed]
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871-90. [PubMed]
- Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev 2009;28:15-33. [PubMed]
- Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science 2013;342:1234850. [PubMed]
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15. [PubMed]
- Asiedu MK, Ingle JN, Behrens MD, et al. TGFbeta/TNF(alpha)-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res 2011;71:4707-19. [PubMed]
- Tsai JH, Donaher JL, Murphy DA, et al. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012;22:725-36. [PubMed]
- Bednarz-Knoll N, Alix-Panabières C, Pantel K. Plasticity of disseminating cancer cells in patients with epithelial malignancies. Cancer Metastasis Rev 2012;31:673-87. [PubMed]
- Baumann H, Nudelman E, Watanabe K, et al. Neutral fucolipids and fucogangliosides of rat hepatoma HTC and H35 cells, rat liver, and hepatocytes. Cancer Res 1979;39:2637-43. [PubMed]
- Wang X, Chen J, Li QK, et al. Overexpression of alpha (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology 2014;24:935-44. [PubMed]
- Desiderio V, Papagerakis P, Tirino V, et al. Increased fucosylation has a pivotal role in invasive and metastatic properties of head and neck cancer stem cells. Oncotarget 2015;6:71-84. [PubMed]
- Okeley NM, Alley SC, Anderson ME, et al. Development of orally active inhibitors of protein and cellular fucosylation. Proc Natl Acad Sci U S A 2013;110:5404-9. [PubMed]
- St Hill CA, Baharo-Hassan D, Farooqui M. C2-O-sLeX glycoproteins are E-selectin ligands that regulate invasion of human colon and hepatic carcinoma cells. PLoS One 2011;6:e16281. [PubMed]
- Deng Y, Zhang Y, Sun S, et al. An integrated microfluidic chip system for single-cell secretion profiling of rare circulating tumor cells. Sci Rep 2014;4:7499. [PubMed]
- Pantel K, Alix-Panabières C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res 2013;73:6384-8. [PubMed]
- Pantel K, Alix-Panabières C. The clinical significance of circulating tumor cells. Nat Clin Pract Oncol 2007;4:62-3. [PubMed]
- Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 2010;16:398-406. [PubMed]
- Rao CG, Chianese D, Doyle GV, et al. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int J Oncol 2005;27:49-57. [PubMed]
- Ignatiadis M, Rothé F, Chaboteaux C, et al. HER2-positive circulating tumor cells in breast cancer. PLoS One 2011;6:e15624. [PubMed]
- Miyamoto DT, Lee RJ, Stott SL, et al. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov 2012;2:995-1003. [PubMed]
- Markou A, Strati A, Malamos N, et al. Molecular characterization of circulating tumor cells in breast cancer by a liquid bead array hybridization assay. Clin Chem 2011;57:421-30. [PubMed]
- Hou HW, Warkiani ME, Khoo BL, et al. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci Rep 2013;3:1259. [PubMed]
- Sollier E, Go DE, Che J, et al. Size-selective collection of circulating tumor cells using Vortex technology. Lab Chip 2014;14:63-77. [PubMed]
- Gertler R, Rosenberg R, Fuehrer K, et al. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. Recent Results Cancer Res 2003;162:149-55. [PubMed]
- Ozkumur E, Shah AM, Ciciliano JC, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med 2013;5:179ra47.
- Sun S, Qiu XS. Cancer stem cells and tumor metastasis. J Cancer Res Ther 2013;9 Suppl:S150-2. [PubMed]
- Li C, Wu JJ, Hynes M, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology 2011;141:2218-27.e5.
- Wang YH, Li F, Luo B, et al. A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma 2009;56:371-8. [PubMed]
- Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res 2006;12:5615-21. [PubMed]
- Reuben JM, Lee BN, Gao H, et al. Primary breast cancer patients with high risk clinicopathologic features have high percentages of bone marrow epithelial cells with ALDH activity and CD44+CD24lo cancer stem cell phenotype. Eur J Cancer 2011;47:1527-36. [PubMed]
- Abraham BK, Fritz P, McClellan M, et al. Prevalence of CD44+/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res 2005;11:1154-9. [PubMed]
- Chen D, Bhat-Nakshatri P, Goswami C, et al. ANTXR1, a stem cell-enriched functional biomarker, connects collagen signaling to cancer stem-like cells and metastasis in breast cancer. Cancer Res 2013;73:5821-33. [PubMed]
- Krohn A, Song YH, Muehlberg F, et al. CXCR4 receptor positive spheroid forming cells are responsible for tumor invasion in vitro. Cancer Lett 2009;280:65-71. [PubMed]
- Zhang SS, Han ZP, Jing YY, et al. CD133(+)CXCR4(+) colon cancer cells exhibit metastatic potential and predict poor prognosis of patients. BMC Med 2012;10:85. [PubMed]
- Todaro M, Gaggianesi M, Catalano V, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014;14:342-56. [PubMed]
- Li M, Zhang B, Zhang Z, et al. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Biomed Res Int 2014;2014:981261.
- Inoue A, Takahashi H, Harada H, et al. Cancer stem-like cells of glioblastoma characteristically express MMP-13 and display highly invasive activity. Int J Oncol 2010;37:1121-31. [PubMed]
- Ho MM, Ng AV, Lam S, et al. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res 2007;67:4827-33. [PubMed]
- Nian WQ, Chen FL, Ao XJ, et al. CXCR4 positive cells from Lewis lung carcinoma cell line have cancer metastatic stem cell characteristics. Mol Cell Biochem 2011;355:241-8. [PubMed]
- Tirino V, Desiderio V, Paino F, et al. Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo. FASEB J 2011;25:2022-30. [PubMed]
- Tirino V, Desiderio V, Paino F, et al. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J 2013;27:13-24. [PubMed]
- Seigel GM, Campbell LM, Narayan M, et al. Cancer stem cell characteristics in retinoblastoma. Mol Vis 2005;11:729-37. [PubMed]
- Sun S, Wang Z. Head neck squamous cell carcinoma c-Met+ cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer 2011;129:2337-48. [PubMed]
- Baba T, Convery PA, Matsumura N, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene 2009;28:209-18. [PubMed]
- Adhikari AS, Agarwal N, Wood BM, et al. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res 2010;70:4602-12. [PubMed]
- Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem 2013;59:110-8. [PubMed]
- Heitzer E, Auer M, Gasch C, et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res 2013;73:2965-75. [PubMed]
- Huang X, Gao P, Song Y, et al. Relationship between circulating tumor cells and tumor response in colorectal cancer patients treated with chemotherapy: a meta-analysis. BMC Cancer 2014;14:976. [PubMed]
- Lianidou ES, Mavroudis D, Sotiropoulou G, et al. What's new on circulating tumor cells? A meeting report. Breast Cancer Res 2010;12:307. [PubMed]
