Comparison of the clinical characteristics and survival between Uyghur patients with hepatitis virus-related and non-B, non-C hepatocellular carcinoma in Xinjiang, China
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent malignancy worldwide, and its mortality ranks third among all malignant tumours. HCC represents 80-90% of primary hepatic malignancies and is among most prevalent and deadly malignancies (1). Recently, some HCC patients have been identified as negative for hepatitis B surface antigen (HBsAg) and anti-hepatitis C virus antibody (anti-HCV); this type of HCC is referred to as non-B, non-C HCC (NBC-HCC). Although the incidence rate of this condition has increased in recent years (2), studies of NBC-HCC are limited. The Uyghur people are one of aboriginal ethnic groups in the Xinjiang region of China that account for a significant proportion of the population in this region. The prevalence of hepatitis infection in Xinjiang is significantly lower than that in other regions of China (3), which suggests the existence of ethnic differences with regard to HCC in Xinjiang. In this study, we summarised the clinical characteristics of the Uyghur HCC patients admitted to our hospital in the past 12 years. We aimed to investigate the possible risk factors and compare the clinical characteristics between NBC-HCC and hepatitis virus-related HCC (viral HCC) in Uyghur patients.
Materials and methods
Patient population
Of the 319 HCC patients admitted to The First Affiliated Hospital of Xinjiang Medical University between 01/01/2000 and 31/12/2012, 18 were excluded from this study. Among them, complete clinical data were not available for 6 patients, and 12 patients were lost to follow up. After these exclusions, 301 patients were enrolled in this study. The study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University, the ethical approval number is 20130725-01.
Diagnosis of HCC
The diagnosis of HCC was based on the 2011 version criteria for primary hepatic malignancies of the Department of Health of the People’s Republic of China. The clinical staging was based on the standards of the American Joint Committee on Cancer (AJCC). HCC was detected via contrast dynamic CT and MRI. A focal lesion ≤2 or >2 cm in diameter displaying arterial hypervascularisation and venous washout as detected by two imaging modalities or a single imaging modality was suggestive of HCC (4). All HCC diagnoses were histopathologically confirmed after resection. Pathological grading was based on the Edmondson-Steiner criteria (5).
Follow-up
Data regarding gender, age, area of residence, maximal tumour diameter, clinical staging, Child-Pugh class, and therapeutic regimen were collected from the two groups. OS was measured in months beginning from the day of the first treatment and ending with the patient’s death. A patient surviving until the most recent follow-up was regarded as a survivor at the end of the study. The end-point for the follow-up of the study was 01/07/2013.
Statistical methods
SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used to analyse the clinical data for the patients included in this study. The categorical data were analysed using the chi-squared test, and the quantitative data were analysed using the t-test. The Kaplan-Meier method was used for univariate analysis of survival, and Cox proportional hazard models were employed for multivariate analysis of survival. All of the tests were two-tailed, and the threshold for significance was P<0.05.
Results
General information and comparisons of the clinical characteristics between the two groups of Uyghur HCC patients
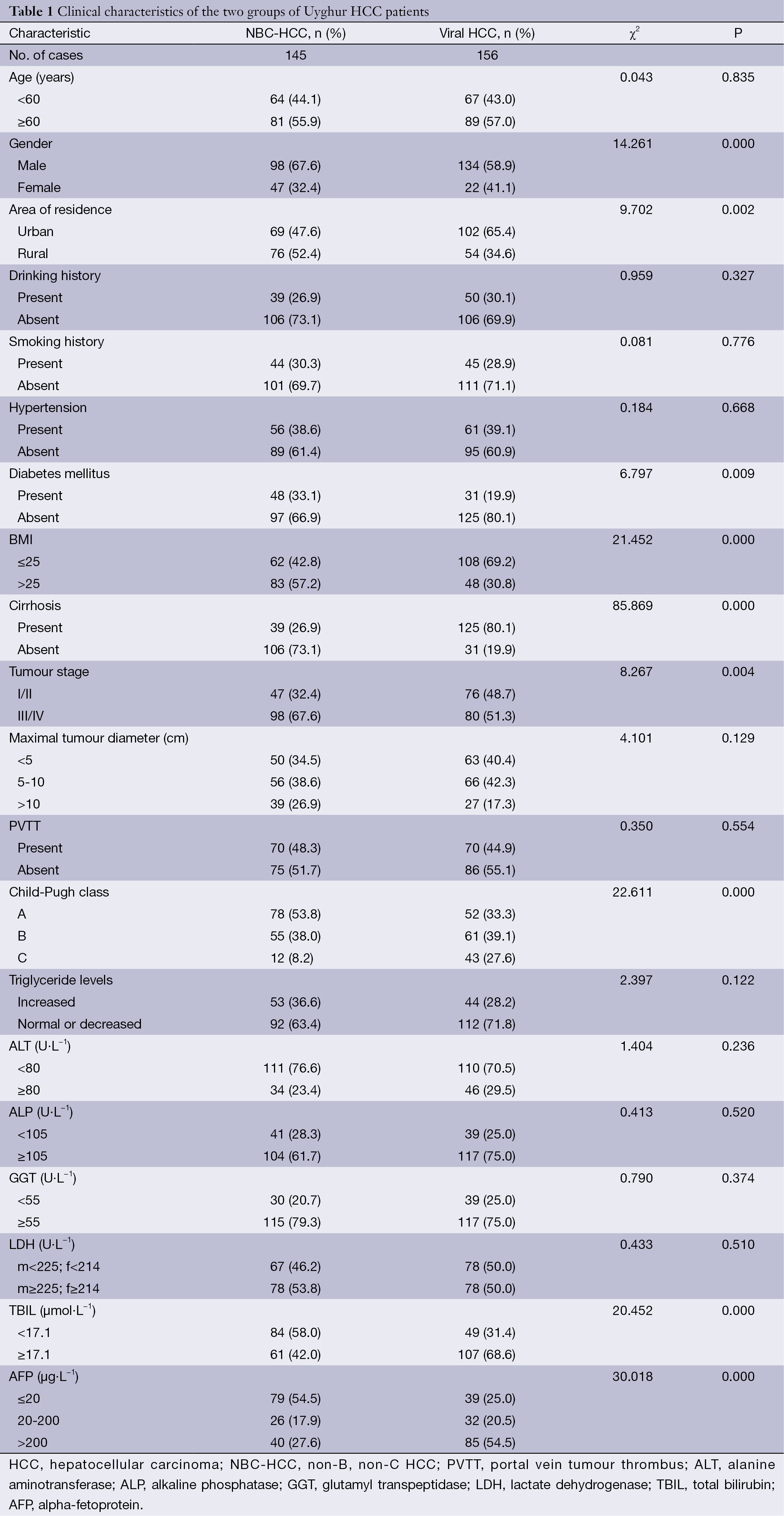
The 301 Uyghur HCC patients were classified into the NBC-HCC (n=145) and viral HCC groups (n=156). In the viral HCC group, hepatitis B (HBV), HCV, and mixed (HAV/HBV, HAV/HCV, or HBV/HCV) infections accounted for 71.2% (111/156), 25.6% (40/156), and 3.2% (5/156) of the cases, respectively. BMI, the average age at diagnosis (NBC-HCC =68.3 years, viral-HCC =63.2 years), and the frequency of concomitant diabetes mellitus were significantly higher in the NBC-HCC group than in the viral HCC group (P<0.05). Other characteristics, such as drinking and smoking history, hypertension, and the plasma triglyceride, ALT, ALP, GGT, and LDH levels, were not significantly different between the two groups (P>0.05). The proportion of patients exhibiting cirrhosis and elevated alpha-fetoprotein (AFP) levels was significantly lower in the NBC-HCC group than in the viral HCC group. The proportion of stage III/IV patients in the NBC-HCC group was higher than that in the viral HCC group; however, the Child-Pugh class was lower in the NBC-HCC group (P<0.05). See Table 1 for details.
Full table
Overall survival (OS)
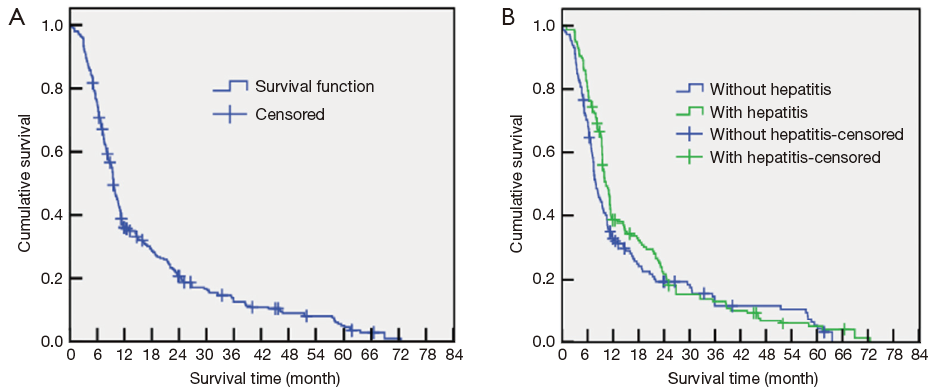
At the end of the follow-up period (the endpoint of the study), the median OS of the entire cohort was 9.5 months (NBC-HCC group median, 7.1 months; viral HCC group median, 10.8 months). The average interval between follow-ups was 9.8±1.1 months. At the study endpoint, 274 of the patients had died (91%); among them, 130 cases (89.7%) were NBC-HCC, and 144 cases were viral HCC (92.3%). The causes of death included tumour recurrence, metastasis, and complications (primarily upper gastrointestinal haemorrhage, haemo-/malignant hydrothorax or ascites, hepatic encephalopathy, hepatorenal syndrome, or tumour rupture and resulting bleeding). The 0.5-, 1-, 3-, and 5-year survival rates of the 301 patients were 35.6%, 20.3%, 12.6%, and 4.5%, respectively (Figure 1A). The corresponding survival rates of the NBC-HCC group were 32.2%, 18.5%, 11.8%, and 3.3%, whereas those of the viral HCC group were 38.0%, 21.1%, 13.5%, and 5.1%, respectively. The survival rates of the two groups were not significantly different (P=0.124) (Figure 1B).
Differences in the management of the two groups of HCC patients
In the NBC-HCC group, 16 patients underwent surgical treatment (11.0%), 50 patients received transcatheter hepatic arterial chemoembolisation (TACE, 34.5%), 6 patients received radiotherapy (4.1%), 17 patients underwent chemotherapy (11.7%), 24 patients received radiofrequency ablation (RAF, 16.6%), and 71 patients received a combined treatment including TACE and radiotherapy or RFA (49.0%).
In the viral HCC group, 10 patients underwent surgical treatment (6.4%), 47 patients received TACE (30.1%), 2 patients received radiotherapy (1.3%), 3 patients underwent chemotherapy (1.9%), 8 patients received RAF (5.1%), and 53 patients received a combined treatment including TACE and radiotherapy or RFA (34.0%). A comparison between the two groups revealed that compared with the viral HCC patients, the NBC-HCC patients significantly more frequently received TACE and radiotherapy or RFA (P<0.05).
Univariate analysis
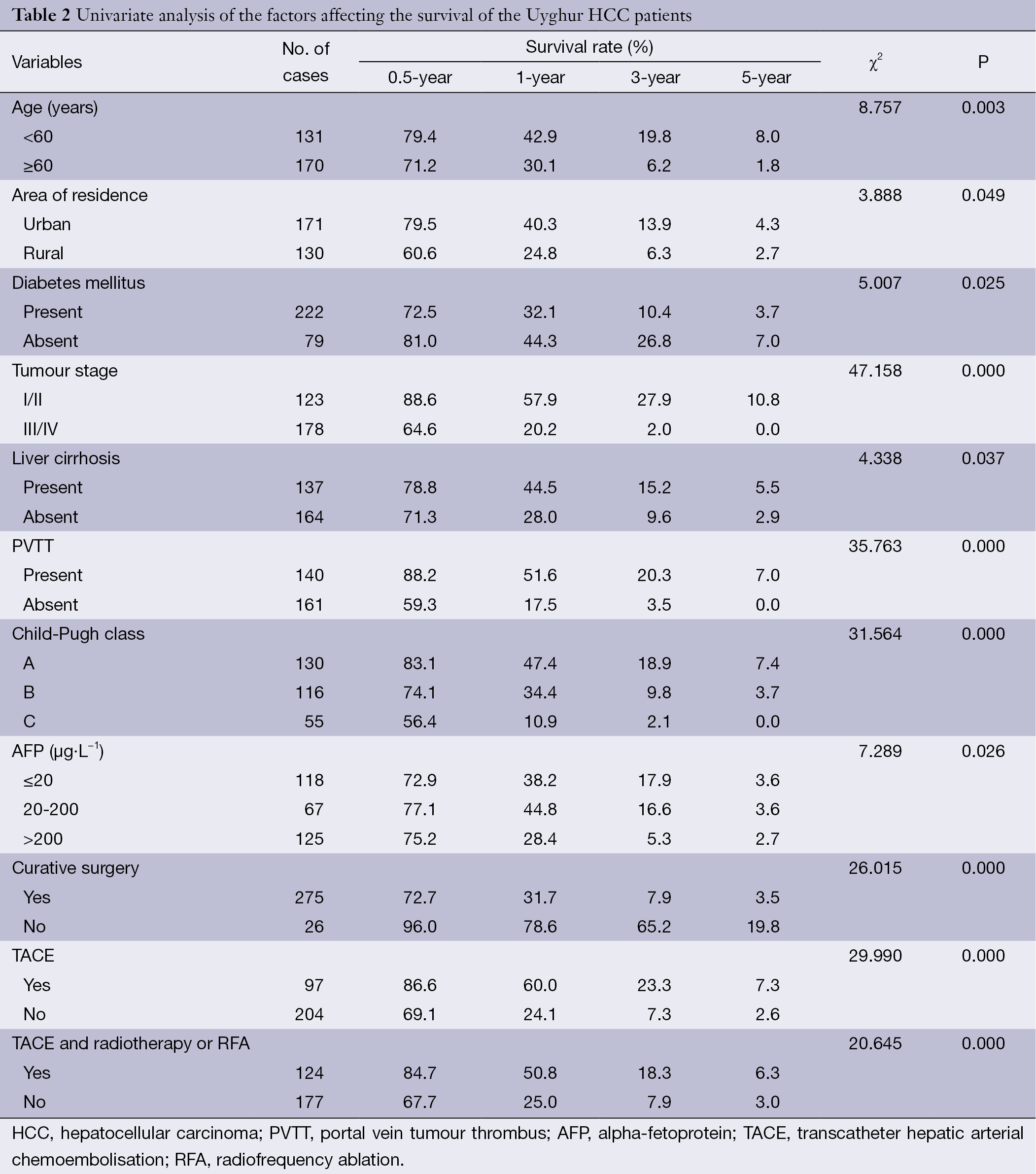
The differences in the survival rates among the patients according to various clinical characteristics were analysed using the log-rank test. Univariate analysis identified three host-related factors, four tumour-related factors, and two therapy-related factors that significantly correlated with the HCC patient survival rate. The host-related factors included age (P=0.003), area of residence (P=0.049), and the presence of diabetes mellitus (P=0.025). The tumour-related factors included tumour stage (P=0.000), the presence of cirrhosis (P=0.037), the presence of portal vein tumour thrombus (PVTT) (P=0.000), and Child-Pugh class (P=0.000). The therapy-related factors included history of curative surgery (P=0.000) and TACE with or without radiotherapy or RFA (P=0.000). The detailed results are listed in Table 2.
Full table
Multivariate analysis
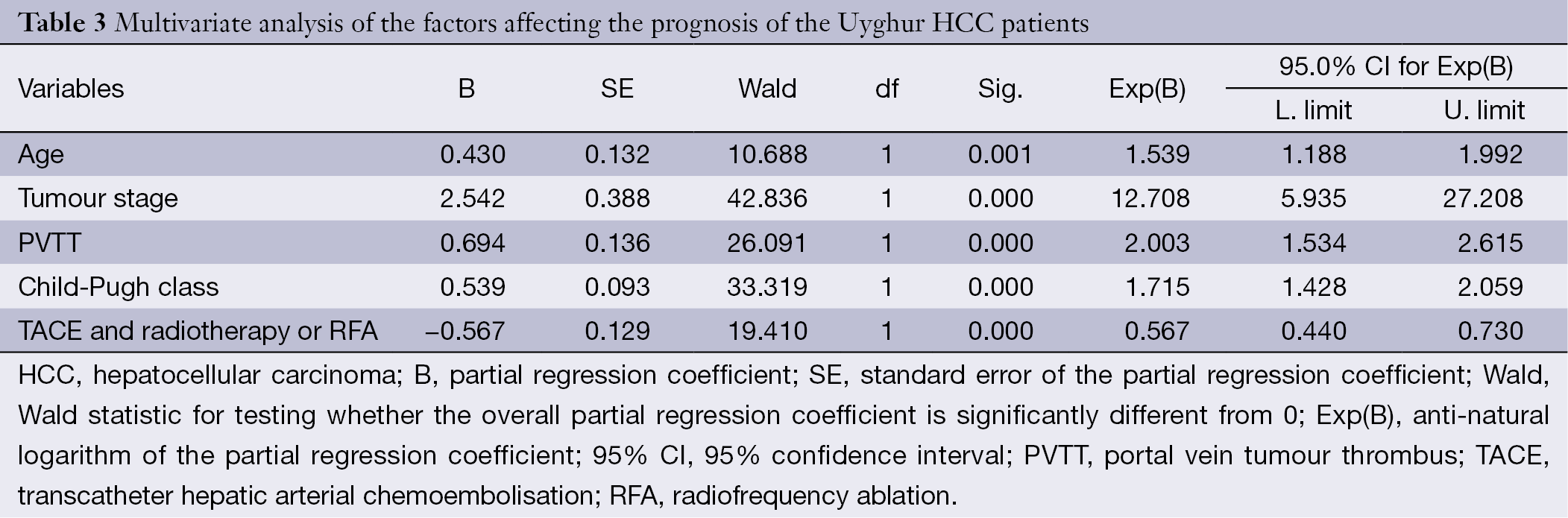
The results of the Cox multivariate proportional hazard model analysis revealed significant independent factors indicating adverse prognosis, which are listed in Table 3. In contrast to the results of the univariate analysis, only five independent risk factors for OS were identified: age (RR =1.539, P=0.001), TNM stage (RR =12.708, P=0.000), PVTT (RR =2.003, P=0.000), Child-Pugh class (RR =1.715, P=0.000), and TACE and radiotherapy or RFA (RR =0.567, P=0.000). The details are listed in Table 3.
Full table
Discussion
More than 90% of primary liver cancer cases are HCC, and infection with a hepatitis virus is understood to be the most common risk factor for the development of HCC in China. The primary viral causes of HCC are HBV and HCV. Globally, 50% and 25% of HCC cases are associated with HBV and HCV infection, respectively (6). However, recent reports (7) have noted that the proportion of NBC-HCC among all HCC cases has increased steadily from 12% to 20% in the past few years. Indeed, we observed an increase in the relative incidence of NBC-HCC among the entire cohort of HCC patients over the duration of this study, which is consistent with previous findings. However, the causes of NBC-HCC remain poorly understood. Currently, alcoholic liver damage, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, and non-alcoholic hepatosteatosis are considered to contribute to NBC-HCC.
Kim et al. (8) proposed correlations between drinking history and diabetes mellitus and the incidence of NBC-HCC. Our findings showed that the proportion of diabetics was significantly higher in the NBC-HCC group than in the viral HCC group (P<0.05). However, no significant difference in drinking history was observed between the two groups (P>0.05); thus, our findings differed from those of Kim et al. (8). This discrepancy may be related to the religious beliefs and dietary habits in the Xinjiang region, which include preferences for meat and noodles, higher alcohol consumption, and a higher intake of fat and salt. The intake of vegetables and fruits is lower in the Xinjiang region than in other regions of China, and it is well known that dietary and living habits are important contributors to metabolic syndrome. The combined prevalence rate of metabolic syndrome in the Han (the major ethnic group in China) and Uyghur peoples in Xinjiang is 30.7% or 28.9% after age standardisation (9). This rate is higher than those reported by the Third American Health and Nutrition Survey (23.7%) and the report by Gu et al. (10). A previous study (11) suggested a causal relationship between obesity and NBC-HCC, and our study showed that the proportion of subjects with a BMI >25 was significantly higher in the NBC-HCC group than in the viral HCC group (P<0.05), which further corroborates the correlation between obesity and NBC-HCC. Therefore, metabolic syndrome may be closely associated with NBC-HCC. Moreover, Kusakabe et al. (12,13) suggested that the rate of liver cancer related to asymptomatic HBV infection has increased in recent years and that asymptomatic HBV infection may directly lead to the development of NBC-HCC.
Previous studies have reported that 75-80% of HCC cases are related to chronic hepatitis virus infection and that 80-90% of HCC cases exhibit concomitant cirrhosis (14). Our study demonstrated a significantly higher proportion of cirrhosis cases in the viral HCC group than in the NBC-HCC group (P<0.05), which is consistent with previous reports (15,16). The currently established theory regarding NBC-HCC pathogenesis is that virus associated hepatic fibrosis-cirrhosis-hepatic cancer is unlikely to represent the predominant pathway of disease development. Based on our finding that a considerable number of NBC-HCC patients exhibited concomitant cirrhosis, the aforementioned theory should be further tested and modified by further research, thus providing new insight for the future prevention and management of HCC. Moreover, several reports (17,18) have suggested the familial aggregation of HCC cases, although dietary and living habits common to family members may explain the observed familial aggregation of HCC cases. In an investigation of chronic HBV infection-related HCC, family history was identified as the predominant risk factor. Thus, the correlation between family history and NBC-HCC requires further investigation.
The 0.5-, 1-, 3-, and 5-year survival rates of the two groups of HCC patients were not significantly different (P>0.05), a result that is somewhat different from that of Kaibori et al. (19). The higher incidence of NBC-HCC in the rural population may be related to a poor diet (aflatoxin-contaminated food or polluted drinking water), poor economic conditions, and a lack of understanding, early intervention, and treatment of HCC. Thus, health education and screening should be promoted in rural villages. The higher 3- and 5-year survival rates of the NBC-HCC patients may be explained by the following reasons: (I) although the proportion of NBC-HCC patients classified as mid- to late-stage at the time of diagnosis was significantly greater than that of viral HCC patients, the Child-Pugh class was significantly less severe in the NBC-HCC group (P<0.05); (II) compared with the viral HCC group, the NBC-HCC group less frequently exhibited concomitant cirrhosis (P<0.05), and at late stages, the effect of the tumour on liver function was reduced (Table 1).
Our study revealed that the predominant factors influencing the prognosis of Uyghur HCC patients include age, tumour stage, PVTT, Child-Pugh class, and history of TACE and radiotherapy or RFA therapy. Patients older than 60; those with an advanced tumour stage, concomitant PVTT, or poor liver function; and those who did not undergo TACE and radiotherapy or RFA therapy exhibited a worse prognosis. This result is largely consistent with that in the literature (20,21) regarding the prognostic factors of HCC. The TACE and radiotherapy or RFA treatment regimen is an independent prognostic factor of HCC, and most HCC cases are initially diagnosed in the mid- to late-stage and are thus largely inoperable. Therefore, a personalised treatment regimen involving a combination of surgical operation, interventional therapy, stereotaxic radiotherapy, chemotherapy, and RFA appears to be essential for improving the survival outcome of the patient, and such combination regimens have been actively investigated for the management of HCC.
In this study, NBC-HCC and viral HCC were significantly different with regard to certain clinical characteristics and concomitant diseases; however, no significant difference in OS was observed. Additionally, the profile of HCC in the Xinjiang area displayed some regional specificity. The independent factors affecting the prognosis of Uyghur HCC patients included age, tumour stage, PVTT, Child-Pugh class, and a history of TACE and radiotherapy or RFA therapy. Notably, the consensus HCC therapy is moving toward this combination therapy.
Acknowledgements
We thank Shasha Zhou for assistance with data analysis.
Authors’ contributions: YX Bao proposed the study. L Xiao and RL Zhang collected the data and wrote the first daft. H Zhang and A Tulahong collected and analysed the data. All authors contributed to the design and interpretation of the study and to further drafts. H Wen and YX Bao are the guarantors.
Funding: This study was supported by a grant from the Urumqi Science and Technology Project Foundation of China (No. H111313001).
Disclosure: The authors declare no conflict of interest.
References
- Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol 2007;4:424-32. [PubMed]
- Abe H, Yoshizawa K, Kitahara T, et al. Etiology of non-B non-C hepatocellular carcinoma in the eastern district of Tokyo. J Gastroenterol 2008;43:967-74. [PubMed]
- Ni YQ, Zhao HR, Mao R, et al. Clinical epidemiological analysis of 3602 cases of primary liver cancer in Xinjiang. Zhonghua Zhong Liu Za Zhi 2012;34:374-7. [PubMed]
- Bruix J, Hessheimer AJ, Forner A, et al. New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene 2006;25:3848-56. [PubMed]
- Du ZG, Wei YG, Chen KF, et al. Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution's experience with 398 consecutive patients. Hepatobiliary Pancreat Dis Int 2014;13:153-61. [PubMed]
- Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist 2010;15 Suppl 4:14-22. [PubMed]
- Nouso K, Kobayashi Y, Nakamura S, et al. Evolution of prognostic factors in hepatocellular carcinoma in Japan. Aliment Pharmacol Ther 2010;31:407-14. [PubMed]
- Kim SK, Marusawa H, Eso Y, et al. Clinical characteristics of non-B non-C hepatocellular carcinoma: a single-center retrospective study. Digestion 2011;84 Suppl 1:43-9. [PubMed]
- Yang T, Ma YT, Yang YN, et al. The prevalence of metabolic syndrome in Han and Uygur in adults from Xinjiang. Journal of Xinjiang Medical University 2011;34:129-32.
- Gu D, Reynolds K, Wu X, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet 2005;365:1398-405. [PubMed]
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9. [PubMed]
- Kusakabe A, Tanaka Y, Orito E, et al. A weak association between occult HBV infection and non-B non-C hepatocellular carcinoma in Japan. J Gastroenterol 2007;42:298-305. [PubMed]
- Shi Y, Wu YH, Wu W, et al. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int 2012;32:231-40. [PubMed]
- Yoshizawa H. Hepatocellular carcinoma associated with hepatitis C virus infection in Japan: projection to other countries in the foreseeable future. Oncology 2002;62 Suppl 1:8-17. [PubMed]
- Akiyama T, Mizuta T, Kawazoe S, et al. Body mass index is associated with age-at-onset of HCV-infected hepatocellular carcinoma patients. World J Gastroenterol 2011;17:914-21. [PubMed]
- Nishikawa H, Arimoto A, Wakasa T, et al. Comparison of clinical characteristics and survival after surgery in patients with non-B and non-C hepatocellular carcinoma and hepatitis virus-related hepatocellular carcinoma. J Cancer 2013;4:502-13. [PubMed]
- Park CH, Jeong SH, Yim HW, et al. Family history influences the early onset of hepatocellular carcinoma. World J Gastroenterol 2012;18:2661-7. [PubMed]
- Turati F, Edefonti V, Talamini R, et al. Family history of liver cancer and hepatocellular carcinoma. Hepatology 2012;55:1416-25. [PubMed]
- Kaibori M, Ishizaki M, Matsui K, et al. Clinicopathologic characteristics of patients with non-B non-C hepatitis virus hepatocellular carcinoma after hepatectomy. Am J Surg 2012;204:300-7. [PubMed]
- Kirchner G, Kirovski G, Hebestreit A, et al. Epidemiology and survival of patients with hepatocellular carcinoma in Southern Germany. Int J Clin Exp Med 2010;3:169-79. [PubMed]
- Gao J, Zhang H, Zhao HR, et al. Analysis of prognosis and influencing factors of 246 Uyghur patients with primary hepatic carcinoma in Xinjiang region. China Oncology 2013;23:362-69.
