Impact of adjuvant treatment modalities on survival outcomes in curatively resected pancreatic and periampullary adenocarcinoma
Introduction
Pancreatic and periampullary adenocarcinoma (PAC) is one of the most lethal human cancers. Although surgical resection remains the only curative intervention for early stage disease, up to 90% of patients with PAC present with unresectable disease at initial diagnosis (1). Even in the most favorable subgroup of patients who have resectable disease, the majority of cases recur after complete tumor resection and the 5-year survival rate after surgery has been reported to be less than 20% (2), demonstrating the need for effective adjuvant therapy.
Although a clear benefit associated with adjuvant therapy has frequently been reported, the optimal choice of treatment modality still remains controversial. Especially, the discrepancy continues regarding the adjuvant intervention whether the optimal treatment modality is with chemotherapy alone (CT) or chemoradiotherapy (CRT) with or without maintenance chemotherapy (CRT/CT). Furthermore, whether CRT with maintenance chemotherapy (CRT-CT) is better than CRT alone is unclear. Therefore, we conducted this retrospective study to investigate whether there is any survival difference between patients who were treated with CT and CRT/CT following resection of PAC.
Patients and methods
Patients
We retrospectively evaluated the records of 563 consecutive PAC patients who were curatively resected for pancreatic or periampullary region tumor between January 2003 and December 2013 in 27 oncology centers. Patients with neoadjuvant therapy (n=6), whose adjuvant therapy was initiated more than 8 weeks (n=4) after surgery, whose adjuvant CT duration was 8 weeks or less (n=10), patients who were given radiotherapy (RT) alone (n=5), and who had no adjuvant therapy (n=35) were excluded. Patients with macroscopic residual tumor (n=6), or patients whose distant metastasis were realized during or 8 weeks after surgery (n=3) were also excluded from the analysis. After an extensive chart review, patients with neuroendocrine tumor (n=6), intraductal papillary mucinous carcinoma (n=5), solid pseudopapillary neoplasm (n=4), acinar cell carcinoma (n=3), undifferentiated carcinoma (n=3), and mucinous cystadenocarcinoma (n=1) were also excluded from the analysis. Hence, these exclusions led to a final count of 472 patients who were included in our statistical analysis.
Adjuvant treatments
Adjuvant treatment options were chosen at the discretion of the attending physician. The adjuvant interventions were CT alone (n=215) and CRT/CT (n=257). Of 257 patients in CRT/CT group, 26 were given CRT alone and 231 were given CRT-CT. However, because there were only 26 patients who were given CRT alone, this small group of patients were included in CRT/CT group, and then compared with CT alone group. The CT regimens after pancreatic resection included 5-fluorouracil plus leucovorin (3), gemcitabine alone (4), gemcitabine plus cisplatin (5), and gemcitabine plus leucovorin plus infusional 5-fluorouracil (6).
Statistical analysis
Recurrence-free survival (RFS) was calculated from the date of primary surgery until the date of proven recurrence of the disease or death from any cause. For patients who were lost to follow-up, data were censored on the date when the patients were last seen alive without recurrence. Overall survival (OS) was calculated from the date of primary surgery to the date of death or to the date of last follow-up. RFS and OS were estimated by the Kaplan-Meier method and compared by the log-rank test. Pearson’s χ2 test was used to compare categorical variables. Cox’s proportional hazards model was used for multivariate analysis and factors with less than 0.10 significance in univariate analysis were recruited into the multivariate analysis. For both univariate and multivariate analyses, P<0.05 was considered statistically significant. All statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
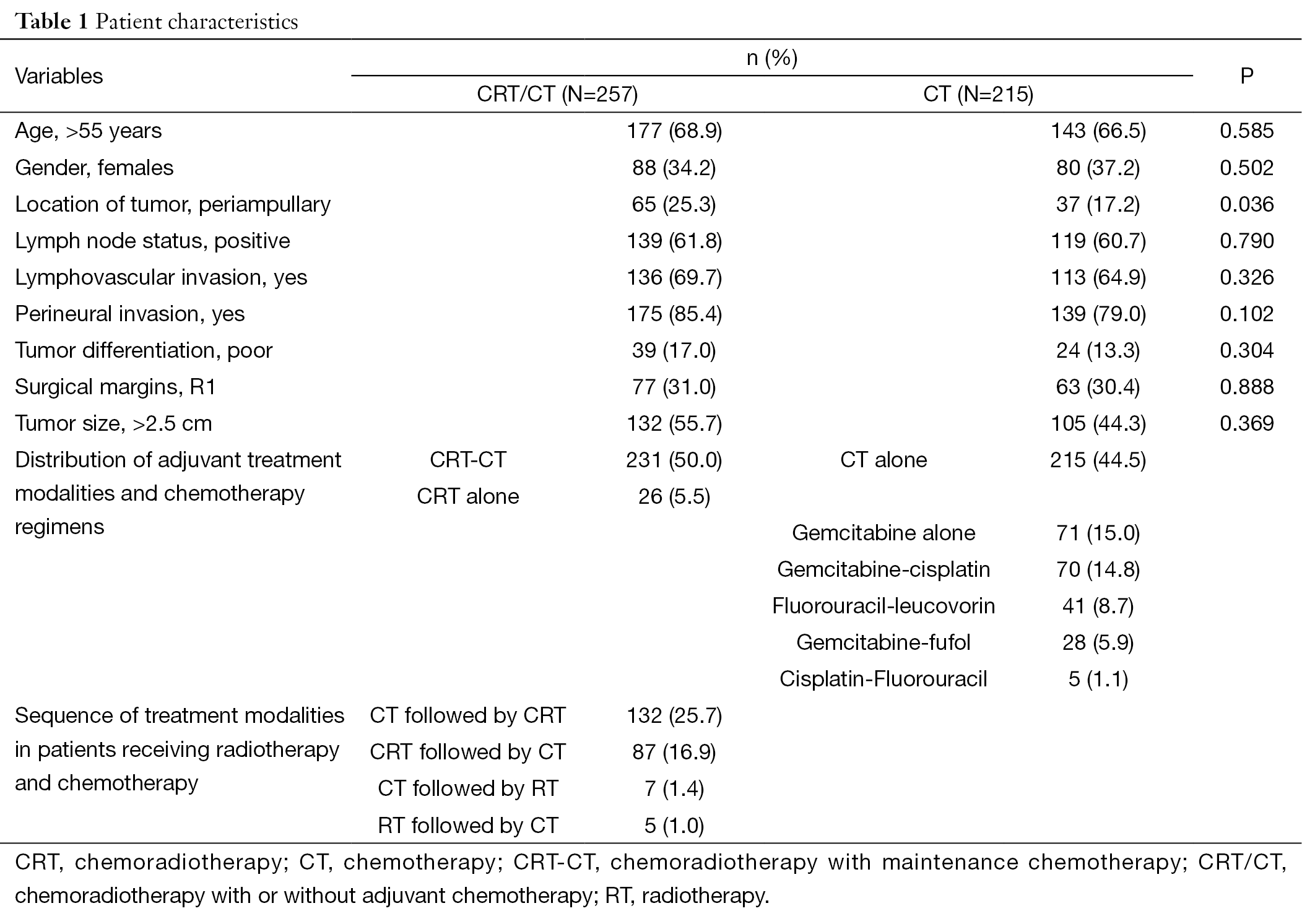
After excluding 91 patients with missing data, 472 patients were available for analysis. The median age was 59 years (range, 29-81 years). The primary tumor site was pancreas in 78.4% of patients and periampullary region in 21.6% of patients. Most patients were males (64.4%). One hundred and forty patients (30.8%) had microscopic residual disease (R1) following surgery. The median number of lymph nodes removed was 12 (range, 3-64). Most patients (61.3%) had one or more lymph node metastasis. The clinical characteristics of the 472 patients are summarized in Table 1.
Full table
Chemotherapeutic regimens
Of 472 patients, 54.4% (n=257) were given CRT/CT and 54.6% (n=215) were given CT alone. Of 215 patients in CT alone, 71 were given gemcitabine alone, 70 were given gemcitabine plus cisplatin, 41 were given 5-fluorouracil plus leucovorin, 28 were given gemcitabine plus leucovorin plus infusional 5-fluorouracil, and 5 were given cisplatin plus 5-fluorouracil. Of 257 patients in CRT/CT group, 15 patients were given sequential CT and radiotherapy while 242 patients were given radiation with concurrent CT. The sequence of treatment modalities in CRT-CT group is detailed in Table 1. The chemotherapeutic agents that accompanied radiation therapy were gemcitabine (n=151), 5-fluorouracil plus leucovorin (n=48), infusional fluorouracil (n=30), and others (n=13).
Survival
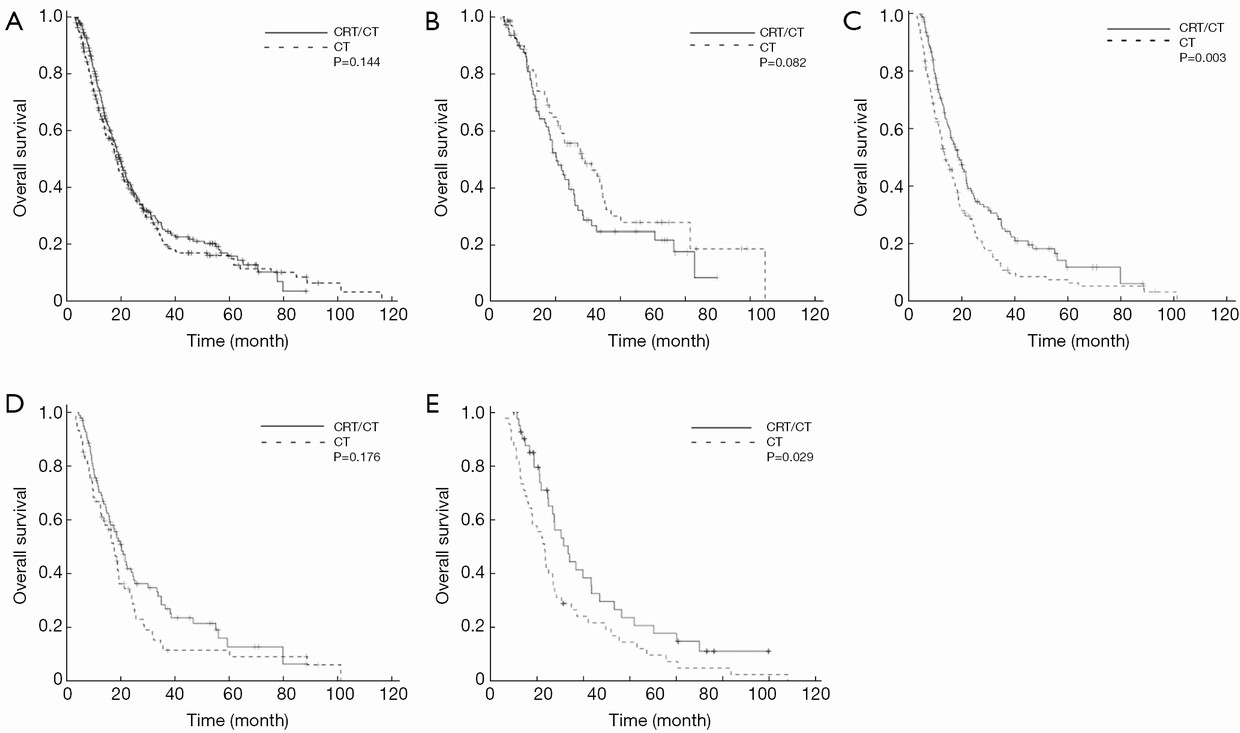
With a median follow-up of 16.3 (range, 3-118) months after surgery, the median RFS and OS were 12 [95% confidence interval (95% CI) 12.8-13.1] months and 19 (95% CI 17.2-20.6) months, respectively. Survival rates at 1st, 3rd, and 5th years were 70%, 23% and 16%, respectively. Figure 1 illustrates the OS curves for patients according to lymph node status stratified by radiotherapy status. When the entire cohort was considered, there was no significant difference between CT and CRT/CT groups with respect to both RFS (P=0.243) and OS (P=0.144) (Figure 1A). When only node-negative patients were considered, a significant RFS (P=0.037) and a non-significant OS (P=0.082) trend favored the CT alone but this trend did not reach significant level for OS (Figure 1B). In contrast, CRT/CT was significantly superior to CT alone for both RFS (P=0.004) and OS (P=0.003), when only node-positive patients were considered (Figure 1C). When CT alone group was considered, there was no RFS (P=0.661) and OS (P=0.676) difference among CT regimens. Similarly, the type of concurrent chemotherapeutic agent was insignificant for both RFS (P=0.635) and OS (P=0.462), when CRT/CT group was considered. Furthermore, there was also no significant RFS (P=0.222) and OS (P=0.274) difference between CRT alone and CRT-CT.
At the time of analysis, 72.2% (n=341) of patients had died (70.9% in the CRT/CT group vs. 74.3% in the CT group; P=0.123), and 76.7% (n=362) of patients had recurred (75.9% in the CRT/CT group vs. 77.7% in the CT group; P=0.726). Data regarding recurrence patterns were available for majority of patients (61%). Overall, 16.3% of recurrences were completely local and 83.7% were distant with the majority of distant recurrences (58%) in the liver. A total of 77 patients (16.3%) had recurred with the first site of failure being local only [35 patients (13.6%) in the CRT-CT group vs. 42 patients (19.5%) in the CT group; P=0.093]. Nine of patients that recurred locally only had been treated with curative intent (3 patients were re-resected and, 6 patients were irradiated).
CRT/CT and CT groups were well-balanced in terms of baseline characteristics (Table 1) except for a higher percentage of patients with periampullary region cancer in CRT/CT compared to CT (P=0.036), which had a potential to influence our results. Analysis comparing CRT/CT and CT did not show any other significant differences in demographics (age and sex), lymphovascular invasion (LVI), perineural invasion (PNI), tumor differentiation (TD), surgical margin (SM), tumor size (TS), and lymph node status (LNS).
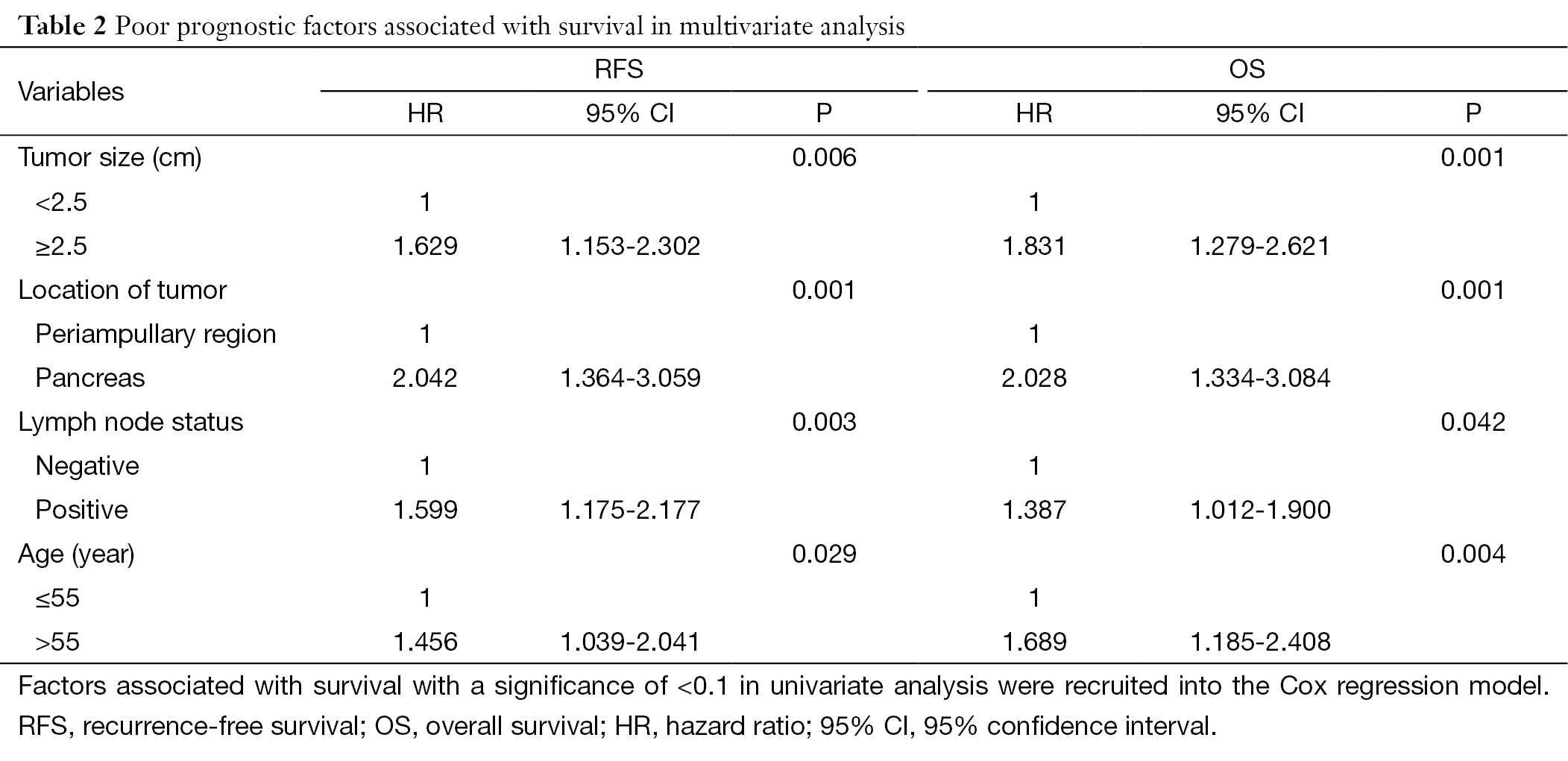
On multivariate analysis, TS≥2.5 cm [hazard ratio (HR) 1.629; 95% CI 1.153-2.302; P=0.006], age >55 years (HR 1.456; 95% CI 1.039-2.041; P=0.029), positive LNS (HR 1.599; 95% CI 1.175-2.177; P=0.003), and pancreatic location (HR 2.042; 95% CI 1.364-3.059; P=0.001) were negative independent prognostic factors associated with RFS. Similarly, TS≥2.5 cm (HR 1.831; 95% CI 1.279-2.621; P=0.001), age >55 years (HR 1.689; 95% CI 1.185-2.408; P=0.004), positive LNS (HR 1.387; 95% CI 1.012-1.900; P=0.042), and pancreatic location (HR 2.028; 95% CI 1.334-3.084; P=0.001) were negative independent prognostic factors associated with OS (Table 2). On the other hand, positive SM, positive LVI or positive PNI was not significant poor prognostic on multivariate analysis neither for RFS nor OS.
Full table
To investigate whether the addition of radiation to CT was associated with survival benefit on subgroups, patients were stratified according to subgroups including: tumor location (TL) (pancreatic vs. periampullary region), LNS (positive vs. negative), SM (R0 vs. R1), TD (well-moderate vs. poor), TS (≤2.5 cm vs. >2.5 cm), LVI (no vs. yes), PNI (no vs. yes), and age (≤55 years vs. >55 years), and then analyzed. There was no difference in RFS and OS between CRT/CT and CT groups when TS, TL, age and SM were considered. In contrast, in patients with positive LVI or PNI, or in patients with poorly differentiated tumor, CRT/CT was significantly superior to CT with respect to RFS, respectively (P=0.009, P=0.004, P=0.006). Similar superiority of CRT/CT on CT group was achieved with respect to OS, respectively (P=0.003, P=0.004, P=0.007). In contrast to this superiority of CRT/CT on CT in these certain subgroups, the superiority of CRT/CT and CT to each other changed according to whether the LNS was positive or not. In other words, CRT/CT was significantly superior to CT in 258 patients with positive LNS with respect to both RFS (P=0.004) and OS (P=0.003) (Figure 1C). In contrast, CT was superior to CRT/CT in 163 patients with negative LNS with respect to both RFS (P=0.037) and OS (P=0.082) (Figure 1B). To further examine the relationship between radiotherapy and survival, the patients with positive LNS were further divided into two subgroups (1-3 lymph nodes vs. ≥4 lymph nodes). In 171 patients with 1-3 positive lymph nodes, CRT/CT was better than CT, but this trend did not reach a significant level for both RFS (P=0.098) and OS (P=0.176) (Figure 1D). In contrast, when 87 patients with ≥4 positive lymph nodes were considered, both RFS (P=0.012) and OS (P=0.029) were significantly longer towards CRT/CT group compared with CT (Figure 1E).
With regard to radiation therapy, 29 of 257 patients who received adjuvant radiation were excluded from the analysis due to having missing radiation data, and the total radiation dose ranged from 14.4 to 60.0 Gy (median, 50.4 Gy) in 8 to 30 fractions with 1.6-2.0 Gy per day. Of the 228 patients who had adequate RT data, 8.4% (n=19) of patients had RT regimens interrupted or modified because of toxicity. Additionally, the interruption rate of concurrent chemotherapeutic agents alone, without a treatment interruption or delay in radiotherapy, was 14.5% (n=33). The percentage of patients who received less than 40.0 Gy of radiation was 5.3% (n=12).
Discussion
The prognosis of pancreatic and periampullary cancers is clearly different, and since the treatment approaches in clinical practice are similar, they were analyzed in a single group instead of two subgroups. When all patients were considered, there was no significant difference between CRT/CT and CT groups for both RFS and OS. Furthermore, when CRT alone was compared with CRT-CT, there was also no significant difference in RFS and OS. On the other hand, subset analysis revealed that there was a significant difference between CRT/CT and CT groups, when stratified by LNS, TD, PNI, or LVI. Our analysis supports the hypothesis that there is an OS benefit for patients who received radiation therapy compared with patients who did not receive radiation therapy.
The first randomized study showing a survival benefit of adjuvant CRT was conducted by the Gastrointestinal Tumor Study Group (GITSG) (7). Then, a similar randomized study was conducted by the European Organization for Research and Treatment of Cancer (EORTC) (8), but this study failed to confirm the earlier results. Then the ESPAC-1 study (9) found a worse survival towards CRT arm when compared with no CRT. However, all of the above-mentioned randomized trials have been criticized for their suboptimal delivery and dosing of RT (10,11). It is very difficult to make a comparison across the GITSG, the EORTC and the ESPAC-1 trials due to significant differences among these trials (12). Recently, several additional randomized trials [CONKO-001 (4), RTOG-9704 (13), ESPAC-3 (14)] have been published in the adjuvant setting. But due to some major differences in study design among these recent trials, it does not provide any further clarification on the role of chemoradiation vs. CT alone. In fact, the only randomized trial that allowed directly comparison of chemoradiation and CT is the ESPAC-1. Almost all other randomized trials using the two therapies have not tested them in a head to head manner, instead both of the therapies have been compared with observation arm. However both the complex design of the ESPAC-1 and the suboptimal dosage and application form of radiotherapy (split course) were criticized too much, and all of these heavily criticized factors had potential to influence outcomes against the chemoradiation arm (15). Therefore, the results of the ESPAC-1 trial are not accepted widely in the United States because of the difficulties in interpreting the results and because of outdated treatment regimens (16).
Despite the risk of selection bias, retrospective trials can provide useful perspectives on the question of whether CRT or CT is superior. Furthermore retrospective trials may include larger samples than randomized trials. So, retrospective trials may guide us to the right way until randomized trials directly addressing this issue are available (17). In addition to the randomized trials, the medical literature includes several large scale retrospective trials (11,16-22) and meta-analyses (23) as well as single institution case series (24-28) addressing the efficacy of adjuvant radiotherapy for resected pancreatic cancer. Most of the large retrospective trials have declared the survival benefit of CRT (11,17,18,20,24,26,27). But, similar to the randomized trials, most of these retrospective trials using the two therapies have not tested them in a head to head manner, except for two recent trials (17,18). One of them (17) which directly compared radiation with CT found radiotherapy had significantly better survival than CT, while the other trial (28) found no benefit from chemoradiation compared with CT. Our survival benefit from radiotherapy is consistent with the GITSG trial and also confirms the results of several single institution studies (24,26,27) as well as national surveillance studies (17,19,20,22).
Subset analysis revealed that there was no significant difference in RFS and OS between CRT/CT and CT group when TS, TL, SM and age were considered. On the other hand, CRT/CT was superior to CT for both OS and RFS when poorly differentiated tumor, or LVI or PNI was present. Furthermore, since the other worse prognostic factors (LNS, poor differentiation, LVI or PNI) except the localization of tumor were equally distributed between CRT/CT and CT groups, this significant difference between the groups could be explained by RT. Although there is no doubt that R1 resection is a sign of poor outcome, the value of SM is still a matter of debate. Our failure to find any difference between R0 and R1 resection may have been caused by a lack of consensus regarding terminology or definition of microscopic margin involvement suggested recently (29). There are many studies that had showed R1 resection was prognostic as well as the studies had showed that it was not prognostic (29). With respect to LNS, there were two possibilities. In patients with node-negative disease, CT was better than CRT/CT for both RFS and OS; however, these differences didn’t reach to significant level. In contrast, in patients with node-positive disease, CRT/CT was significantly better than CT, for both RFS and OS. To further examine the association between node positivity and radiation benefit in this setting, node-positive patients were further divided into two subgroups (1-3 vs. ≥4 lymph nodes). Subset analysis of node-positive disease revealed that an increase in the number of lymph node metastasis was associated with increased radiation benefit for both OS and RFS, when modeled as a categorical variable. In brief, our results confirmed the presence of node metastasis as the most important predictor of inferior outcomes, and we believe that this unpleasant outcome of node metastasis could be reversed with the addition of radiation to CT.
We still had some limitations: first, it was a retrospectively designed study with unavoidable selection bias as in all non-randomized studies. However, all patients who underwent curative resection for pancreatic cancer were included in the study which minimized the likelihood of selection bias. Second, the small number of CRT alone arm has limited our comments on whether maintenance CT following chemoradiation should be given or not following pancreatic resection. And finally, because our study population was treated along for nearly a decade, advances in the treatment administration and radiation therapy over the years might have influenced treatment outcomes. On the other hand, we provided one of the largest study cohorts of pancreatic cancer who were treated with radiation therapy with a more contemporary RT dosing and fractionation schedule in a head to head manner. Furthermore, our percentage of patients with node-positive disease and R1 resection rate were comparable to randomized trials including the GITSG, the ESPAC-1 and the EORTC.
Conclusions
Subset analysis revealed that the benefit of addition of radiation to CT was limited to subgroup patients who had poorly differentiated tumor, positive LNS, PNI, or LVI. Furthermore, this radiation benefit was increased with increasing number of metastatic lymph nodes. In light of the conflicting outcomes from existing randomized trials and meta-analyses as well as retrospective trials, our findings support the use of combined radiotherapy and CT as adjuvant therapy for resected PAC, at least for patients with aforementioned risk groups.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Smaglo BG, Pishvaian MJ. Postresection chemotherapy for pancreatic cancer. Cancer J 2012;18:614-23. [PubMed]
- Murakami Y, Uemura K, Sudo T, et al. Early initiation of adjuvant chemotherapy improves survival of patients with pancreatic carcinoma after surgical resection. Cancer Chemother Pharmacol 2013;71:419-29. [PubMed]
- Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin and levamisole in high-risk stage II and III colon cancer: final report of intergroup 0089. J Clin Oncol 2005;23:8671-8. [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [PubMed]
- Colucci G, Giulani F, Gebbia V, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’ltalia Meridionale. Cancer 2002;94:902-10. [PubMed]
- Unal OU, Oztop I, Unek IT, et al. Two-week combination chemotherapy with gemcitabine, high-dose folinic acid and 5 fluorouracil (GEMFUFOL) as first-line treatment of metastatic biliary tract cancers. Asian Pac J Cancer Prev 2013;14:5263-7. [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82. [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [PubMed]
- Antoniou G, Kountourakis P, Papadimitriou K, et al. Adjuvant therapy for resectable pancreatic adenocarcinoma: review of the current treatment approaches and future directions. Cancer Treat Rev 2014;40:78-85. [PubMed]
- Merchant NB, Rymer J, Koehler EA, et al. Adjuvant chemoradiation therapy for pancreatic adenocarcinoma: who really benefits? J Am Coll Surg 2009;208:829-38. [PubMed]
- Twombly R. Adjuvant chemoradiation for pancreatic cancer: few good data, much debate. J Natl Cancer Inst 2008;100:1670-1. [PubMed]
- Regine WF, Winter K, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019-26. [PubMed]
- Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 2012;308:147-56. [PubMed]
- Wang F, Kumar P. The role of radiotherapy in management of pancreatic cancer. J Gastrointest Oncol 2011;2:157-67. [PubMed]
- Artinyan A, Hellan M, Mojica-Manosa P, et al. Improved survival with adjuvant external-beam radiation therapy in lymph node-negative pancreatic cancer: a United States population-based assessment. Cancer 2008;112:34-42. [PubMed]
- Kooby DA, Gillespie TW, Liu Y, et al. Impact of adjuvant radiotherapy on survival after pancreatic cancer resection: an appraisal of data from the national cancer data base. Ann Surg Oncol 2013;20:3634-42. [PubMed]
- Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol 2010;17:981-90. [PubMed]
- Hazard L, Tward JD, Szabo A, et al. Radiation therapy is associated with improved survival in patients with pancreatic adenocarcinoma: results of a study from the Surveillance, Epidemiology, and End Results (SEER) registry data. Cancer 2007;110:2191-201. [PubMed]
- Mellon EA, Springett GM, Hoffe SE, et al. Adjuvant radiotherapy and lymph node dissection in pancreatic cancer treated with surgery and chemotherapy. Cancer 2014;120:1171-7. [PubMed]
- McDade TP, Hill JS, Simons JP, et al. A national propensity-adjusted analysis of adjuvant radiotherapy in the treatment of resected pancreatic adenocarcinoma. Cancer 2010;116:3257-66. [PubMed]
- Opfermann KJ, Wahlquist AE, Garrett-Mayer E, et al. Adjuvant radiotherapy and lymph node status for pancreatic cancer: results of a study from the Surveillance, Epidemiology, and End Results (SEER) Registry Data. Am J Clin Oncol 2014;37:112-6. [PubMed]
- Stocken DD, Büchler MW, Dervenis C, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer 2005;92:1372-81. [PubMed]
- Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: The Mayo Clinic experience (1975-2005). J Clin Oncol 2008;26:3511-6. [PubMed]
- Miller RC, Iott MJ, Corsini MM. Review of adjuvant radiochemotherapy for resected pancreatic cancer and results from Mayo Clinic for the 5th JUCTS symposium. Int J Radiat Oncol Biol Phys 2009;75:364-8. [PubMed]
- Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997;225:621-33. [PubMed]
- Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol 2008;26:3503-10. [PubMed]
- Martin LK, Luu DC, Li X, et al. The addition of radiation to chemotherapy does not improve outcome whencompared tochemotherapy in the treatment of resected pancreas cancer: the results of a single-institution experience. Ann Surg Oncol 2014;21:862-7. [PubMed]
- Verbeke CS. Resection margins in pancreatic cancer. Pathologe 2013;34:241-7. [PubMed]
