Parasympathetic neurogenesis is strongly associated with tumor budding and correlates with an adverse prognosis in pancreatic ductal adenocarcinoma
Introduction
Parasympathetic neurogenesis is a morphologic phenomenon of the tumor microenvironment that presents as distribution of parasympathetic nerves in the tumor stroma (1). Moreover, parasympathetic neurogenesis is associated with progression of invasion and metastasis in prostate cancer (1). Although neurogenesis is generally considered as a poor risk factor for tumor prognosis (2, 3, 4, 5), the phenomenon of parasympathetic neurogenesis in pancreatic ductal adenocarcinoma (PDAC) has not been studied.
Tumor budding reflects a type of diffusely infiltrative growth frequently observed in gastrointestinal carcinomas and is defined as the presence of detached isolated single cells or small cell clusters (up to five cells) scattered in the stroma at the invasive tumor front (6, 7, 8, 9, 10, 11). Tumor budding has been suggested to reflect the process of epithelial-mesenchymal transition (12). In a previous study, it showed that tumor budding occurs frequently in pancreatic cancer and may be used as a parameter of tumor aggressiveness and an indicator of unfavorable outcomes (13). In following years, our results were further confirmed in other studies (14, 15, 16).
Based on previous findings in prostate cancer (1), we hypothesized that parasympathetic neurogenesis may be associated with the frequent occurrence of tumor budding in PDAC and may play a role in PDAC invasion and metastasis. We therefore analyzed parasympathetic neurogenesis at the invasive front of the tumor in 59 PDAC patients with available information on clinicopathologic data, follow-up, and adjuvant therapy. Parasympathetic neurogenesis was correlated with clinicopathologic data, especially the presence of tumor budding and prognosis.
Methods
Patients and tissue samples
With the approval of the Institutional Review Board, the data of 76 consecutive patients who were treated with intended radical pancreaticoduodenectomy at the Department of General Surgery, Peking University Third Hospital between 2005 and 2010 were retrospectively reviewed. After excluding patients who did not have a diagnosis of PDAC, 59 patients (77.6%) with PDAC who had available pathologic specimens and 3 specimens of normal pancreatic tissues were enrolled in this study.
The clinicopathologic characteristics and the outcomes of these patients were recorded. The observation period was from February 2005 to December 2014, with a median follow-up time of 15 months (range, 3-84 months). Clinical and pathologic factors, including demographic information, clinical stage, and presence of vessel invasion and neural invasion, were evaluated. The clinical stages of all patients were classified according to the 2009 TNM staging of the International Union against Cancer.
Immunohistochemistry
Paraffin-embedded PDAC sections (4 μm) were deparaffinized with xylene and rehydrated. Antigen retrieval was performed by heating in an autoclave in 10 mmol/L sodium citrate buffer (pH 6.0). The sections were treated with 3% hydrogen peroxide in methanol to quench the endogenous peroxidase activity and with 1% bovine serum albumin to block non-specific binding, followed by incubation with a rabbit polyclonal antibody against vesicular acetylcholine transporter (VAChT) (1:100, ab-68984; Abcam, CA, USA) overnight at 4 °C. After washing with phosphate-buffered saline (PBS), the sections were incubated with a biotinylated secondary antibody followed by incubation with the streptavidin-horseradish peroxidase complex. The sections were then immersed in 3,3’-diaminobenzidine for 6 min, counterstained with 10% Mayer’s hematoxylin, dehydrated, and mounted in crystal mount. PBS was used instead of a primary antibody for the negative control. To minimize variation in immunopositivity, all sections were stained in 3,3’-diaminobenzidine for the same amount of time. One pathologist, who was blinded to the clinical outcome, independently scored the results of the staining.
Evaluation of immunohistochemistry and tumor budding
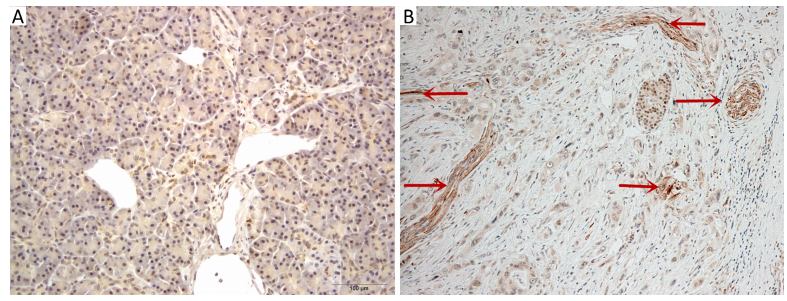
Parasympathetic neurogenesis was defined as a morphologic phenomenon in the tumor microenvironment, presenting as the distribution of parasympathetic nerves in the tumor stroma (Figure 1). For the VAChT-stained tissue sections, a pathologist first selected the area with the highest density of nerve fibers at a low magnification (5×), which was defined as the hotspot field. Subsequently, the number of nerve fibers was counted using 20× magnification (surface 0.785 mm2) in high-power fields (200×) and scoring was performed based on the number of nerve fibers in five hotspot fields at 200× magnification.
Tumor budding was defined as detached single cells or clusters of fewer than five cells. Cases were evaluated for tumor budding as described in our previous study (6).
Follow-up and statistical analysis
Patients were followed up clinically with physical examinations and abdominal-pelvic computed tomography (CT) performed every 3-6 months. Patients with stage II or III disease received adjuvant chemotherapy depending on their preference. Overall survival (OS) was defined as the time from the first surgery to the date of death (all-cause mortality). Disease-free survival (DFS) was defined as the time from the first surgery to the date of the first evidence of recurrence (local, regional, or metastatic). Follow-up time was defined as the time from the first surgery to the date of the last follow-up. Follow-up information was obtained from office charts, hospital records, and telephone interviews. Follow-up and survival times were recorded in months.
Study data were collected on standard forms, checked for completeness, and double keyed into an Epidata 3.1 database. Continuous variables that did not meet the normal distribution were expressed as median (P25, P75), and the Mann–Whitney U test was used to analyze differences between groups. Categorical variables were described by frequencies and proportions and tested by the Chi-square test or Fisher’s exact test. The cut-off value of parasympathetic nerve fibers was determined by receiver operating characteristic (ROC) curves. The Kaplan–Meier method was used to represent survival curves and the log-rank test was used to test significant differences in survival time. Multivariate Cox regression models (Backward Stepwise Likelihood Ratio) were used to analyze factors influencing survival and reported with a hazard ratio (HR) of 1.0 as baseline and 95% confidence interval (95% CI). All analyses were carried out using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA). A two-sided P value of <0.05 was considered statistically significant in all analyses.
Results
Patient demographics
The study cohort of 59 patients with resected PDAC had a median age of 63 years (range: 23-77 years). There was a slight male predominance 1.4:1 (male, n=34). The majority of tumors were histological grade Ⅱ [32 (48%)] or grade Ⅲ [21 (31%)]. Thirty-nine (58%) of the tumors were pT3 and 29 patients (43%) had lymph node metastases. Thirty (50.8%) patients were treated with adjuvant chemotherapy. Twenty-six patients showed recurrence within 6 months, of whom 6 cases had local recurrence, 12 had liver metastasis, 1 had pulmonary metastasis, 1 had abdominal wall metastasis, and 6 had multiple organ metastasis. All of these patients formed the early recurrence group. The observation period was from February 2005 to December 2014, with a median follow-up time of 15 months (range, 3-84 months).
Frequency of parasympathetic neurogenesis and association of parasympathetic neurogenesis with clinicopathologic parameters
VAChT-positive parasympathetic nerve fibers were not seen in the stroma of 3 cases of normal pancreatic tissues whereas they were presented in all cases of PDAC [median 18 fibers/(5×0.785) mm2, range 4-38 fibers/(5×0.785) mm2] (Figure 1).
The diagnostic potential of parasympathetic neurogenesis for predicting death within 2 years is shown in Figure 2. In general, parasympathetic neurogenesis was found to be a significant indicator for distinguishing patients who died within 2 years from survivors (area under the ROC curve=0.815, 95% CI: 0.701-0.929, P=0.000). We chose the cut-off value when the Youden’s index was the biggest. The ROC curve showed an optimal cut-off value of 15 fibers/(5×0.785) mm2 for parasympathetic neurogenesis with 84% sensitivity and 67% specificity (Figure 2). Therefore, we divided the cohort into two groups according to the number of parasympathetic neurogenesis: a low-grade parasympathetic neurogenesis group [parasympathetic neurogenesis ≤15 fibers/(5×0.785) mm2] and a high-grade parasympathetic neurogenesis group [parasympathetic neurogenesis >15 fibers/(5×0.785) mm2]. The high-grade parasympathetic neurogenesis group represented 66.1% of cases (39/59).
The presence of high-grade parasympathetic neurogenesis was associated with a high tumor budding number (P=0.001) and early recurrence (P=0.035). Parasympathetic neurogenesis was not found to be associated with pT stage (P=0.174), N stage (P=0.314), American Joint Committee on Cancer (AJCC) stage (P=0.586), vessel invasion (P=0.626), neural invasion (P=0.233), or histological grade (P=0.522). A complete summary of clinicopathologic associations with parasympathetic neurogenesis is provided in Table 1.

Full table
Prognostic significance of parasympathetic neurogenesis
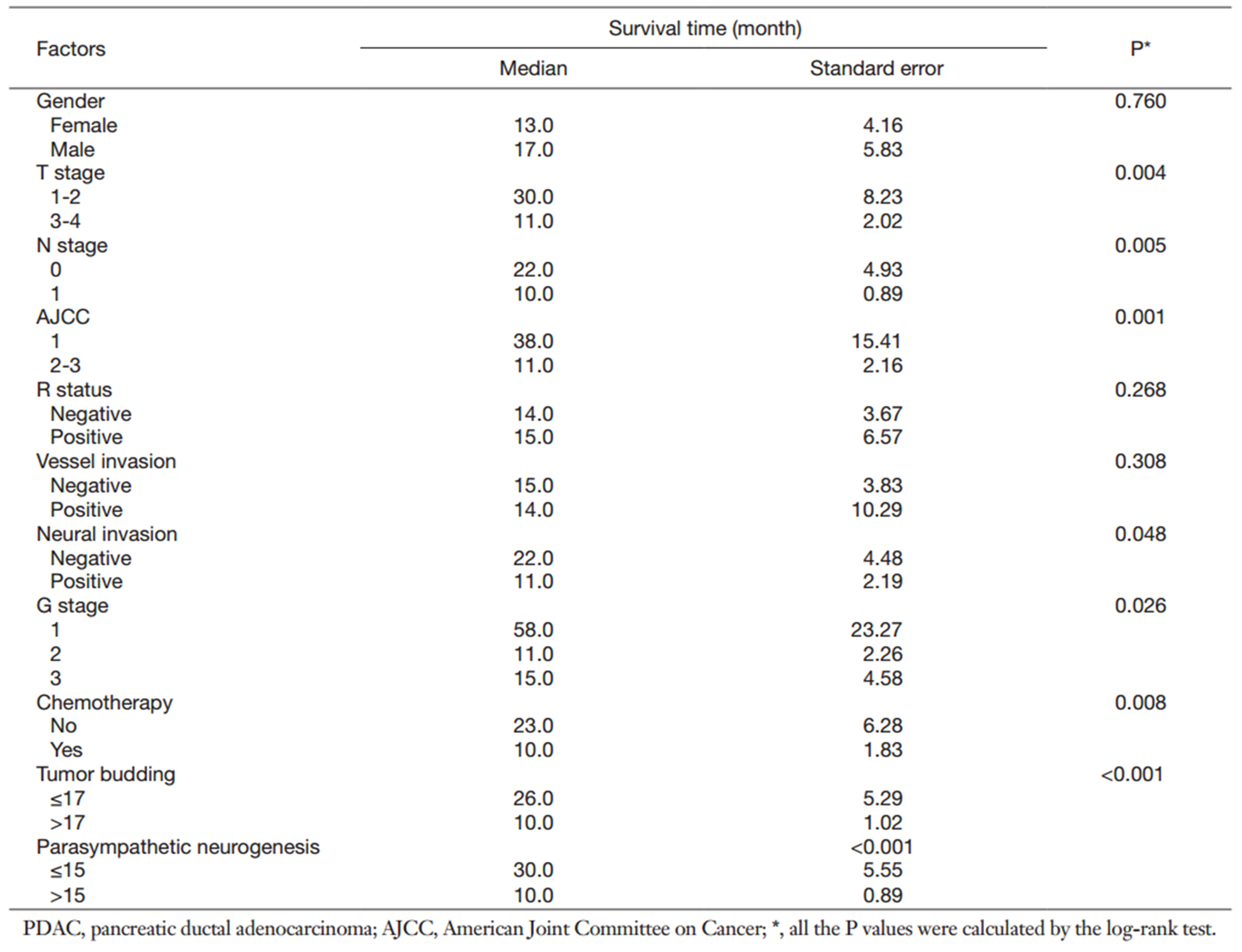
Median DFS was 10 and 30 months in patients with high-grade and low-grade parasympathetic neurogenesis, respectively. Univariable OS analysis showed that T stage (log-rank test, P=0.004), N stage (log-rank test, P=0.005), AJCC stage (log-rank test, P=0.001), neural invasion (log-rank test, P=0.048), G stage (log-rank test, P=0.026), chemotherapy (log-rank test, P=0.008), tumor budding (log-rank test, P<0.001), and the presence of parasympathetic neurogenesis (log-rank test, P<0.001) were associated with an inferior prognosis (Table 2, Figure 3).
Full table

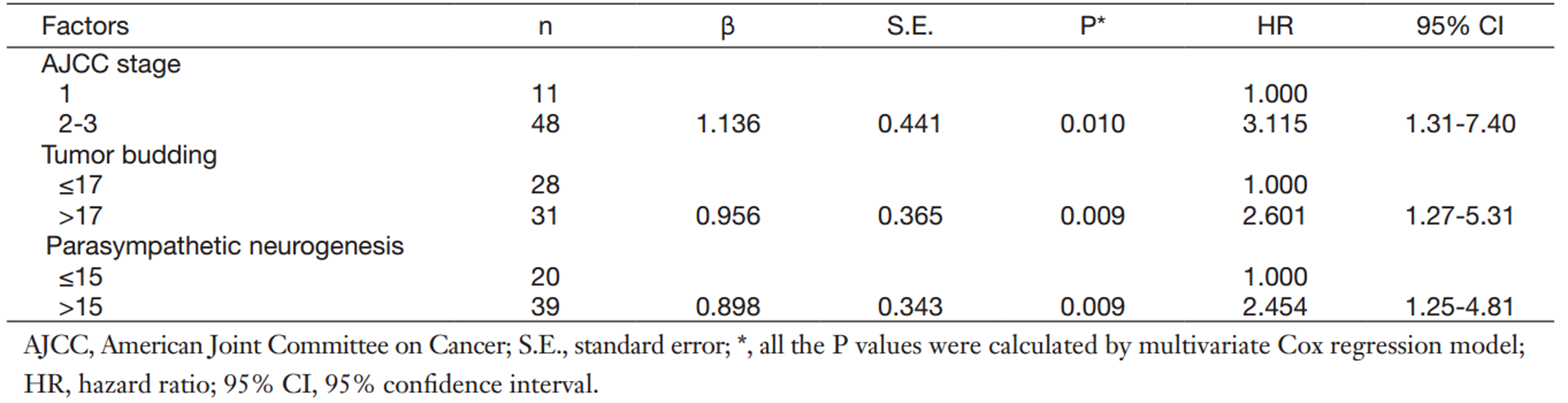
The multivariate Cox regression model using Backward Stepwise (likelihood ratio) was employed to analyze the prognostic factors of OS in PDAC patients. Tumor budding and parasympathetic neurogenesis were included as dependent variables in the model. The results indicated that the presence of high-grade parasympathetic neurogenesis maintained its unfavorable impact on prognosis (P=0.009). Patients with high-grade parasympathetic neurogenesis had a 2.45-fold elevated risk of death after adjusting for the remaining features compared with patients with low-grade parasympathetic neurogenesis (Table 3).
Full table
Discussion
The present study identified the association of high-grade parasympathetic neurogenesis with frequent occurrence of tumor budding and its role as an independent and highly unfavorable prognostic factor in PDAC, and deepens our understanding of the role of parasympathetic neurogenesis in PDAC in several aspects.
First, we found a significant progressive increase in the occurrence of parasympathetic neurogenesis from normal pancreas to PDAC. This implies that a progressive increase in parasympathetic neurogenesis may play a key role in pancreatic neoplastic transformation and that it is taking place even before invasion occurs. Similar findings were observed in previous studies, in which a high density of parasympathetic neurogenesis was found in high-risk prostate cancer (1) and gastric cancer (17).
Second, the role of the autonomic system in pancreatic cancer has been studied in recent years (18, 19, 20), but to our knowledge no studies have assessed the presence of parasympathetic neurogenesis in PDAC. Our findings might help to better stratify PDAC patients into prognostic subgroups and lead to better clinical decision making in terms of intraoperative or postoperative treatment modalities. According to the distribution of parasympathetic neurogenesis in our cases of PDAC, we propose two categories of parasympathetic neurogenesis: high grade and low grade. In the present study, high-grade parasympathetic neurogenesis was associated with aggressive clinicopathologic features of tumors such as the presence of tumor budding and early recurrence. Therefore, our data suggest that high-grade parasympathetic neurogenesis correlates with a more aggressive phenotype in PDAC. Moreover, parasympathetic neurogenesis was found to be the most important prognostic factor in PDAC patients, showing a higher prognostic ability than TNM stage in our series.
Third, prior studies have described a distinct phenomenon possibly analogous to angiogenesis (1, 17), that the tumor itself is infiltrated by a network of newly developed autonomic nerve projections that regulate cancer initiation and progression (1, 17). Studies of mouse models revealed that branches of the parasympathetic nervous system play an important role in tumor cell invasion, migration, and distant metastases through stromal Chrm1-mediated signals. In this context, parasympathetic neurogenesis can be considered not just as a simple distribution of parasympathetic nerves in the tumor, but through its association with invasion and metastasis can rather be regarded as a promoter of PDAC progression from local to systemic disease. Moreover, we observed that parasympathetic neurogenesis was strongly associated with a frequent occurrence of tumor budding. This finding probably reflects the fact that the rich parasympathetic neurogenesis of PDAC may actively participate in and promote the development of invasion and metastasis, although the contribution and role of parasympathetic neurogenesis in invasion and metastasis should be further confirmed.
Our study might be limited by the relatively small number of PDAC patients and the fact that all cases came from a single center. Nonetheless, our study benefits from complete clinicopathologic data with information on follow-up. Our findings need to be validated in larger series from multiple centers to find their proper place in everyday practice. These studies will allow us to gain further insight into the process of parasympathetic neurogenesis and provide us with a platform on which to build future in vitro and in vivo studies, with the aim of identifying new candidate molecules for future therapeutic interventions focusing on the destruction of parasympathetic neurogenesis.
Conclusions
Our study provides the evidence that parasympathetic neurogenesis occurs frequently in PDAC and may be used as a parameter of tumor aggressiveness and as an indicator of unfavorable outcomes. Moreover, parasympathetic neurogenesis was strongly associated with the frequent occurrence of tumor budding, although the underlying mechanism is yet to be elucidated.
Acknowledgements
Funding: This work was supported by grants from China Cancer Research Foundation Y-N2013-008 and the Doctoral Program of the Ministry of Education 20130001110089 to DR Xiu; the National Natural Science Foundation of China 81272709 to W Fu; and Peking University Third Hospital Grant Y81524-01 to LF Zhang.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science 2013;341:1236361. [PubMed]
- Ayala GE, Dai H, Powell M, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 2008;14:7593-603. [PubMed]
- Albo D, Akay CL, Marshall CL, et al. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 2011;117:4834-45. [PubMed]
- Olar A, He D, Florentin D, et al. Biologic correlates and significance of axonogenesis in prostate cancer. Hum Pathol 2014; 45:1358-64. [PubMed]
- Zhao Q, Yang Y, Liang X, et al. The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer 2014;14:484. [PubMed]
- Prall F. Tumour budding in colorectal carcinoma. Histopathology 2007;50:151-62. [PubMed]
- Brown M, Sillah K, Griffiths EA, et al. Tumour budding and a low host inflammatory response are associated with a poor prognosis in oesophageal and gastro-oesophageal junction cancers. Histopathology 2010;56:893-9. [PubMed]
- Koike M, Kodera Y, Itoh Y, et al. Multivariate analysis of the pathologic features of esophageal squamous cell cancer: tumor budding is a significant independent prognostic factor. Ann Surg Oncol 2008;15:1977-82. [PubMed]
- Miyata H, Yoshioka A, Yamasaki M, et al. Tumor budding in tumor invasive front predicts prognosis and survival of patients with esophageal squamous cell carcinomas receiving neoadjuvant chemotherapy. Cancer 2009;115:3324-34. [PubMed]
- Roh MS, Lee JI, Choi PJ. Tumor budding as a useful prognostic marker in esophageal squamous cell carcinoma. Dis Esophagus 2004;17:333-7. [PubMed]
- Ohike N, Coban I, Kim GE, et al. Tumor budding as a strong prognostic indicator in invasive ampullary adenocarcinomas. Am J Surg Pathol 2010;34:1417-24. [PubMed]
- Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest 2009;119:1417-9. [PubMed]
- Zhang LF, Tao M, Guo LM, et al. Multivariate analysis of predictors of pancreatic head cancer after total resection: tumor budding is an independent factor. Zhongguo Wei Chuang Wai Ke Za Zhi (in Chinese) 2012;12:604-7.
- Karamitopoulou E, Zlobec I, Born D, et al. Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur J Cancer 2013;49:1032-9. [PubMed]
- Kohler I, Bronsert P, Timme S, et al. Detailed analysis of epithelial-mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J Gastroenterol Hepatol 2015;30:78-84. [PubMed]
- O’Connor K, Li-Chang HH, Kalloger SE, et al. Tumor budding is an independent adverse prognostic factor in pancreatic ductal adenocarcinoma. Am J Surg Pathol 2015;39:472-8. [PubMed]
- Zhao CM, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med 2014;6:250ra115.
- Stopczynski RE, Normolle DP, Hartman DJ, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res 2014;74:1718-27. [PubMed]
- Kim-Fuchs C, Le CP, Pimentel MA, et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun 2014;40:40-7. [PubMed]
- Chang A, Kim-Fuchs C, Le CP, et al. Neural regulation of pancreatic cancer: a novel target for intervention. Cancers (Basel) 2015;7:1292-312. [PubMed]
