Phase Ⅰ study of chimeric anti-CD20 monoclonal antibody in Chinese patients with CD20-positive non-Hodgkin's lymphoma
Introduction
Non-Hodgkin’s lymphomas (NHLs) are a heterogeneous group of lymphatic proliferative disorders,80%-85% of which are B-cell lymphomas. CD20 is a transmembrane cellular protein that is expressed by pre-B and mature B lymphocytes,but is absent on hematopoietic stem cells,pro-B-cells,normal plasma cells,or other normal tissues. More than 90% of B-cell NHLs express CD20,which has been validated as a therapeutic target for treating NHL with successful commercialized monoclonal antibodies (mAbs).
In 1997,rituximab (MabThera®,Rituxan®; Roche/Genentech/Biogen IDEC),a chimeric,human-mouse,IgG1 anti-CD20 mAb,was the first mAb approved by United States Food and Drug Administration for the treatment of lymphoma. Rituximab was introduced as monotherapy for relapsed or refractory low-grade B-cell lymphoma (1). Thereafter,rituximab in combination with chemotherapy showed greater efficacy compared with chemotherapy alone in multiple subtypes of B-cell NHL,including chronic lymphocytic leukemia (CLL),which generally expresses low levels of CD20 antigen (2, 3, 4, 5, 6, 7, 8). More recently,rituximab maintenance therapy has been shown to further improve outcomes in patients with follicular lymphoma (FL) and mantel cell lymphoma (MCL) (9, 10, 11). Thus,rituximab has revolutionized the treatment of NHL.
Recently,several new anti-CD20 mAbs have been developed. Ofatumumab (Arzerra®,Genmab/GlaxoSmithKline,Type Ⅰ) and obinutuzumab (Gazyva®,Gazyvaro®; Roche/Genentech; Type Ⅱ) have been approved for the treatment of CLL (12, 13). Preclinical studies support the relative potency of the new anti-CD20 mAbs over rituximab (14, 15, 16),but the clinical data have been mixed. Obinutuzumab is superior to rituximab in patients with CLL (12),but no difference was observed in relapsed indolent lymphoma between the two mAbs in a phrase Ⅱ study [Sehn LH,2011 (unpublished data)]. Thus far,it is unclear whether or not these new mAbs are more efficacious than rituximab in NHL subtypes other than CLL. At present,rituximab is considered as the standard-of-care therapy in the treatment of B-cell NHL.
Due to the prominent efficacy and good tolerability of rituximab in the treatment of lymphoma,considerable effort has been made in exploring similar versions of this antibody. SCT400 is a recombinant,human-mouse chimeric anti-CD20 IgG1 mAb produced by Sinocelltech Ltd. (Beijing,China). SCT400 has 1,328 amino acids and a molecular weight of approximately 145 kD. SCT400 is expressed in Chinese hamster ovary (CHO) cells,as is rituximab. SCT400 has the same variable regions and antigen-binding site as rituximab. SCT400 has the heavy and K light chain constant regions of human IgG1 allotype G1m (1, 17) which is common in Asians. There is only one amino acid difference between SCT400 and rituximab (V219A in the CH1 domain of the heavy chain). Extensive preclinical studies in vitro and in vivo have shown that SCT400 has similar biological activities to rituximab (unpublished data).
We present here a phase Ⅰ clinical study of SCT400 in Chinese patients with CD20-positive (CD20+) B-cell NHL. SCT400 was administered as 4 once-weekly infusions at doses from 250 mg/m2 to 500 mg/m2 with up to 24 weeks of follow-up. This study provided information regarding the safety,immunogenicity,pharmacokinetics (PK),pharmacodynamics (PD) and preliminary efficacy of SCT400. Moreover,the results of the study provided a design rational for its future clinical studies.
Materials and methods
Patients
Eligible patients were 18-75 years of age with histologically-confirmed NHL that expressed the CD20 antigen,according to the 2008 WHO Classification of Lymphoma. Eligible patients received at least one course of standard therapy and were expected to benefit from treatment with anti-CD20 mAbs. All patients met the following criteria: Eastern Cooperative Oncology Group (ECOG) performance status score of 0-1,a life expectancy ≥3 months,leukocyte count ≥3×109/L,neutrophil count ≥1.5×109/L,hemoglobin ≥80 g/L,platelet count ≥75×109/L,serum IgG ≥6 g/L,serum creatinine ≤1.5× the upper normal limit (UNL),total bilirubin ≤1.5×ULN,and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤2.5×ULN.
Patients were excluded if they received rituximab or another anti-CD20 mAb within 1 year before enrollment. Patients who had prior surgery, chemotherapy or radiotherapy within 4 weeks of enrollment were not selected. Patients with central nervous system involvement of lymphoma, a history of hypersensitivity to similar mAb therapy, active infections, other severe internal diseases, and/or serologic evidence of hepatitis C virus (HCV) or human immunodeficiency virus (HIV) were excluded. Patients who were positive for hepatitis B virus surface antigen (HBsAg) or core antibody (HBcAb) and who were serum hepatitis B viral deoxyribonucleic acid (HBV DNA) polymerase chain reaction (PCR)-negative were permitted to enter the study.
The study was reviewed and approved by the Ethics Review Boards of the participating institutions and was conducted in accordance with the revised Declaration of Helsinki and the Guidelines for Good Clinical Practice Requirements. All patients were provided informed consent before participation. This study was registered at www.clinicaltrials.gov (identifier: NCT02206308).
Study design
This study was designed as a multicenter,open-label,dose-escalating phase Ⅰ clinical trial for evaluating the safety,PK and biologic effects of SCT400. Infusions were administered once weekly for 4 consecutive weeks with up to 24 weeks of follow-up. SCT400 doses were escalated in a standard 3+3 design (250 mg/m2,375 mg/m2 and 500 mg/m2). A dose expansion cohort of 9 patients was assigned to the 375 mg/m2 group for further evaluation because the recommended dose of rituximab is 375 mg/m2 for the treatment of NHL.
Treatment regimen
SCT400 was supplied in vials containing 50 mg/5 mL by Sinocelltech Ltd. (Beijing,China). SCT400 was diluted to a concentration of 1 mg/mL with normal saline,and administered as 4 weekly intravenous infusions by an infusion pump with an in-line filter. Patients were pre-medicated with oral acetaminophen (500 mg),intramuscular diphenhydramine (20 mg) and intravenous dexamethasone (10 mg) 30-60 min before each SCT400 infusion.
In this first in-human study,SCT400 was administered at a lower infusion rate in the 250 mg/m2 group for monitoring infusion-related reactions (IRRs). If SCT400 was well-tolerated in the 250 mg/m2 group,the infusion rate was escalated in the 375 and 500 mg/m2 groups. For the 250 mg/m2 dose group,the first infusion was started at a rate of 25 mg/h for 1 h,then 50 mg/h for 1 h,and escalated in increments of 50 mg/h every 1 h to a maximum rate of 200 mg/h. If well-tolerated,subsequent infusions were administered at an initial rate of 50 mg/h and increased by 50 mg/h every 30 min to a maximum rate of 200 mg/h. For the 375 mg/m2 and 500 mg/m2 dose groups,the initial infusion rate was 50 mg/h,and escalated in increments of 50 mg/h every 1 h to a maximum rate of 400 mg/h. If well-tolerated,subsequent infusions were administered at an initial rate of 100 mg/h,and increased by 100 mg/h every 30 min to a maximum rate of 400 mg/h.
If IRRs occurred,the infusion was decreased or discontinued,and symptomatic treatments,including steroids,were administered at the discretion of the investigator depending on the severity. The infusion was resumed at a 50% reduction in the previous rate after the IRR was relieved.
Dose-limiting toxicity (DLT) was defined as any of the following that was at least possibly related to SCT400 during all 4 infusions and at the one-week follow-up evaluation: grade 3 or 4 adverse events [AEs; graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events,version 4.0 (NCI CTCAE v4.0)] on the day of infusion despite premedication with glucocorticosteroids,grade 3 or 4 non-hematologic AEs,or grade 4 hematologic AEs on non-infusion days.
The maximum tolerated dose (MTD) was defined as the dose level below the dose in which any of the following occurred: 1 treatment-related,fatal,life threatening,or a permanently disabling serious adverse event (SAE); or 2 cases of DLT.
Evaluation of safety and tolerability
Disease history,physical examination,electrocardiogram (ECG),laboratory assessments,including hematology,clinical chemistry,serum immunoglobulin levels (IgG,IgA,and IgM),complement C3 level and T cell count (CD3+,CD4+ and CD8+) and natural killer (NK) cell count (CD16+ and CD56+),were carried out at the time of screening weekly during treatment,and every 4 weeks during follow-up. Serum markers for HBV,HCV,and HⅣ were assessed at baseline. Patients who were positive for HBV (surface antigen or core antibody) and PCR-negative for HBV DNA,had HBV DNA tested at weeks 1,4,8,11,15 and 24 after treatment. Vital signs were monitored before and every 15 min during infusion until 1 h after infusion. ECG monitoring (before,during,and until 1 h from the end of infusion) was performed during the infusions and every 4 weeks during the follow-up period. AEs were documented throughout the study and graded according to the NCI CTCAE v4.0.
Human anti-chimeric antibodies (HACAs) assay
Titers of HACAs in serum were evaluated quantitatively at the time of screening and weeks 1,12,and 24 weeks after treatment by a validated bridging enzyme-linked immunosorbent assay (ELISA) co-developed by the Beijing Key Laboratory of Clinical Study on Anticancer Molecular Targeted Drugs (China) and Sinocelltech Ltd. (Beijing,China). The lower limit of quantification using the method was determined to be 125 ng/mL.
Pharmacokinetics
Blood samples were obtained for determination of serum SCT400 concentrations. In the first infusion,blood samples were obtained pre-infusion,1,3,and 6 h (if applicable) after the beginning of the infusion,and 0,2,4,8,24,48,72,96,and 120 h after the end of the infusion. During the second and third infusions,blood samples were obtained pre-infusion,and 0 and 120 h after the end of the infusion. During the fourth infusion,in addition to the time points during the first infusion,blood samples were also obtained at 168 h and on days 14,21,28,56,84,and 168 after the infusion. Serum SCT400 levels were measured by an ELISA-based assay co-developed by the Beijing Key Laboratory of Clinical Study on Anticancer Molecular Targeted Drugs (China) and Sinocelltech Ltd. (Beijing,China). The lower limit of quantification was 1.56 μg/mL. Pharmacokinetic parameters,including the area under the curve (AUC),maximum concentration (Cmax),volume of distribution (Vz),terminal half-life (T1/2),and clearance (CL),were estimated by non-compartmental pharmacokinetic analysis using Phoenix WinNonlin 6.3 (Tripos,St Louis,MO,USA).
Biological effects
Quantification of B cells (CD19+ and CD20+) was performed using flow cytometry at the time of screening,72 and 96 h after the first infusion,before the second,third and fourth infusions and at weeks 1,4,8,12 and 24 after treatment.
Response to SCT400 was evaluated according to International Workshop Criteria. The overall response rate (ORR) was defined as complete remission (CR),unconfirmed complete remission (CRu) and partial remission (PR). Patients were assessed with computed tomography (CT) scans of the neck,thorax,abdomen,and pelvis at months 1,2,3,and 6 after treatment. A bone marrow (BM) biopsy was obtained to confirm CR if positive at screening.
Statistical analysis
Statistical analyses were performed with SPSS software (version 16.0; SPSS Inc.,Chicago,IL,USA). The Wilcoxon matched-pairs signed-ranks test was used to assess the statistical significance of changes in the B cell count,T cell count,immunoglobulin levels and C3 level,compared with the baseline values. The same test was used to compare serum concentration by clinical response. Time to progression (TTP) was measured from the first day of SCT400 infusion and was estimated according to the Kaplan-Meier method. All tests were two-sided and P<0.05 was considered statistically significant.
Results
Demographics
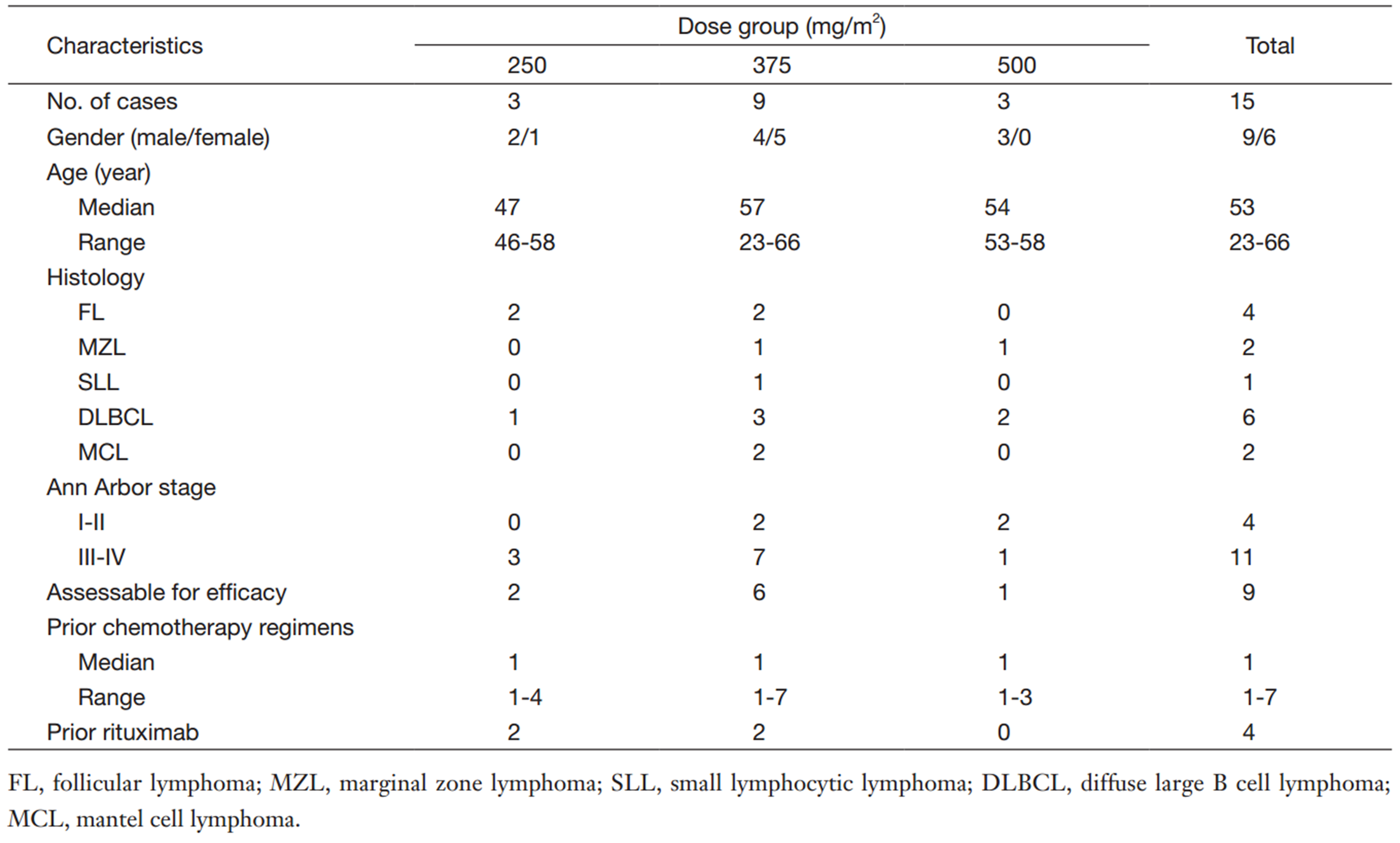
Between June 2012 and July 2013,15 patients with CD20+ NHL were enrolled in the study. The patient characteristics are listed in Table 1. The median age was 53 years (range,23-66 years) and 9 patients (60%) were males. The histological subtypes were as follows: diffuse large B cell lymphoma (DLBCL),n=6; FL,n=4; marginal zone lymphoma (MZL),n=2; MCL,n=2; and small lymphocytic lymphoma (SLL),n=1. Three patients had BM infiltration,and the majority of patients (11 of 15) had Ann Arbor Stage Ⅲ or Ⅳ disease. Four patients received previous rituximab-containing chemotherapy; the median interval between the last rituximab treatment and the first SCT400 treatment was 21 months (range,18-31 months). None of the patients was refractory to rituximab (no response or progression within 6 months of treatment). No patients had undergone prior high-dose therapy and autologous stem cell transplantation (HDT/ASCT). All 15 patients completed treatment and the safety evaluation. Nine patients were included in efficacy analysis,and the other 6 patients were not evaluable for clinical response because of no measurable lesions at the time of screening.
Full table
Treatment
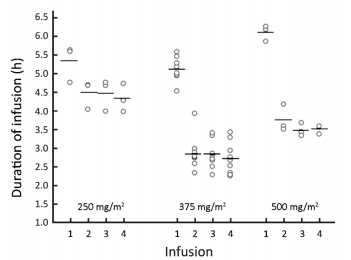
All 15 patients completed 4 weekly infusions of SCT400. The median weekly dose was 522.5 mg (range,412.5-532.5 mg),648.8 mg (range,596.3-817.5 mg),and 925.0 mg (range,855.0-945.0 mg) for the 250 mg/m2,375 mg/m2,and 500 mg/m2 dose groups,respectively. The median duration of the first infusion was 5.58,5.17,and 6.25 h for the 250,375,and 500 mg/m2 dose groups,respectively,and the median duration for the subsequent 3 infusions was 4.67,2.75,and 3.58 h for the 3 groups,respectively (Figure 1).
Safety
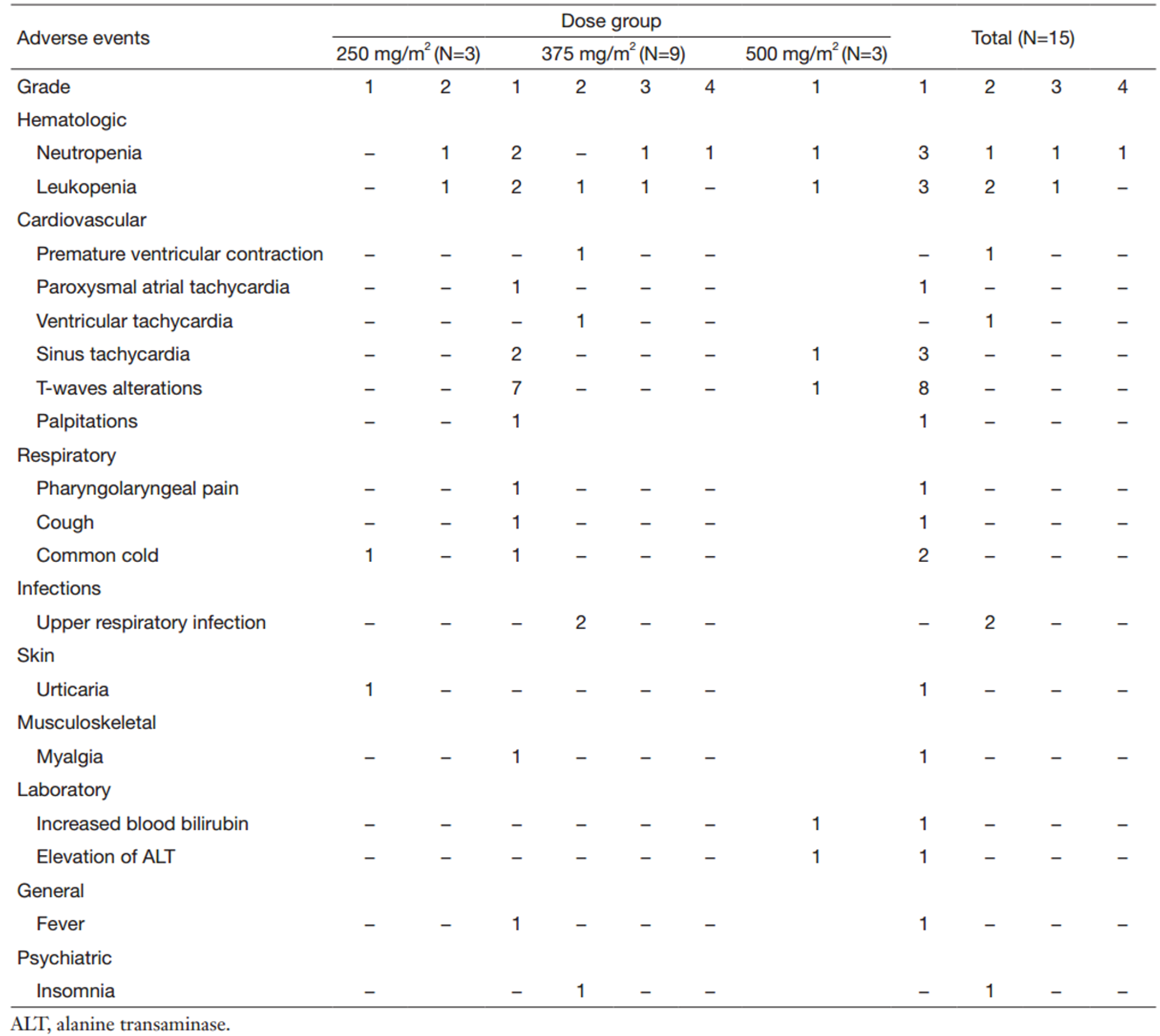
All 15 patients were evaluated for safety. A total of 66 AEs that were possibly related to SCT400 were reported in 12 patients during the course of the study (Table 2). Among the AEs,61 (92%) were grade 1 or 2,and the majority resolved without medication. The most frequent AEs were in the category of “hematology”,“cardiac disorders” and “respiratory disorders”.
Full table
With premedication of antihistamine and glucocorticosteroids,IRRs were recorded in only 2 patients (13%) with manifestations of urticaria and muscle pain,which were all grade 1 and resolved without medication,and did not require a decreased infusion rate or an interruption in the infusion.
A total of 5 patients (33%) developed neutropenia in the study,and 2 patients (13%) in the 375 mg/m2 dose group had grade 3 or 4 neutropenia. One patient with normal BM had 2 episodes of grade 4 neutropenia at weeks 8 and 14 after treatment; the patient recovered after the treatment with recombinant human granulocyte-colony stimulating factor (rhG-CSF). The other patient with BM infiltrated by lymphoma,had grade 3 neutropenia 2 weeks after treatment and recovered within 28 d without medical intervention. The above grade 3 or 4 neutropenia all occurred during the follow-up period and did not meet the definition of a DLT. No anemia or thrombocytopenia was recorded in the study.
One patient in the 375 mg/m2 dose group had palpitations shortly after the first SCT400 infusion. The ECG revealed paroxysmal atria tachycardia,ventricular tachycardia,and premature ventricular complexes. The heart rate and blood pressure were in the normal ranges,so close monitoring continued and no intervention was given to the patient. The palpitations resolved 1 d later,and the ECG was normal 5 d later. This patient developed transient palpitation after the next three infusions,but arrhythmias never occurred. The palpitations were graded as 1 and the arrhythmias were graded as 2; both cardiac AEs were judged to be related to SCT400. An occasional premature ventricular complex was noted in the baseline ECG of the patient. Eight patients (7 in the 375 mg/m2 dose group and 1 in the 500 mg/m2 dose group) experienced asymptomatic T-flattening or inversion in 2 or 3 leads in the ECG,which returned to baseline status spontaneously from 1 d to 2 weeks later. The T-wave alterations were judged to be possibly related to the treatment and graded as level 1.
Two episodes of upper respiratory infections were noted during follow-up in 2 patients in the 375 mg/m2 dose group. One patient had grade 4 neutropenia and the other patient had a normal neutrophil count. Both upper respiratory infections resolved completely with oral antibiotics.
Only 1 patient developed a transient grade 1 elevation of ALT and bilirubin after SCT400 treatment,and recovered without treatment. No SAEs were reported. No DLT events were observed,and no MTD was identified in the study. Given the small number of patients in this trial,an association between dose,safety and tolerability could not be confirmed.
HBV reactivation
One patient was HBsAg-positve and 7 patients was HBsAg-negtive but HBcAb-positive. All the 8 patients were PCR-negative for HBV DNA at the time of screening. The patient who was HBsAg-positve took entecavir (0.5 mg daily) during the study,and the patients who were HBcAb-positive received no anti-HBV therapy. The serum HBV DNA of the 8 patients remained PCR-negative throughout the study.
Immunology
No significant changes in T or NK cell counts were observed throughout the study. No significant changes in serum levels of immunoglobulin and complement C3 were noted. All patients were tested negative for HACAs at baseline,and no patient developed anti-SCT400 antibodies after treatment.
Treatment response
All 15 patients had radiologic assessments during the study. Of the 9 assessable patients (2 with FL,2 with MZL,3 with DLBCL and 2 with MCL),the best overall response was 33.3% (3/9). One patient with MZL in the 375 mg/m2 dose group reached CR,and 1 patient with DLBCL in the 250 mg/m2 dose group and 1 patient with FL in the 375 mg/m2 dose group achieved PR. Another patient with MZL in the 375 mg/m2 dose group had a 46% reduction in tumor mass at the end of the study. The duration of response in responding patients ranged from 8-20 weeks. In the 6 patients without target lesions at baseline,2 with DLBCL relapsed at 4 and 8 weeks after treatment,respectively. The median TTP for the 9 assessable patients was 28 weeks (range,16-28+ weeks). The median TTP for the entire cohort of patients was not reached as of the median follow-up time of 28 weeks.
Pharmacokinetics
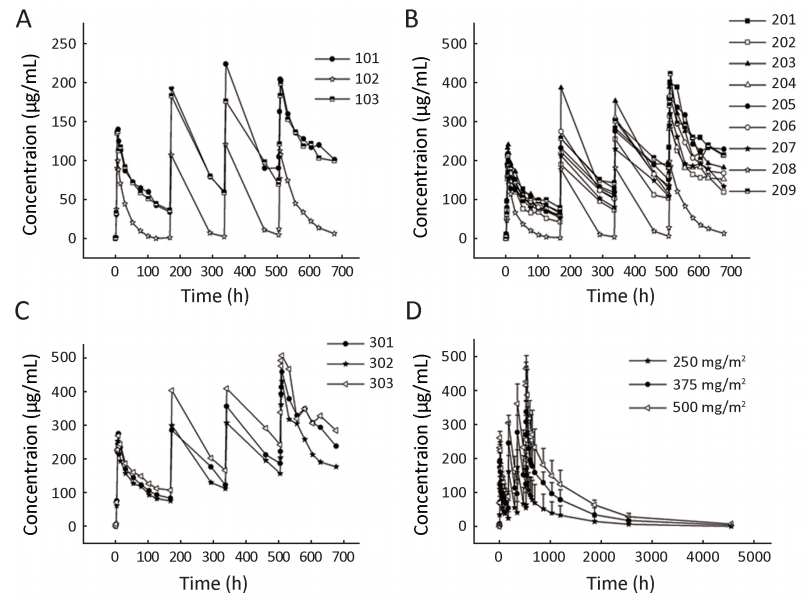
Serum concentrations of SCT400 in the individual dose groups are shown in Figure 2,and the pharmacokinetic parameters of SCT400 following the first and the fourth doses are summarized in Table 3. From the first to the fourth dose,the Cmax,AUC and T1/2 of SCT400 increased significantly (P<0.05),and the decrease of CL during the course of treatment was significant (P<0.05). From 250 mg/m2 to 500 mg/m2,the Cmax and AUC increased significantly in a dose-dependent manner (P<0.05),but the increase of T1/2 and decrease in CL across dose levels were not significant (P>0.05). There was no significant difference in the SCT400 concentration between responders and non-responders (data not shown).

Full table
In the present study,2 patients [patient No.102 (250 mg/m2) and patient No.208 (375 mg/m2)] had different pharmacokinetic profiles,compared with the other 13 patients. For these 2 patients,the peak SCT400 concentrations (Cpeak) after each infusion were markedly lower compared with those of the other patients in the same dose group,and the trough concentration (Ctrough) decreased to nearly zero before subsequent infusions (Figure 2). In the other 13 patients,the Cpeak and Ctrough increased in parallel with the course of infusions,and an accumulating effect was observed.
Depletion of B cells in peripheral blood
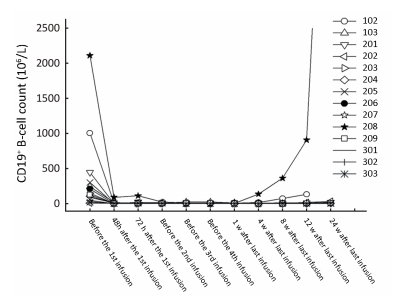
The median peripheral blood CD19+ B-cell count was 126×106/L [range,(8-2,112)×106/L] at baseline which was in the normal range (50-610×106/L). A rapid,profound and durable depletion of circulating CD19+ B cells was observed in the patients in all dose groups following the treatment with SCT400. As shown in Figure 3,the CD19+ B cell counts decreased rapidly in all patients following the first infusion,and the depletion status (<50×106/L) remained through week 24 after the final dose in 13 of the 15 patients. Because of progression of BM disease,the other 2 patients [patient No.102 (250 mg/m2) and patient No.208 (375 mg/m2)] had early B-cell recovery at week 4 and 8,respectively,following the last infusion. The same changes were observed respect to CD20+ B-cell counts. With the limited number of patients at each dose level,it is not possible to draw meaningful conclusions regarding the correlations between SCT400 dose levels and the effect on peripheral blood B-cell counts.
Discussion
This phrase Ⅰ study demonstrated that SCT400 is generally safe and well-tolerated when administered as 4 once-weekly infusions at doses from 250 mg/m2 to 500 mg/m2. Most AEs were mild-to-moderate in intensity,with 2 patients experiencing grade 3 or 4 treatment-related neutropenia and no patients experiencing grade 3 or 4 infections. No HACA was detected. No effects were noted in immunoglobulin levels,complement levels or T-cell counts,which are in agreement with the reports involving rituximab (1, 17, 18, 19). No SAEs were reported,no DLTs were identified,and the MTD was not reached.
The IRRs of SCT400 were mild in the study,and were less common than the IRRs reported in the studies involving rituximab (1, 17, 18, 19, 20). IRRs tend to be proportional to the patient’s tumor load and can be ameliorated by premedication with glucocorticosteroids (21). Six out of the 15 patients in our study had no tumor burden,which could be an explanation for the low incidence of IRRs. Premedication,including glucocorticosteroids,is mandatory according to the label of rituximab distributed in China. Due to ethical considerations,dexamethasone was used in this study,which may have prevented some of IRRs.
In the present study,2 out of 15 patients experienced grade 3 or 4 neutropenia. Neutropenia is one of the common side effects of rituximab,and often occurred in the patients with BM involvement,bulky disease,elevated lactate dehydrogenase (LDH),extranodal diseases,or a history of ASCT (1, 19, 22, 23). In our study with SCT400,1 patient with grade 3 neutropenia had BM involvement of lymphoma. Grade 3 or 4 neutropenia was observed during the follow-up and resulted in one episode of infection. Delayed neutropenia is the most common late toxicity from rituximab treatment and could result in severe infections,such as respiratory infections and herpes zoster (1, 23, 24). The experience in this study,as described in previous reports,suggests that patients following anti-CD20 mAbs treatment,especially patients with high risk factors,warrant regular complete blood count monitoring after the treatment.
In this study,1 patient developed multiple arrhythmias with palpitation after the first SCT400 infusion,which resolved without medical intervention. Arrhythmias are reported in a minority of patients treated with rituximab,and appear most often in the initial infusions,during the infusions,or immediately afterwards (24, 25). The precise pharmacologic mechanism underlying arrhythmia related with rituximab is unknown. Indeed,the arrhythmias may simply relate to the concomitant cardiac risk factors of the patients. The patient,who had arrhythmias in our study,had premature ventricular complexes in the baseline ECG,which might be a clinical manifestation of underlying disease of cardiac conduction system. Because arrhythmias can be life-threatening,cardiac monitoring during and after infusions of anti-CD20 mAbs,such as rituximab and SCT400,should be performed,especially for patients with high-risk factors such as high tumor burden,a history of cardiac diseases,or cardiac risk factors (25).
Asymptomatic ECG alteration (T-flattening or inversion) was observed in 8 patients. Asymptomatic T-wave alteration was only reported in 1 patient in a phase Ⅰ/Ⅱ study under a maximal rituximab infusion rate of 700 mg/h (26). As indicated,the patient had no pre-existing cardiac conditions,and there was no cardiac enzyme elevation in the patient. No further ECG alterations appeared after a reduction of the infusion rate. It is unfortunate that cardiac enzyme was not tested in the present study involving SCT400. An extensive cardiologic evaluation will be performed concomitantly in the phase Ⅱ study to evaluate whether or not SCT400 infusions have an impact on ECG.
Analysis of pharmacokinetic data indicated that exposure (Cmax and AUC) to SCT400 increased in parallel with the course of infusions and an accumulating effect was observed; the Cmax and AUC of SCT400 increased in a dose-dependent manner from 250 mg/m2 to 500 mg/m2. The above findings were consistent with the findings reported for rituximab (1, 17, 18, 20).
The key pharmacokinetic parameters of SCT400 at 375 mg/m2 in the present study (n=9) and the corresponding results (n=14) in the phrase Ⅲ study of rituximab monotherapy are summarized and compared in Table 4 (1, 27). The PK profiles of SCT400 in this population of Chinese patients are broadly comparable to the PK profiles of rituximab in Caucasian patients,although the PK parameters of anti-CD20 mAbs had a high degree of inter-individual variability. These data also support the notion that the PK profiles of anti-CD20 mAbs are similar between Asians and Caucasians,which has been reported in the studies involving rituximab and obinutuzumab in Japanese patients (18, 28).

Full table
In the present study,2 patients [patient No.102 (250 mg/m2) and patient No.208 (375 mg/m2)] had markedly lower SCT400 exposure compared with the other patients in the same dose group. Through further analysis of clinical characteristics,we found that these 2 patients had higher tumor burdens. Using patient No.208 (375 mg/m2) as an example,a high number of CD19+ B cells were noted in peripheral blood (2,112×106/L) due to BM involvement,and large tumor masses [sum of products of the perpendicular diameters (SPD) of target lesions=77.38 cm2] existed at the start of the study. The patient did not achieve responses to treatment. In contrast,the other 8 patients in 375 mg/m2 dose group had normal CD19+ B-cell counts [median,136×106/L; range,(7-445)×106/L]. Three of them had no tumor burden,and the majority of the other 5 patients had small tumor masses (median SPD of target lesions,15.96 cm2; range,2.40-47.09 cm2) at baseline. Two of the 5 assessable patients achieved PR after SCT400 treatment. The same finding was noted for patient No.102 (250 mg/m2). The above analysis showed that tumor burden influenced exposure and response to SCT400,which was in agreement with the results of studies involving rituximab and ofatumumab in murine xenograft models (29, 30). Thus,individual adjustment of anti-CD20 mAb dose to tumor burden or the same over-saturated dose for all the patients warrants further study.
All doses of SCT400 resulted in a rapid depletion of B cells within 48 h after the first infusion,and the depletion persisted for up to 24 weeks after treatment for most of the patients. These results were consistent with the reports involving rituximab (1, 17, 18, 19, 20). In the present study,2 patients (No.102 and No.208),who had lymphoma infiltration of the BM at baseline,displayed early B cell (CD19+ and CD20+) recovery 4 and 8 weeks,respectively,after treatment. At those time points,the leukocyte and lymphocyte counts in the 2 patients were in the normal ranges,and tumor masses were stable based on CT scans,but the SCT400 concentrations could not be detected. Disease progression in the 2 patients was showed by CT scans and BM biopsy at 15 and 24 weeks after treatment. Therefore,after anti-CD20 mAb treatment,early B cell recovery may indicate a marked decrease in anti-CD20 mAb concentration and disease progression afterwards.
Three of 9 assessable patients achieved objective responses (CR,n=1; PR,n=2) after 4 once-weekly infusions of SCT400,and responders had FL,MZL and DLBCL. In addition,at the end of the study,1 patient with MZL had a 46% reduction in the SPD compared with baseline. Three of the 4 assessable indolent lymphoma patients responded,which indicated encouraging activity of SCT400.
Conclusions
The present study demonstrated that SCT400 is safe and well-tolerated in Chinese patients with CD20+ B-cell NHL. The PK/PD results of SCT400 are comparable to rituximab,and the preliminary efficacy data are encouraging. Given the above similarity between SCT400 and rituximab plus the extensive in vitro and preclinical bio-similarity assessments,the same dosing schedule as rituximab is recommended for SCT400,but further clinical studies are warranted.
Acknowledgements
The authors thank D Li,DD Zhao,Z Lin and XB Zhou for their contributions in the preclinical studies of SCT400,and KL Chong and XB Xiao for their contributions in the clinical study. The authors also thank XL Yang for editing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 1998;16:2825-33. [PubMed]
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010;116:2040-5. [PubMed]
- Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008;9:105-16. [PubMed]
- Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the Mab Thera International Trial (MInT) Group. Lancet Oncol 2011;12:1013-22. [PubMed]
- Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005;106:3725-32. [PubMed]
- Marcus R, Imrie K, Solal-Celigny P, et al. Phase Ⅲ study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol 2008;26:4579-86. [PubMed]
- Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2004;104:3064-71. [PubMed]
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010;376:1164-74. [PubMed]
- Martinelli G, Schmitz SF, Utiger U, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol 2010;28:4480-4. [PubMed]
- Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011;377:42-51. [PubMed]
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med 2012;367:520-31. [PubMed]
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014;370:1101-10. [PubMed]
- Hillmen P, Robak T, Janssens A, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet 2015;385:1873-83. [PubMed]
- Dalle S, Reslan L, Besseyre de Horts T, et al. Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther 2011;10:178-85. [PubMed]
- Rafiq S, Butchar JP, Cheney C, et al. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol 2013;190:2702-11. [PubMed]
- Herter S, Herting F, Mundigl O, et al. Preclinical activity of the type Ⅱ CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther 2013;12:2031-42. [PubMed]
- Maloney DG, Grillo-López AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase Ⅰ multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol 1997;15:3266-74. [PubMed]
- Tobinai K, Kobayashi Y, Narabayashi M, et al. Feasibility and pharmacokinetic study of a chimeric anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab) in relapsed B-cell lymphoma. The IDEC-C2B8 Study Group. Ann Oncol 1998;9:527-34. [PubMed]
- Igarashi T, Kobayashi Y, Ogura M, et al. Factors affecting toxicity, response and progression-free survival in relapsed patients with indolent B-cell lymphoma and mantle cell lymphoma treated with rituximab: a Japanese phase Ⅱ study. Ann Oncol 2002;13:928-43. [PubMed]
- Maloney DG, Grillo-López AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood 1997;90:2188-95. [PubMed]
- Lim SH, Levy R. Translational medicine in action: anti-CD20 therapy in lymphoma. J Immunol 2014;193:1519-24. [PubMed]
- Davis TA, White CA, Grillo-López AJ, et al. Single-agent monoclonal antibody efficacy in bulky non-Hodgkin's lymphoma: results of a phase Ⅱ trial of rituximab. J Clin Oncol 1999;17:1851-7. [PubMed]
- Tobinai K, Igarashi T, Itoh K, et al. Japanese multicenter phase Ⅱ and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Ann Oncol 2004;15:821-30. [PubMed]
- Foran JM, Rohatiner AZ, Cunningham D, et al. European phase Ⅱ study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol 2000;18:317-24. [PubMed]
- Poterucha JT, Westberg M, Nerheim P, et al. Rituximab-induced polymorphic ventricular tachycardia. Tex Heart Inst J 2010;37:218-20. [PubMed]
- Siano M, Lerch E, Negretti L, et al. A phase Ⅰ-Ⅱ study to determine the maximum tolerated infusion rate of rituximab with special emphasis on monitoring the effect of rituximab on cardiac function. Clin Cancer Res 2008;14:7935-9. [PubMed]
- Berinstein NL, Grillo-López AJ, White CA, et al. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol 1998;9:995-1001. [PubMed]
- Ogura M, Tobinai K, Hatake K, et al. Phase Ⅰ study of obinutuzumab (GA101) in Japanese patients with relapsed or refractory B-cell non-Hodgkin lymphoma. Cancer Sci 2013;104:105-10. [PubMed]
- Daydé D, Ternant D, Ohresser M, et al. Tumor burden influences exposure and response to rituximab: pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20. Blood 2009;113:3765-72. [PubMed]
- Bleeker WK, Munk ME, Mackus WJ, et al. Estimation of dose requirements for sustained in vivo activity of a therapeutic human anti-CD20 antibody. Br J Haematol 2008;140:303-12. [PubMed]