Evolution of radical hysterectomy for cervical cancer along the last two decades: single institution experience
Introduction
Radical hysterectomy (RH) remains as the preferred surgical option for the management of early stage cervical cancer (1, 2, 3). Other indications include recurrence after radiation therapy,selected patients with upper vaginal carcinoma and other rare malignancies of the cervix (1, 2). The goal of this surgery is to remove the tumor with free margins,to identify and to remove possible nodal metastases in order to plan the most appropriate adjuvant treatment (4).
The first surgical approach to RH [abdominal RH,(ARH)] was made by Osiander and colleagues in the nineteenth century (1). Although it is known that the first truly RH for cervical cancer was performed by John Clark in 1895,this surgery is mostly linked to Ernst Wherteim,due to his report on 500 radical hysterectomies and partial lymphadenectomies performed from 1898 to 1911 (1, 5, 6). After that,Shauta described the first vaginal RH and Meigs established this surgery as the treatment of choice incorporating complete pelvic lymphadenectomy (1, 5). With the introduction of laparoscopy,Dargent reported in 1987 the first laparoscopic assisted vaginal RH (LAVRH). After that,other surgeons such as Kadar and Querleu described combinations of laparoscopic and vaginal surgeries in 1993 (7, 8, 9). The first total laparoscopic RH (LRH) with pelvic lymphadenectomy was reported by Nezhat in 1992 and Spirtos in 1996 (10, 11). Currently,endoscopic RH is the gold standard (12, 13) although abdominal RH is an option in non-expertise centers (14, 15). Among different types of RH included in the published classifications (6, 12, 16, 17),currently the less radical options are preferred,including nerve sparing if possible in order to decrease the complication rates observed in the past (18, 19, 20).
The aim of the present study was to evaluate the historical evolution of RH in three periods: abdominal RH,combination of laparoscopic and vaginal techniques and totally laparoscopic RH; as well as to compare perioperative and oncological outcomes.
Methods
After IRB approval,we performed a retrospective review of medical records from patients who underwent RH for gynaecological cancer,mainly cervical cancer,between years 1990-2013 at La Paz University Hospital in Madrid,Spain. We divided the patients according to the year of surgery in three periods: from 1990 to 1999,the second one from 2000 to 2009 and the last one from 2010 to 2013. This was made because of the different approaches for this surgery in the different periods: open surgery during the first period,during the second one we found the introduction of laparoscopic approach,and well established laparoscopy and the end.
Data collected included: age,parity,smoking,co-morbid medical conditions,previous surgeries,body mass index (BMI),International Federation of Gynecology and Obstetrics (FIGO) stage,pathologic details,perioperative data and follow-up. Moreover,presence of necrosis was reported after year 2000. Nerve sparing technique was introduced in year 2011,before it,all radical hysterectomies were Piver type Ⅲ.
To analyze the disease-free survival rate,we computed from the day of surgery to the date of relapse/death or censored at the date of last follow-up visit in event-free subjects.
Descriptive statistics were carried out using mean and standard deviation for quantitative variables,and proportion and absolute values for qualitative variables. Comparisons for qualitative variables were carried out by chi-square test and for qualitative variables t-test and ANOVA were used. Disease free interval and overall survival were done using the Kaplan-Meier curves. Alpha error was set at 5%. All analyses were carried out using the software SPSS 15.0 (SPSS Inc.,Madrid,Spain).
Results
We revised 102 cases of RH performed at our center during the study period. We divided the total of surgeries according to three time frames corresponding to the different decades: 1990-1999,2000-2009 and 2010-2013. The baseline characteristics are shown in Table 1.

Full table
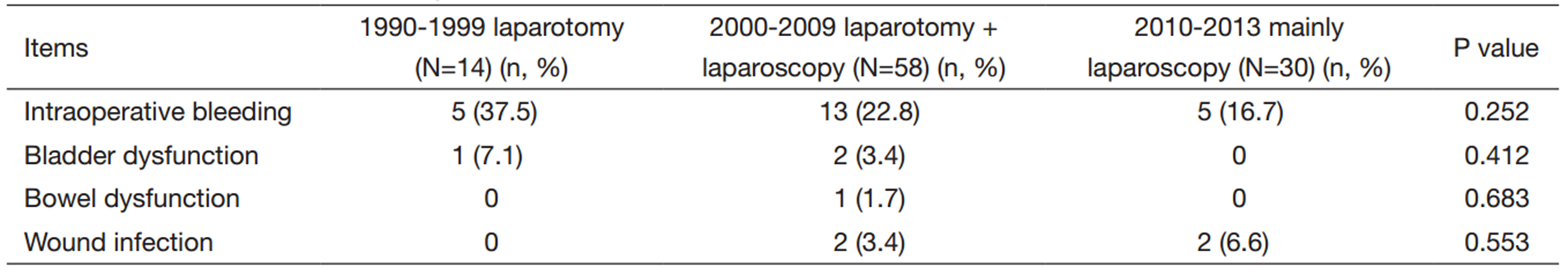
The conversion to laparotomy rate was significantly different between groups. Four cases (19%) were observed between 2000 and 2009 compared to the absence of cases (0%) between 2010 and 2013 (P=0.001). No laparoscopies were performed between 1990 and 1999. The rest of data regarding complications are shown in Table 2.
Full table
No significant differences (P=0.124) were observed in the adjuvant treatment received among the three different groups. No adjuvant treatment was administered in 11 (78.5%),36 (62.1%) and 15 (50%) cases,respectively; although radiotherapy was administered in 2 (14.2%),8 (13.7%) and 8 (26.6%) patients,respectively; chemotherapy was administered in 0,1 (1.7%) and 3 (10%) cases,respectively; whereas,concurrent chemoradiation was given to 1 (7.1%),13 (22.4%),and 2 (6.6%) patients,respectively. Moreover,additional brachytherapy was administered to 1 (7.1%),14 (24.1%) and 10 (33.3%) patients among the different periods of time,respectively (P=0.253).
The mean follow-up time was 109.9±83.3 months. According to the different groups the mean follow-up time was 207.3±30.3,105.5±33.5 and 30.1±13.2 months respectively. No significant differences were observed in the rate of recurrences between the last two groups: 6 (10.3%) recurrences between 2000 and 2009 and 5 (16.6%) relapses between 2010 and 2013 (P=0.347). At the time of the last contact the patients free of disease were 12 (85.7%),53 (91.3%) and 26 (86.6%),respectively (P=0.406).
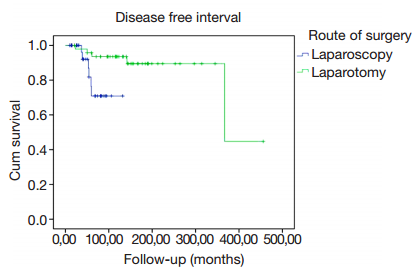
When we analyzed the disease free interval we observed significant better outcomes in the group of laparotomy compared to laparoscopy (P=0.015) (Figure 1).
Discussion
In this study we analyzed the evolution of RH in our center through the last 20 years. We found that the mean age and BMI increased through the study periods,although the last one did not reach statistical significance. A possible explanation could be that in the second and third period we increased the number of obese patients treated surgically referred from other centers due to the complexity of surgery.
There was a significant change in the route of surgery preferred in the last decade,in the second period only 36% were laparoscopic surgeries compared to the 87% in the last 4 years. As we have seen above,in the last study period the conversion rate was 0 compared to the second period when it reached the 19%. It is important to notice how the learning curve influences the outcomes in the laparoscopic approach of this surgery,as described by many previous papers (13, 15, 21). In our study the route of surgery was significantly different among groups.
In a prospective study of 234 open radical hysterectomies from 12 European institutes including patients with cervical and endometrial cancer (22),the mean operating time was 240 minutes,the median number of lymph nodes removed was 26,and they reported a 22% of postoperative morbidity (mainly urinary tract infection). Moreover,they found 30% of blood transfusion and a median hospital stay of 13 days. When comparing these findings to ours during the first period of study,we observed the same hospital stay and blood transfusion rate; however,our operating time and harvested lymph nodes were lesser. About this last point,we found a significant difference in the number of lymph nodes between the periods according with other authors who found that the number increased with laparoscopy (8, 13, 15).
With the introduction of laparoscopic technique,we found that the operating time was longer but the hospital stay shorter (P<0.05). We also encountered decreased intraoperative estimated blood loss and less transfusion requirements using this technique (P<0.05). Authors have been tried to find possible explanations to these improvements,such as better visualization of small vessels,better hemostasis control,and the use of electro-cautery (10, 15, 21).
In a review of ten studies comparing LAVRH vs. ARH including 1,019 patients,it was found that mean blood loss,major postoperative complications and hospital stay were significantly lower for LAVRH (15). The finding of less blood loss was consistently reported in other studies (7, 8, 10). They also described an increased mean number of lymph nodes removed with LAVRH (15). Salicrú et al.’s review of LRH for early stage cervical cancer showed similar results although they reported no significant differences in the number of lymph nodes obtained (21). Regarding complications,we found no significant differences but in the last period there was a decrease in bladder dysfunction,a relatively frequent complication in ARH (2, 3, 14),probably caused by the introduction of nerve sparing technique in the last period. We did not observe differences in the rate of intraoperative complications comparing ARH to LRH as reported in other studies (7, 21). It was intriguing that we found a not significant increase in wound infection rates during the last period; perhaps,this could be caused by the longer operating time,which needs to be investigated in the future.
Our median tumoral size was similar in all the periods,bigger than 2 cm,which represents an important prognostic factor in cervical cancer (1, 2, 3, 4, 23). However,its value is controversial for other authors (24). Lymphovascular space invasion (LVSI) was presented in nearly 30% of patients for each period although it was not statistically significant. The presence of necrosis was significantly higher in the first period when we comparing it to the other two. An interesting study comparing multivariate to univariate analysis on prognostic factors for cervical cancer reported clinical stage,cell differentiation,depth of cervical stromal invasion,parametrial tissue involvement,and lymph node metastasis when univariate analysis was carried out; and non-scamous histological type,poor differentiation,parametrial tissue involvement and stromal invasion when multivariate analysis was performed (25).
We found no significant differences in the rate of recurrences among the last two groups. Other studies have described recurrence rates ranging from 7% to 16% for ARH (23) and from 6% to 8% in LARVH and LRH (8). However,we found a significant difference in the disease-free interval between laparotomy and laparoscopy. In the study of Kato et al. (26),the 5-year overall survival depending on the FIGO stage showed a decrease comparing the early stages to the advanced stages. In our study,10% of patients in the third period were FIGO IIA1 stage,thus this could explain partially the differences in the disease-free intervals and the increment of recurrences that we observed among patients underwent laparoscopy. It also correlates with the increased number of patients who required adjuvant therapy,this could represent an interpretation bias of disease-free survival that needs to be taken into consideration,which disagrees with the general literature results. In spite of these findings,the percentage of disease-free patients at the last contact was similar for all periods.
Conclusions
In conclusion,laparoscopic RH is a feasible procedure that shows truly advantages such as decreased surgical complications,shorter hospital stay,and earlier resumption to daily activities. Further studies are needed in order to clarify the oncological out comes of the technique.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors declare no conflict of interest.
References
- Hughes SH, Steller MA. Radical gynecologic surgery for cancer. Surg Oncol Clin N Am 2005;14:607-31, viii. [PubMed]
- Webb MJ. Radical hysterectomy. Baillieres Clin Obstet Gynaecol 1997;11:149-66. [PubMed]
- Ware RA, van Nagell JR. Radical hysterectomy with pelvic lymphadenectomy: indications, technique, and complications. Obstet Gynecol Int 2010;2010. pii: 587610. [PubMed]
- Verleye L, Vergote I, Reed N, et al. Quality assurance for radical hysterectomy for cervical cancer: the view of the European Organization for Research and Treatment of Cancer--Gynecological Cancer Group (EORTC-GCG). Ann Oncol 2009;20:1631-8. [PubMed]
- Rock JA, Jones HW Ⅲ, editors. Te Linde’s Operative Gynecology.10th Edition. Philadelphia: Lippincott Williams & Wilkins, 2008.
- Cibula D, Abu-Rustum NR, Benedetti-Panici P, et al. New classification system of radical hysterectomy: emphasis on a three-dimensional anatomic template for parametrial resection. Gynecol Oncol 2011;122:264-8. [PubMed]
- Kucukmetin A, Biliatis I, Naik R, et al. Laparoscopically assisted radical vaginal hysterectomy versus radical abdominal hysterectomy for the treatment of early cervical cancer. Cochrane Database Syst Rev 2013;10:CD006651. [PubMed]
- Koehler C, Gottschalk E, Chiantera V, et al. From laparoscopic assisted radical vaginal hysterectomy to vaginal assisted laparoscopic radical hysterectomy. BJOG 2012;119:254-62. [PubMed]
- Querleu D. Laparoscopically assisted radical vaginal hysterectomy. Gynecol Oncol 1993;51:248-54. [PubMed]
- Pikaart DP, Holloway RW, Finkler NJ, et al. Clinical-pathologic and morbidity analyses of Types 2 and 3 abdominal radical hysterectomy for cervical cancer. Gyn Oncol 2007;107:205-10.
- Nezhat CR, Burrell MO, Nezhat FR, et al. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet Gynecol 1992;166:864-5. [PubMed]
- Salicrú SR, de la Torre JF, Gil-Moreno A. The surgical management of early-stage cervical cancer. Curr Opin Obstet Gynecol 2013;25:312-9. [PubMed]
- Hong JH, Choi JS, Lee JH, et al. Can laparoscopic radical hysterectomy be a standard surgical modality in stage IA2-ⅡA cervical cancer? Gynecol Oncol 2012;127:102-6. [PubMed]
- Landoni F, Maneo A, Cormio G, et al. Class Ⅱ versus class Ⅲ radical hysterectomy in stage IB-ⅡA cervical cancer: a prospective randomized study. Gynecol Oncol 2001;80:3-12. [PubMed]
- Pergialiotis V, Rodolakis A, Christakis D, et al. Laparoscopically assisted vaginal radical hysterectomy: systematic review of the literature. J Minim Invasive Gynecol 2013;20:745-53. [PubMed]
- Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol 1974;44:265-72. [PubMed]
- Querleu D, Morrow CP. Classification of radical hysterectomy. Lancet Oncol 2008;9:297-303. [PubMed]
- Matsuo K, Mabuchi S, Okazawa M, et al. Utility of risk-weighted surgical-pathological factors in early-stage cervical cancer. Br J Cancer 2013;108:1348-57. [PubMed]
- Mahawerawat S, Charoenkwan K, Srisomboon J, et al. Surgical outcomes of patients with stage IA2 cervical cancer treated with radical hysterectomy. Asian Pac J Cancer Prev 2013;14:5375-8. [PubMed]
- Kokka F, Bryant A, Brockbank E, et al. Surgical treatment of stage IA2 cervical cancer. Cochrane Database Syst Rev 2014;5:CD010870. [PubMed]
- Salicrú S, Gil-Moreno A, Montero A, et al. Laparoscopic radical hysterectomy with pelvic lymphadenectomy in early invasive cervical cancer. J Minim Invasive Gynecol 2011;18:555-68. [PubMed]
- Trimbos JB, Franchi M, Zanaboni F, et al. 'State of the art' of radical hysterectomy; current practice in European oncology centres. Eur J Cancer 2004;40:375-8. [PubMed]
- Ditto A, Martinelli F, Ramondino S, et al. Class Ⅱ versus Class Ⅲ radical hysterectomy in early cervical cancer: an observational study in a tertiary center. Eur J Surg Oncol 2014;40:883-90. [PubMed]
- Xie XZ, Song K, Cui B, et al. Clinical and pathological factors related to the prognosis of chinese patients with stage Ib to Ⅱb cervical cancer. Asian Pac J Cancer Prev 2012;13:5505-10. [PubMed]
- Minig L, Patrono MG, Romero N, et al. Different strategies of treatment for uterine cervical carcinoma stage IB2-ⅡB. World J Clin Oncol 2014;5:86-92. [PubMed]
- Kato T, Watari H, Takeda M, et al. Multivariate prognostic analysis of adenocarcinoma of the uterine cervix treated with radical hysterectomy and systematic lymphadenectomy. J Gynecol Oncol 2013;24:222-8. [PubMed]