Glasgow prognostic score after concurrent chemoradiotherapy is a prognostic factor in advanced head and neck cancer
Introduction
Concurrent chemoradiotherapy (CCRT) has become one of the standard therapies for patients with locally advanced head and neck cancer and has been demonstrated to result in significant improvements in the survival and disease control rates compared to radiation alone (1). Importantly, CCRT-induced toxicities, including emesis, anorexia, mucositis, dysphagia and xerostomia, have a negative impact on the nutrition status and functional abilities of the patients, thereby compromising the treatment tolerance and efficacy, and increasing healthcare costs (2). Malnutrition before CCRT is associated with increased risks of complications, toxic deaths, and poor survival (3,4). Hence, early and intensive nutrition support during CCRT is important and may be beneficial for minimizing body weight loss, potentially resulting in better treatment tolerance and survival benefits in advanced head and neck cancer undergoing CCRT, as demonstrated in a previous study (5).
There is an increasing recognition that patient-related factors, especially nutritional and functional declines, are correlated with poorer outcome in addition to the tumor stage. The inflammation-based Glasgow prognostic score (GPS) has been used to reflect the degree of tumor-associated inflammation and malnutrition (6), with patients with an elevated GPS considered to have pre-cachexia (7). Thus, it is reasonable to use the GPS as a biomarker for malnutrition surveillance. Further, the pretreatment GPS has been shown to be a prognostic factor in head and neck cancer (8), with the overall survival (OS) and recurrence-free survival (RFS) being higher when the GPS was low.
CCRT is known to have a negative impact on nutritional status (2,9). However, to our knowledge, changes in the GPS after CCRT relative to the pretreatment GPS have never been investigated, and the prognostic impact of post-CCRT GPS remains unknown. Therefore, the aims of this study were to evaluate the changes in the GPS during CCRT and the impact and potential prognostic roles of the pre- and post-treatment GPSs and the change thereof in patients with advanced head and neck cancer undergoing CCRT.
Materials and methods
Patient selection
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital. Patients with biopsy-proven stage III, IVA, or IVB head and neck squamous cell carcinoma [TNM stage reclassified according to the American Joint Committee on Cancer (AJCC) staging system published in 2002] of the oral cavity, oropharynx, and hypopharynx, and who were treated with primary CCRT were eligible for this study. Patients with recurrent cancer, distant metastasis, another concomitant active cancer, or toxic death during the treatment course were excluded from the study. We retrospectively reviewed the records of 161 patients with stage III, IVA, and IVB head and neck squamous cell carcinoma who underwent CCRT at the Department of Internal Medicine, Chang Gung Memorial Hospital between January 2008 and December 2011. All patients were followed up until March 2015. Twenty-two patients were excluded from the study. Ten patients dropped out during the CCRT regimen and were lost to follow-up because of unavoidable family reasons, such as poor financial support (6 patients) and moving out of town (4 patients). Six patients died during the treatment course. Four patients were treated with re-chemoradiation for recurrent disease and two were diagnosed with a concomitant active cancer upon diagnosis. Thus, 139 patients receiving CCRT were included in the final analysis. All patients received intensity-modulated or arc technique radiotherapy on 5 consecutive days per week at a conventional fractionated daily dose of 1.8 or 2 Gy. The total prescribed dose of radiotherapy was 70–74 Gy. The initial treatment volume included the tumor bed and regional lymphatics. After receiving 46–50 Gy, the treatment area was reduced to irradiate the tumor bed and regional nodes. The chemotherapy regimens, which included cisplatin 40 mg/m2 every week or 100 mg/m2 every 3 weeks, were administered according to the treatment guidelines at the Department of Internal Medicine, Chang Gung Memorial Hospital. All patients are required to place the feeding tube placement including nasogastric tube or percutaneous gastrostomy if their weight loss is more than 5% during treatment period.
Data collection
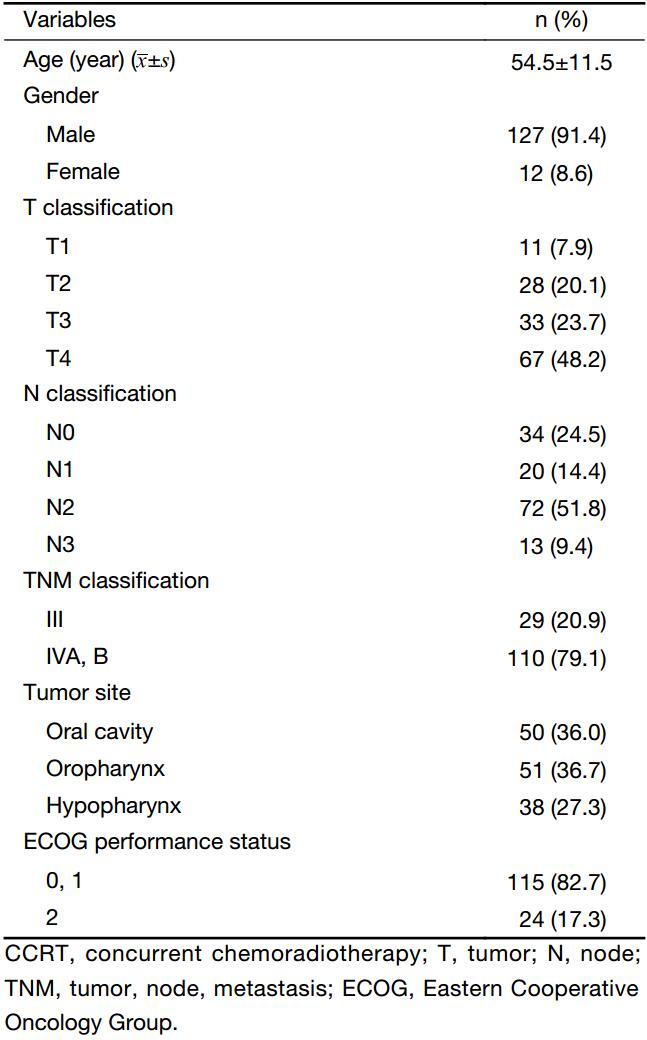
The following clinical and demographic parameters were retrospectively analyzed in this study: age, gender, primary site of disease, TNM stage at diagnosis (AJCC), Eastern Cooperative Oncology Group performance status, and serum albumin and C-reactive protein (CRP) levels before and at the end of the treatment. The treatment outcomes were assessed by the RFS and OS rates. RFS was defined as the time from the initiation of CCRT to the first evidence of recurrence of the primary tumor. OS was defined as the time from the initiation of CCRT to death from any cause.
GPS
The GPS was calculated as follows in our study: patients with elevated serum CRP (>10 mg/L) and hypo-albuminemia (<3.5 mg/L) were allocated a score of 2, patients with only one of these biochemical abnormalities were allocated a score of 1, and patients with neither of these abnormalities were allocated a score of 0.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA). The Kaplan-Meier method was used to analyze survival, and the log-rank test was used to examine the differences in survival between the study groups. Multivariate analysis was performed using Cox’s proportional hazards model. Differences were considered statistically significant when P<0.05.
Results
Study population
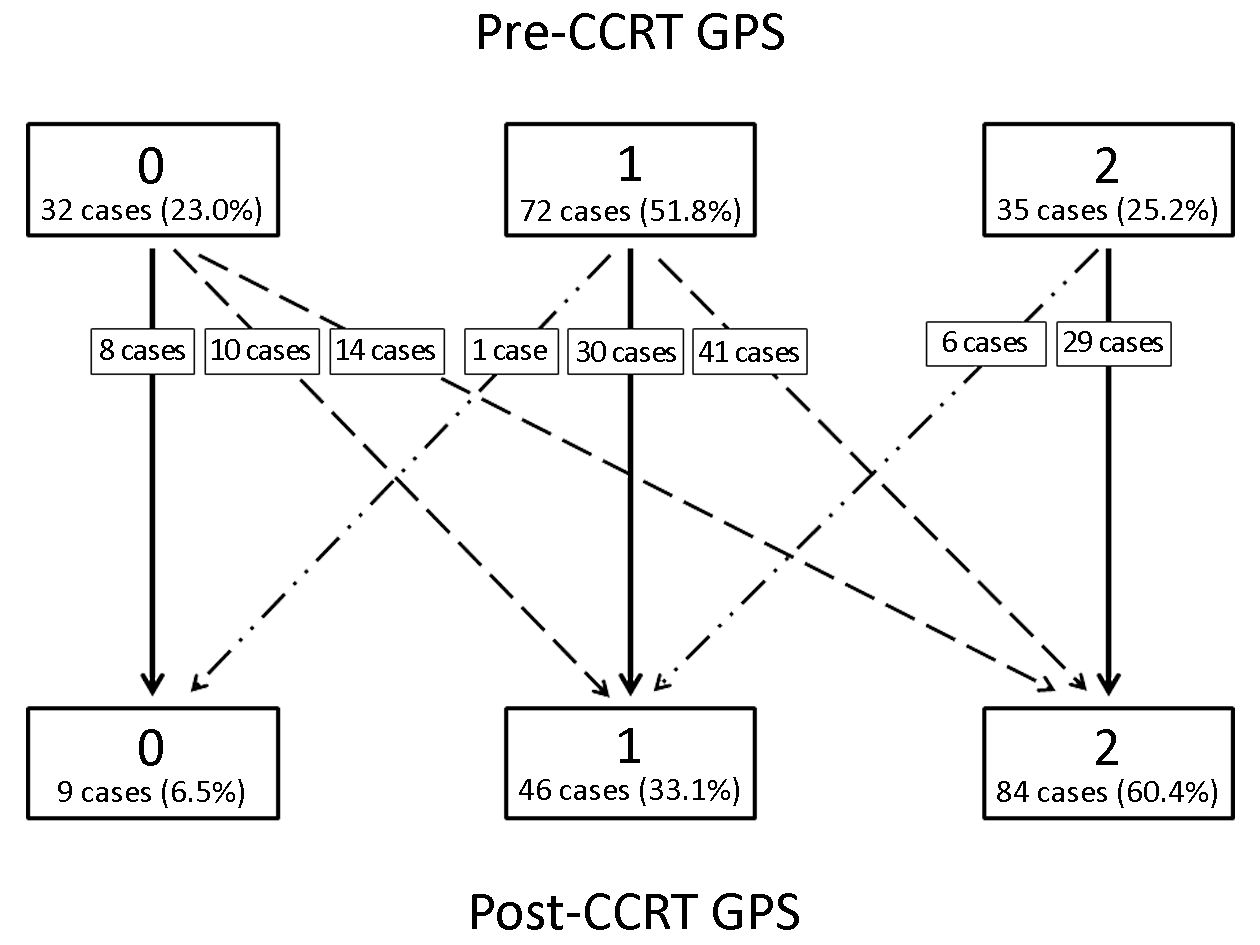
The baseline characteristics of the study patients are shown in Table 1. Among the 139 patients who underwent CCRT for advanced head and neck cancer during the study period, the age at diagnosis ranged from 26 to 79 years (median, 53 years), and the dominant gender was male (91.4%). Twenty-nine (20.9%) and 110 (79.1%) patients were diagnosed with stage III and IVA–B disease, respectively. Figure 1 shows the changes in the GPS after CCRT as compared to the pretreatment values. The number of patients with pretreatment GPSs of 0, 1 and 2 were 32 (23.0%), 72 (51.8%) and 35 (25.2%), respectively. Of all patients, 72 (51.8%) showed changes in the GPS after CCRT; the GPS worsened in 65 (46.8%) patients and was improved in only 7 (5.0%) patients. After CCRT, the numbers of patients with GPSs of 0, 1, and 2 were 9 (6.5%), 46 (33.1%), and 84 (60.4%), respectively (Figure 1).
Full table
Survival analysis according to GPS
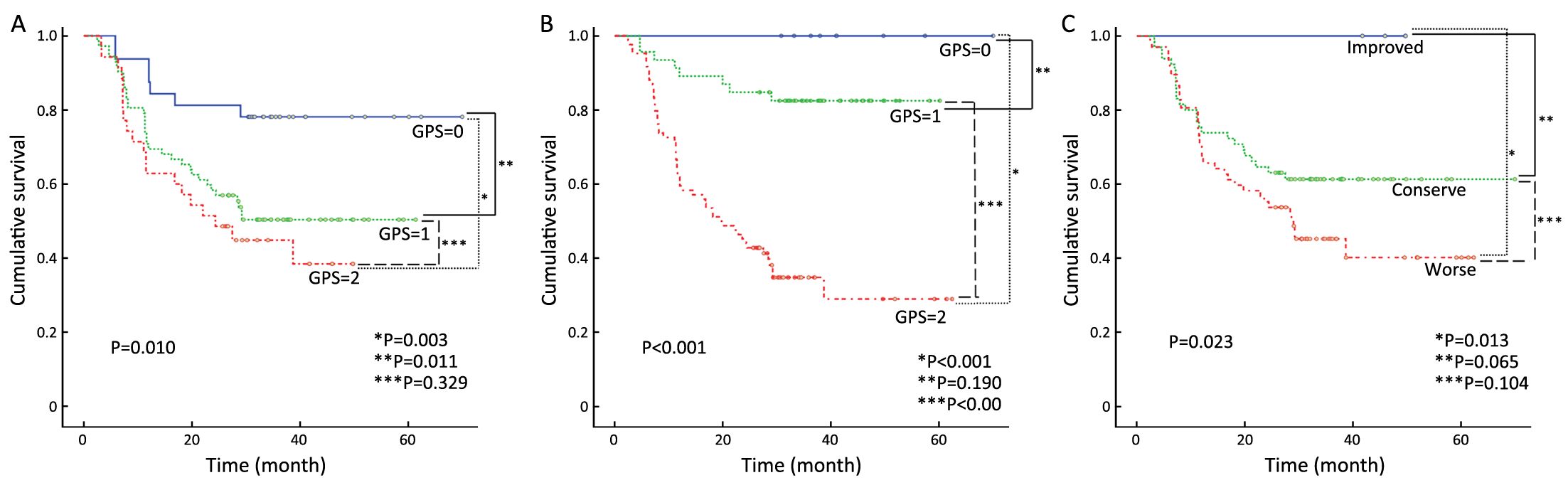
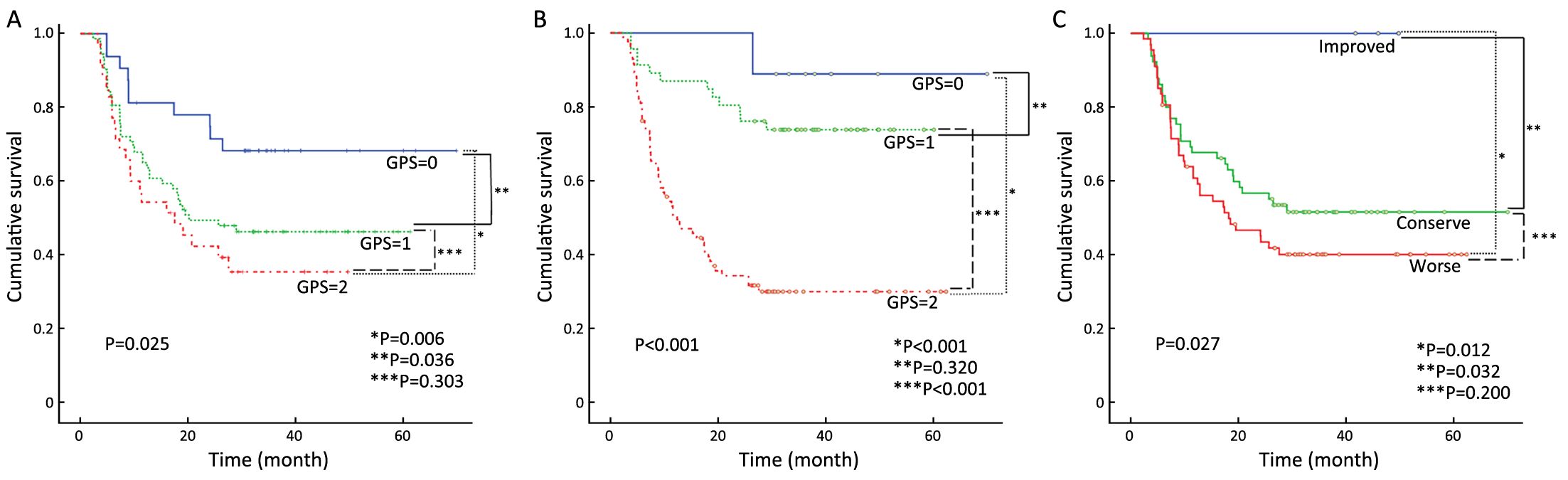
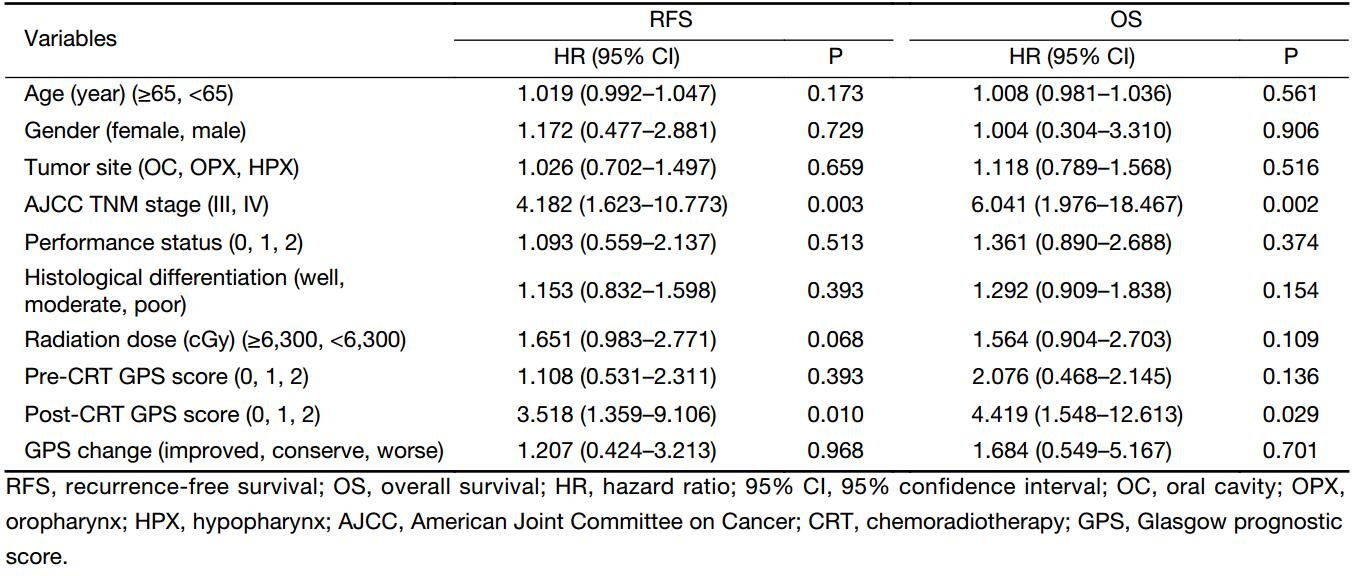
After a median follow-up duration of 38 (interquartile range, 5.9–62.3) months, there were 70 (50.4%) and 62 (44.6%) cases of disease recurrence and death, respectively. The OS according to the pre- and post-CCRT GPSs are shown in Figure 2A, B. There were significant difference in the OS according to both the pre-CCRT GPS (P=0.010) and post-CCRT GPS (P<0.001). We further classified the patients into three groups according to the changes in the GPS after CCRT relative to at pretreatment (improved, conserved, and worse GPS). There was no death in the improved GPS group. The highest OS was observed in the improved GPS group, while the lowest OS was observed in the worse GPS group. The group with improved GPS had a higher OS rate compared to the worse GPS group (P=0.013) (Figure 2C). Similar results were observed for the relationship between RFS and GPS. Patients with a GPS of 0 had significantly better RFS when classified according to the pre-CCRT (P=0.025) and post-CCRT GPS scores (P<0.001) (Figure 3A, B). When classified according to the changes in the GPS after CCRT relative to the pretreatment GPS, the highest and lowest RFSs were observed in the groups with improved and worse GPSs, respectively. The group with improved GPS showed a higher RFS rate compared to the group with worse GPS (P=0.012) and conserve GPS (P=0.032) (Figure 3C). When the pretreatment GPS, posttreatment GPS, change in GPS, tumor stage, tumor site, patient age, patient gender and patient performance status were analyzed as prognostic scores for RFS and OS by multivariate analysis, the post-CCRT GPS was shown to be an independent prognostic parameter, in addition to tumor stage (Table 2).
Full table
Discussion
Tumor stage is widely accepted as the cornerstone of cancer prognosis. However, there is increasing evidence that patient-related factors, such as nutritional and functional declines, are associated with a poorer prognosis, independent of the tumor stage. Several reports have demonstrated that the presence of a systemic inflammatory response is associated with increased weight loss, elevated resting energy expenditure, loss of lean tissue, and functional decline (10-14). Cancer cachexia is associated with complex metabolic, molecular, and cellular alterations, and an increased plasma level of CRP, combined with reduced food intake and weight loss, has been used as a clinical marker of cancer cachexia (15). In addition, inflammation appears to also play a significant role in cancer anorexia. In animal models of cancer anorexia, increased brain levels of various cytokines, including interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) have been demonstrated (16,17), and blocking of circulating TNF-α or intra-hypothalamic IL-1 receptors in anorexic tumor-bearing mice has been shown to improve food intake (18,19). Further, serum albumin is known to correlate with systemic inflammation through increased levels of pro-inflammatory cytokines. Increased catabolism, chronic malnutrition, and chronic inflammatory reactions due to cancer eventually lead to hypoalbuminemia, which has been shown to be associated with poor survival in various cancers (20). The GPS is calculated from the serum CRP and albumin concentrations. It is simple to measure, routinely available, and well-standardized worldwide; as a result, it has been the subject of prognostic studies in a wide variety of cancers (10). MacDonald et al. (7) reported that the prognosis of patients with an elevated GPS was extremely poor and that these patients should thus be given pre-cachexia status and offered multimodal therapy, which may delay the onset of cachexia and death. McMillan et al. (10) concluded that the GPS may be included in addition or in preference to the current definitions of cachexia, along with tumor staging as part of the routine assessment of all cancer patients. As a consequence, using GPS as an objective prognostic nutritional marker is considered reasonable.
The majority of patients (51.8%) in our study showed changes in the GPS after CCRT. Of patients with changes in the GPS, 65 of 72 patients (90.3%) had a worsened GPS post-CCRT. This result implies that most patients’ nutritional status declined after CCRT, since GPS reflects the degree of malnutrition (6). We further analyzed the relationship between the GPS change and survival. The results showed that patients with improved GPS tended to have better OS (P=0.013) and RFS (P=0.012) compared with those with worse GPS (Figure 2C, 3C). These findings suggest that we need to pay attention to the GPS, and that maintaining or improving the patients’ nutritional status is critical during CCRT. Furthermore, our findings also demonstrated that a higher pretreatment GPS was associated with poor disease-free survival (DFS) and OS, which is in accordance with a previous study reported by Nakayama et al. (8). Since the GPS can reflect the degree of cachexia and malnutrition (6), and considering that the post-CCRT GPS declined in most patients in our study, we further analyzed the prognostic role of post-CCRT GPS as a biomarker for the follow-up of the patients’ nutritional status and found that the post-CCRT GPS level was an independent prognostic factor in the multivariate analysis, rather than the pre-CCRT GPS (Table 2), implying that poor nutrition after CCRT can predict survival.
To our knowledge, the changes in the GPS after treatment and the association between the post-CCRT GPS and survival outcome have never been investigated previously, and these factors deserve further discussion. In our study, the post-CCRT GPS was a better prognostic factor than the pre-CCRT GPS. Using the GPS as a nutritional prognostic marker, our study showed that malnutrition after CCRT was associated with poor survival outcome. Accordingly, the malnutrition status following therapy completion deserves more attention, and evaluating the treatment influence on patient outcomes following effect-induced malnutrition is important.
The post-treatment effects of CCRT on the nutrition status are often long-lasting (21). Therefore, nutrition surveillance should continue during the recovery and follow-up period to ensure adequate nutrition intake. However, the post-CCRT nutrition status is often overlooked, and thus, the efficacy of curative therapy may be compromised, consequently leading to undue adverse effects and potentially shortened duration of life. In a previous study, low body mass index at 3 months following CCRT completion was found to strongly associate with poorer survival outcomes (22). Taken together with our study results, this indicates that special attention should be paid continuously, from pretreatment to post-treatment, to the nutrition status of patients undergoing CCRT, and that an intensive nutritional intervention schedule should be implemented and maintained. Yeh et al. (23) conducted a randomized study and reported that the addition of several micronutrients and probiotics to an omega-3 fatty acid containing oral nutritional supplement improved the maintenance of body weight, serum albumin, and prealbumin levels in head and neck cancer patients with cachexia who were undergoing CCRT. Nutrient support, via eicosapentaenoic acid (EPA) food components, therefore attracted attention as a potential anti-tumor immunonutrition therapy and is a potential treatment strategy to improve GPS. As shown in this study, improving GPS may therefore help improve patients’ survival in advanced head and neck cancer patients who are undergoing CCRT. Hence, further large-scale prospective studies are required to fully determine how to reduce the worsening of the GPS during CCRT in order to improve patients’ outcomes.
Some limitations of this study should be acknowledged. First, this study was a retrospective study, conducted in a single institute without blindness or control groups. Second, the number of patients studied was small. Only 7 patients were in the improved GPS group for analysis. In the future, larger prospective studies are needed to establish the significance of the GPS in advanced head and neck cancers.
Conclusions
Nutritional intervention and maintenance before, during, and especially after CCRT are important and affect the survival of patients with advanced head and neck cancer undergoing CCRT. GPS is an objective prognostic nutritional marker for survival and provides additional value in these patients and should hence be included in clinical practice.
Acknowledgements
The authors thank all members of the Cancer Center, Chang Gung Memorial Hospital, Keelung, for their invaluable help.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ramos M, Benavente S, Giralt J. Management of squamous cell carcinoma of the head and neck: updated European treatment recommendations. Expert Rev Anticancer Ther 2010;10:339–44. [PubMed] DOI:10.1586/era.10.6
- Ravasco P, Monteiro-Grillo I, Marques Vidal P, et al. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005;27:659–68. [PubMed] DOI:10.1002/hed.20221
- Chang PH, Yeh KY, Huang JS, et al. Pretreatment performance status and nutrition are associated with early mortality of locally advanced head and neck cancer patients undergoing concurrent chemoradiation. Eur Arch Otorhinolaryngol 2013;270:1909–15. [PubMed] DOI:10.1007/s00405-012-2290-2
- van Bokhorst-de van der Schuer, van Leeuwen PA, Kuik DJ, et al. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer 1999;86:519–27. [PubMed] DOI:10.1002/(SICI)1097-0142(19990801)86:3<519::AID-CNCR22>3.0.CO;2-S
- Wang CH, Wang HM, Pang YP, et al. Early nutritional support in non-metastatic stage IV oral cavity cancer patients undergoing adjuvant concurrent chemoradiotherapy: analysis of treatment tolerance and outcome in an area endemic for betel quid chewing. Support Care Cancer 2012;20:1169–74. [PubMed] DOI:10.1007/s00520-011-1192-y
- Forrest LM, McMillan DC, McArdle CS, et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 2003;89:1028–30. [PubMed] DOI:10.1038/sj.bjc.6601242
- MacDonald N. Terminology in cancer cachexia: importance and status. Curr Opin Clin Nutr Metab Care 2012;15:220–5. [PubMed] DOI:10.1097/MCO.0b013e328352a895
- Nakayama M, Tabuchi K, Hara A. Clinical utility of the modified Glasgow prognostic score in patients with advanced head and neck cancer. Head Neck 2015;37:1745–9. [PubMed] DOI:10.1002/hed.23823
- Nayel H, el-Ghoneimy E, el-Haddad S. Impact of nutritional supplementation on treatment delay and morbidity in patients with head and neck tumors treated with irradiation. Nutrition 1992;8:13–8. [PubMed]
- McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–40. [PubMed] DOI:10.1016/j.ctrv.2012.08.003
- Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer 2008;44:1124–32. [PubMed] DOI:10.1016/j.ejca.2008.02.033
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223–6. [PubMed] DOI:10.1097/MCO.0b013e32832a7902
- MacDonald N. Chronic inflammatory states: their relationship to cancer prognosis and symptoms. J R Coll Physicians Edinb 2011;41:246–53. [PubMed] DOI:10.4997/JRCPE.2011.315
- Muliawati Y, Haroen H, Rotty LW. Cancer anorexia — cachexia syndrome. Acta Med Indones 2012;44:154–62. [PubMed]
- Fearon KC, Voss AC, Hustead DS, et al. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 2006;83:1345–50. [PubMed]
- Guijarro A, Laviano A, Meguid MM. Hypothalamic integration of immune function and metabolism. Prog Brain Res 2006;153:367–405. [PubMed] DOI:10.1016/S0079-6123(06)53022-5
- Plata-Salamán CR, Ilyin SE, Gayle D. Brain cytokine mRNAs in anorectic rats bearing prostate adenocarcinoma tumor cells. Am J Physiol 1998;275:R566–73. [PubMed]
- Torelli GF, Meguid MM, Moldawer LL, et al. Use of recombinant human soluble TNF receptor in anorectic tumor-bearing rats. Am J Physiol 1999;277:R850–5. [PubMed]
- Laviano A, Gleason JR, Meguid MM, et al. Effects of intra-VMN mianserin and IL-1ra on meal number in anorectic tumor-bearing rats. J Investig Med 2000;48:40–8. [PubMed]
- Dixon MR, Haukoos JS, Udani SM, et al. Carcinoembryonic antigen and albumin predict survival in patients with advanced colon and rectal cancer. Arch Surg 2003;138:962–6. [PubMed] DOI:10.1001/archsurg.138.9.962
- Murphy BA, Gilbert J, Cmelak A, et al. Symptom control issues and supportive care of patients with head and neck cancers. Clin Adv Hematol Oncol 2007;5:807–22. [PubMed]
- Chang PH, Wang CH, Huang JS, et al. Low body mass index at 3 months following adjuvant chemoradiation affects survival of postoperative locally advanced oral cavity cancer patients. Laryngoscope 2012;122:2193–8. [PubMed] DOI:10.1002/lary.23450
- Yeh KY, Wang HM, Chang JW, et al. Omega-3 fatty acid-, micronutrient-, and probiotic-enriched nutrition helps body weight stabilization in head and neck cancer cachexia. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:41–8. [PubMed] DOI:10.1016/j.oooo.2013.01.015