Neck observation versus elective neck dissection in management of clinical T1/2N0 oral squamous cell carcinoma: a retrospective study of 232 patients
Introduction
Oral squamous cell carcinoma (OSCC) is the most common type of oral cancer with a high potential to metastasize even in the early stage. Though the incidence and mortality of oral cancer are not high in China, it has remained a low 5-year overall survival (OS) rate at approximately 50% for the past decades (1). According to the newest National Comprehensive Cancer Network (NCCN) Guidelines, head and neck cancer, version 1.2017, the management of early-stage OSCC (cT1/2N0) can be mainly divided into two policies: surgery and radiation therapy. However, when advocating surgery first, there is still no consensus on whether elective neck dissection (END) should be performed simultaneously with primary site resection or neck observation (OBS) (2,3). The core problem leading to the argument is the existence of occult metastases, which may lead to locoregional failure and poor survival. Specifically, occult metastases are defined as lymph nodes metastases that are not detected initially by neck palpation and imaging examination, but they are detected by histological examination after neck dissection, or they are presented as delayed regional recurrence if they were untreated after OBS (4-6). However, few articles have compared the survival rates of patients with occult metastases between the END and OBS groups up to now.
Some surgeons advocate END because of the high incidence of occult metastases in patients with early-stage OSCC, ranging from 8.2% to 46.3% (7,8). However, others support OBS policy because it avoids overtreatment to those without occult metastases, and once regional recurrence is detected during follow-up, therapeutic neck dissection can also be performed in time (9-12). Nowadays, both policies have their proponents in different centers around the world.
In this retrospective study, we report our experience in the treatment of 232 patients with OSCC by comparing survival outcomes between END and OBS, as well as those of patients with occult metastases between the two treatments.
Materials and methods
Patients
This study was approved by the Ethics Committee of Institution Research of Hospital of Stomatology, Sun Yat-sen University. A total of 232 consecutive patients with clinical T1/2N0 OSCC were underwent initial surgery in the Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Sun Yat-sen University, from January 2001 to June 2011. The TNM classification was established according to the Union for International Cancer Control (UICC) 2010 guidelines. The inclusion criteria were as follows: 1) the primary lesions were on tongue, buccal mucosa, floor of the mouth, or mandibular gingiva; 2) clinically N0 neck: physical examination (neck palpation) and imaging examination, such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography-computed tomography (PET-CT), found no enlarged lymph node or lymph node less than 1.0 cm that was soft and movable; 3) the tumor was pathologically confirmed OSCC; and 4) the pathological results of the lymph nodes were recorded if patients received neck dissection. The exclusion criteria were as follows: 1) patients had previous head and neck treatment history (surgery, radiotherapy, or chemotherapy); or 2) patients had several kinds of systemic diseases before treatment [such as: grade III–IV cardiac function according to the New York Heart Association (NYHA) Functional Classification; stage 2–3 hypertension: higher than 160 mmHg systolic or 100 mmHg diastolic (1 mmHg=0.133 kPa); diabetes mellitus].
Treatment
Among the 232 patients, 181 patients were treated with END and 51 patients were treated with OBS. Specifically, in the END group, local excision of primary tumor were performed simultaneously with END; reconstruction of tissue defect was also performed, if necessary. Neck specimens were examined by pathologists to identify the potential occult metastases. Postoperative radiotherapy (RT), which was scheduled within 4–6 weeks after the operation, was carried out if patients presented with pathologically positive lymph nodes. In the OBS group, patients were treated only with local excision of primary tumor and then, were observed under close follow-up.
Follow-up
For the END group, follow-up was established every 3 months for at least 2 years, and then, every 3 or 6 months later. For the OBS group, the follow-up interval was suggested to be 1–3 months for the first 3 years, and then, 3 or 6 months later. CT or MRI was performed as a routine inspection every 6 months for the OBS group and 6 months to 1 year for the END group. In the OBS group, patients with occult metastases were defined as delayed regional recurrence (apparent in the neck, detected by imaging examination or identified by pathologists after surgery) in the follow-up. If suspected regional recurrence was detected by physical or imaging examination, therapeutic neck dissection or salvage surgery on the affected side or both sides was established. In addition, RT, chemotherapy, or chemoradiotherapy were also taken into consideration.
Statistical analysis
The OS was calculated from the date of operation to the date of the last follow-up or death. Disease-specific survival (DSS) was calculated from the date of operation to the date of last follow-up or death related to OSCC. Recurrence-free survival (RFS) was defined from the date of operation to the date of finding local, regional, or distant recurrence; LRFS for local RFS, and RRFS for regional RFS.
Statistical analysis was performed using IBM SPSS Statistics (Version 20.0; IBM Corp., New York, USA). The baseline demographic data between the END and OBS groups were compared using the Pearson Chi-square test for categorical variable. The best cut-off value was calculated using the receiver operating characteristic curve (ROC) analysis for continuous variable. The Cox multivariate regression analysis was used to adjust for confounding factors. The survival curves of OS, DSS, RFS, LRFS, and RRFS were plotted using the Kaplan-Meier method for each group, and compared using the Log-rank test. The level of significance was set at P<0.05 for two tails.
Results
General characteristics
Of the 232 patients, 181 (mean age: 57.5±12.6 years) were in the END group and 51 patients (mean age: 58.6±12.5 years) were in the OBS group. According to the ROC analysis, the best cut-off value was 55.5 years. The baseline demographic and clinical pathological characteristics showed that patients were well-matched in age, gender, alcohol/tobacco habit, site of tumor, clinical T classification, growth pattern, and histological grade between END and OBS groups (Table 1).
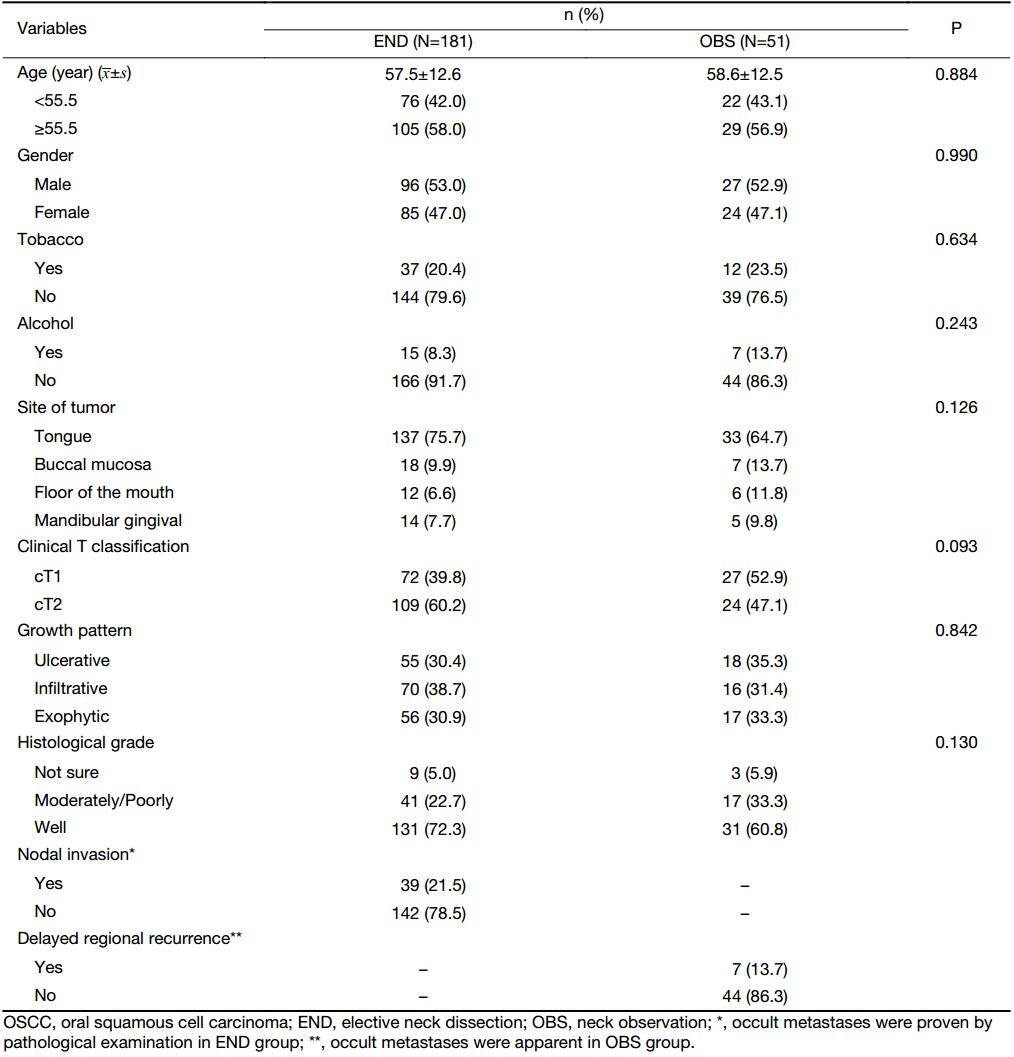
Full table
Clinical outcomes of END and OBS groups
This cohort of patients was followed up to January 31, 2016, with a median follow-up period of 68 (range: 5–175) months in the END group and 68 (range: 12–175) months in the OBS group. The study flow chart (Figure 1) illustrates the study population with two types of neck management from initial treatment to follow-up. During the follow-up period, tumor recurrence was significantly higher in the OBS group compared to the END group (P=0.013); 25.5% (13/51) and 11.6% (21/181) of patients in the OBS and END groups, respectively. As for regional recurrence, the incidence of 13.7% of patients (7/51) observed in the OBS group was significantly higher than that of 5.0% of patients (9/181) in the END group (P=0.029).
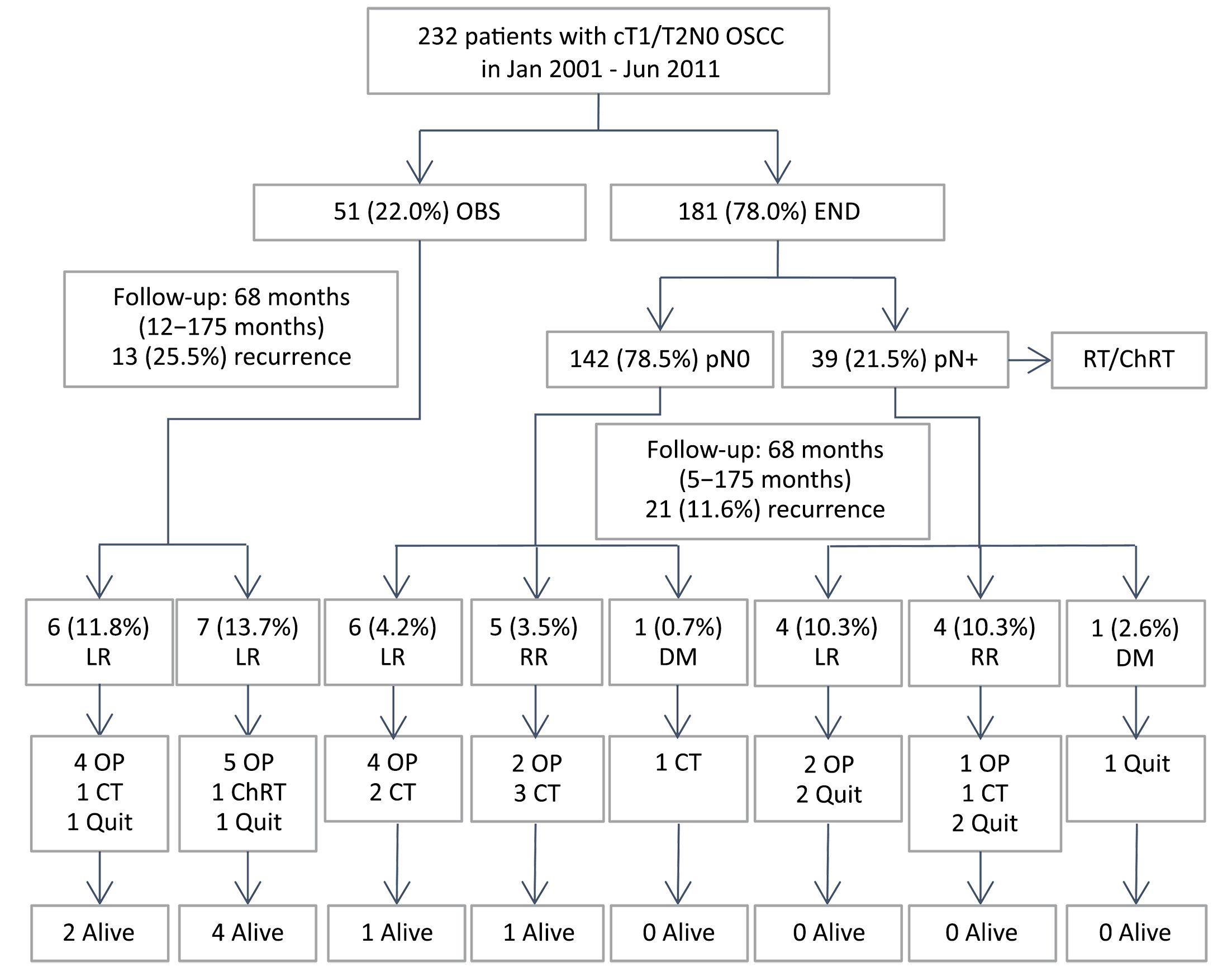
As shown in Figure 1, 142 (78.5%) patients in the END group were confirmed as having pathological nodal-negative diseases (pN0), while 39 (21.5%) patients in the END group were diagnosed with pathological nodal-positive diseases (pN+). Among the 142 pN0 patients in the END group, 6 (4.2%) patients, 5 (3.5%) patients, and 1 (0.7%) patient developed local recurrence, regional recurrence, and distant metastasis, respectively. Six patients (4 patients with local recurrence and 2 patients with regional recurrence) accepted salvage surgery, 2 (2/6, 33.3%) of them survived in the follow-up. Among 39 pN+ patients in END group, 4 (10.3%) patients, 4 (10.3%) patients and 1 (2.6%) patient developed local recurrence, regional recurrence, and distant metastasis, respectively. Three patients (2 patients with local recurrence and 1 patient with regional recurrence) accepted salvage surgery, but none of them survived in the follow-up. For OBS group, 6 (11.8%) patients developed local recurrence, while 7 (13.7%) patients reached regional recurrence. Nine patients (4 patients with local recurrence, 5 patients with regional recurrence) accepted salvage surgery, 6 (6/9, 66.7%) of them survived in the follow-up.
Unfortunately, 12 (12/21, 57.1%) recurrent patients (4 patients with local recurrence, 6 patients with regional recurrence, and 2 patients with distant metastases) in the END group were in poor physical condition or in low intentions to accept surgery. Unlike the recurrent cases in the END group, only 4 (4/13, 30.8%) patients (2 patients with local recurrence and 2 patients with regional recurrence) in the OBS group gave up surgery (Figure 1).
Comparison of survival rates between END and OBS groups
For adjusting for confounding factors, the balanced variables from Table 1 showed no significant survival differences according to the Cox multivariate regression analysis, except surgery treatment for 5-year RFS rate (P=0.012, HR=0.329, 95% CI: 0.153–0.707). It was specifically shown that END could decrease the risk of recurrence to 0.329 compared with OBS (Table 2).
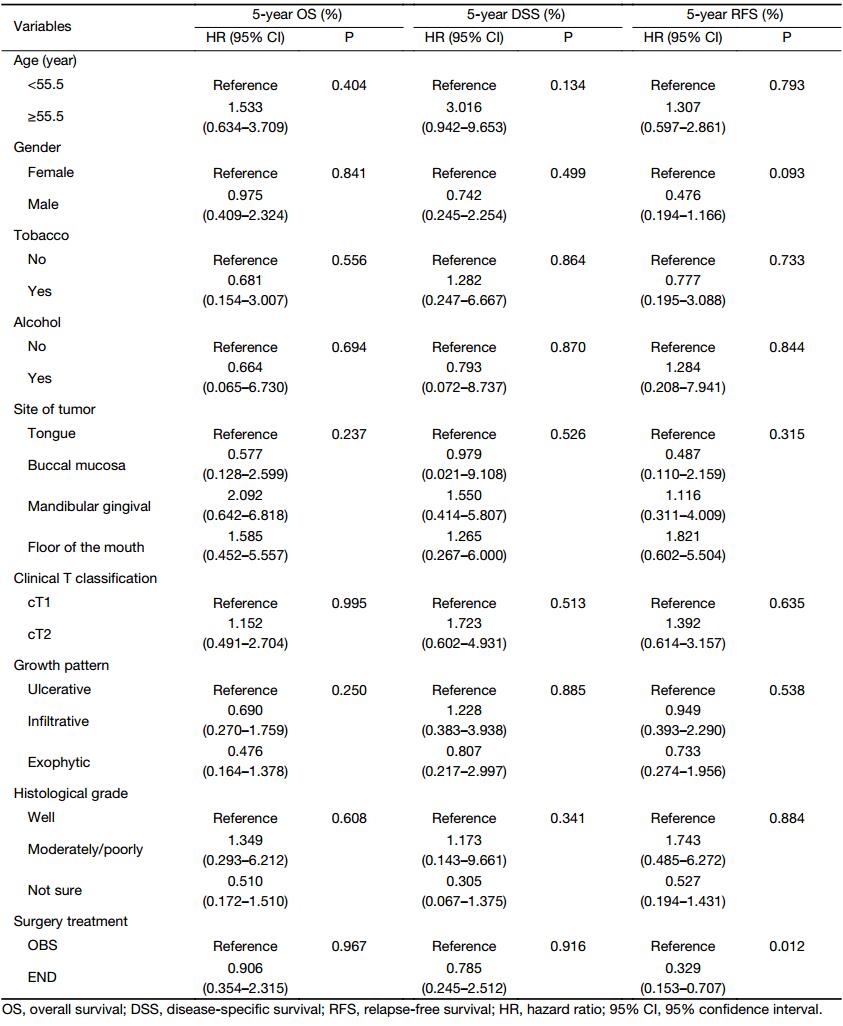
Full table
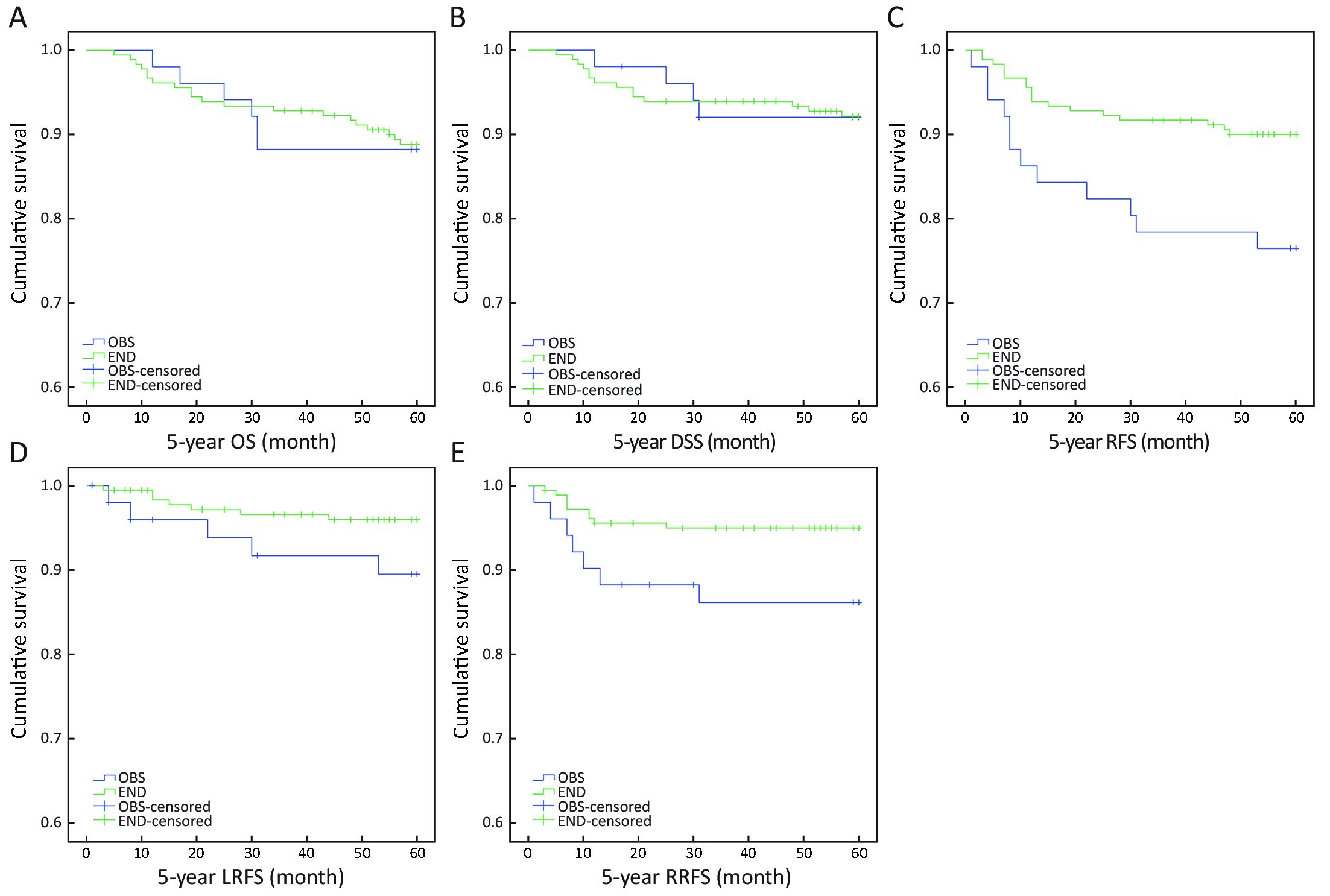
The 5-year OS, DSS, RFS, LRFS and RRFS rates between END and OBS groups were 89.0% vs. 88.2%, 92.3% vs. 92.2%, 90.1% vs. 76.5%, 96.1% vs. 90.2%, and 95.0% vs. 86.3%, respectively (Figure 2). Significantly lower 5-year RFS and RRFS rates could be seen in the END group than the OBS group (Log-rank, P=0.009 and P=0.028, respectively), while no statistical difference was found in the 5-year OS (P=0.906), DSS (P=0.998), and LRFS (P=0.081) rates.
The survival rates of cT1 and cT2 patients between the END and OBS groups are shown in Table 3. For cT1 patients, the 5-year OS, DSS, RFS, LRFS, and RRFS rates had no significant differences between the two groups. Similar findings were also investigated for cT2 patients.
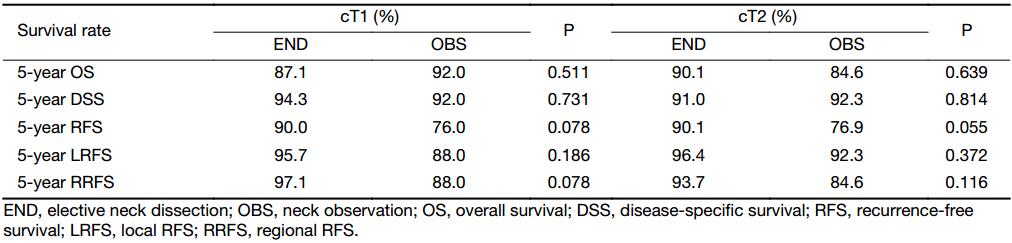
Full table
For patients with occult metastases, comparisons of the 5-year OS and DSS rates between the two groups are shown in Table 4. A total of 46 patients (46/232, 19.8%) had occult metastases, including 7 patients (delayed regional recurrence) in the OBS group (7/51, 13.7%) and 39 patients (pN+) in the END group (39/181, 21.5%). Among these patients, no significant difference was observed in the incidence of occult metastases between the two groups (P=0.216). Moreover, the 5-year OS rate (57.1% vs. 64.1%, P=0.839) and DSS rate (71.4% vs. 74.4%, P=0.982) also showed no significant difference.
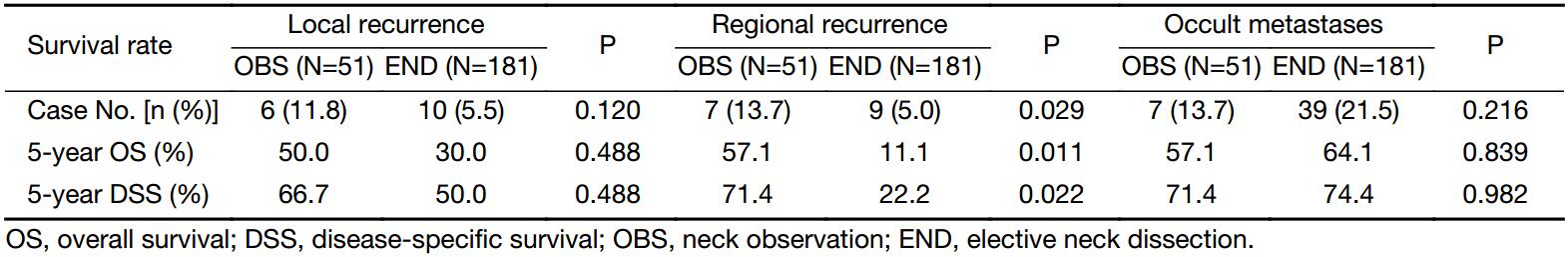
Full table
Table 4 presents the 5-year OS and DSS rates of the recurrent patients including 21 patients in END group and 13 in OBS group. Results demonstrated no significant difference in the local recurrence rate (11.8% vs. 5.5%, P=0.120), 5-year OS rate (50.0% vs. 30.0%, P=0.488), and 5-year DSS rate (66.7% vs. 50.0%, P=0.488) between the two groups. However, for the regional recurrence patients, the 5-year OS (57.1% vs. 11.1%, P=0.011) and DSS (71.4% vs. 22.2%, P=0.022) rates in the OBS group (7/51, 13.7%) were higher than those in the END group (9/181, 5.0%).
Discussion
According to the newest NCCN guidelines, OBS and END are the two main surgery treatment policies for patients with early-stage OSCC. However, which of them is more effective still remains a controversial issue. This study analyzed the survival outcomes of patients with clinical T1/2N0 OSCC treated with OBS or END. We showed that OBS-treated patients obtained similar 5-year OS, DSS, and LRFS rates, but lower 5-year RFS and RRFS rates when compared with the END-treated patients. It was worthy to note that patients with regional recurrence in the OBS group had higher 5-year OS and DSS rates than those in the END group. Moreover, patients with occult metastases in the OBS group achieved similar 5-year OS and DSS rates with those in the END group.
As far as we know, only five prospective studies have compared the survival outcomes between patients treated with END and OBS (13-17). In 1994, Kligerman et al.(15) demonstrated that patients who underwent END had a significantly higher 3-year disease-free survival rate than those who received OBS. Vandenbrouk et al. (14), Fakih et al. (13), and Yuen et al. (16) reported that there was no significant difference in OS between the two treatments, which is consistent with our study results. However, all these four prospective studies lacked adequate samples to detect a meaningful difference (3). Hence, in another prospectively randomized clinical trial with a large sample size from India, D’Cruz et al. (17) enrolled 500 patients with early-stage OSCC in their study and found that the END group had higher 3-year OS and disease-free survival rates than the OBS group, which was different from our findings. This difference might result from their inadequate 5-year follow-up, regional differences, diet structure, or other influencing factors (8). In our study, although END had a good control on regional recurrence, it did not influence the 5-year OS and DSS rates.
Clinical T stage and occult metastases were the significant tumor-related predictive factors for performing END (2). Ganly et al. (18) found that patients with cT2 OSCC treated with OBS had lower 5-year OS and DSS rates than those treated with END, but similar results were not found for cT1 patients; thus, they concluded that neck dissection should be performed in cT2 patients. However, Flach et al. (19) observed 234 patients with early-stage OSCC using ultrasound guided fine needle aspiration cytology followed by OBS. This study found that these patients had similar survival outcomes as those treated with END. Currently, some studies still considered that cT2 patients should receive END because of the high occult metastases and poor survival outcomes (7,8,20). In our study, since patients treated with OBS could have a close follow-up and receive therapeutic neck dissection in time, we found similar survival rates in cT1 and cT2 patients between the OBS and END groups. Thus, in our opinion, both cT1 and cT2 patients could be treated with OBS policy under close follow-up.
The incidence of occult metastases in cN0 OSCC varied in different studies (7-11,18-20). Since 1994, the probability of 20% for occult metastases was recognized as the common threshold value for performing END (21). In 2009, a study demonstrated that OBS policy was recommended when the risk was lower than 44% (22). In our study, the incidence of occult metastases was 19.8%, which indicated that OBS policy may be appropriate for patients with early-stage OSCC. In a further investigation, we explored the 5-year OS and DSS rates of patients with proven nodal metastases, and results showed no significant differences between the OBS group and END group. This was consistent with a study performed by Flach et al. (19), which demonstrated that patients treated with OBS and presented with delayed metastases had similar 5-year OS and DSS rates to those treated with END and presented with proven metastases. Some other researches also indicated that END turned out to be unnecessary and was an overtreatment method, as after comparing the two policies in patients with occult metastases, similar OS and DSS rates were observed (16,23). Moreover, END could cause a number of complications, such as shoulder disability and scarring (9,12). To avoid overtreatment, sentinel lymph node biopsy (SLNB) had been introduced in clinical treatment. Compared to conditional examinations, SLNB could improve the detection of potential nodes and reduce recurrence rate (24,25). However, there was still no accurate technique for the detection of all the occult nodes in neck (26).
Regional recurrence had been the most common cause of failure in early-stage oral cancer. Multiple studies indicated that patients with early-stage OSCC treated with OBS had a higher rate of regional failure than END (7,8,17,20). In a retrospective study, Liu et al. (12) found that the 5-year DSS rate of cN0 patients with proven neck metastases would decrease by 35% when compared with patients with proven negative results for neck metastases. However, there was still lack of data on the survival outcomes of patients with neck recurrence between the END and OBS groups (8). In our study though, the OBS group had a higher rate of regional recurrence than the END group, and the 5-year OS and DSS rates in the OBS group were higher than those in the END group. This indicates that the patients with delayed metastases in the OBS group could achieve even longer survival time since patients in the OBS group were under closer follow-up and it was easier to perform active interventions in these patients.
Some limitations can be noticed in this retrospective study, which may lead to selection bias for the patients included in the END or OBS group. Firstly, there were unbalanced sample sizes between the two study groups. For this reason, the Cox multivariate regression analysis was used to adjust for potential confounding factors. Secondly, tumor thickness or depth of invasion was not recorded or analyzed. In patients with cN0 OSCC, depth of invasion has been considered in future versions of the AJCC TNM staging system according to high incidence of occult metastasis (27,28). Thirdly, considering the economic status or emotional reasons of the patients in developing countries, including China, END is more extensively used than OBS. Considering the possible selection bias mentioned above, observed better 5-year OS and DSS in the OBS group may be based on patients with low-risk potential to metastasis (such as smaller T size, smaller tumor thickness or other clinical variables). However, selection bias cannot be eliminated in the retrospective study, which urges us to carry out multicenter prospective randomized cohort study in the future.
Conclusions
Our study demonstrates that OBS could obtain similar 5-year OS and DSS rates as END. Moreover, in the regional recurrence patients, OBS can result in higher 5-year OS and DSS rates than END. Even though END can provide effective neck recurrence control, END still needs close follow-up and active interventions as OBS. Under close follow-up, OBS policy may be an available treatment option for patients with clinical T1/2N0 OSCC.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (No. 81372884 and No. 81672679), and 5010 Project of Clinical Study, Sun Yat-sen University (No. 2010018).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhang SK, Zheng R, Chen Q, et al. Oral cancer incidence and mortality in China, 2011. Chin J Cancer Res 2015;27:44–51. [PubMed] DOI:10.3978/j.issn.1000-9604.2015.01.03
- Rodrigo JP, Shah JP, Silver CE, et al. Management of the clinically negative neck in early-stage head and neck cancers after transoral resection. Head Neck 2011;33:1210–9. [PubMed] DOI:10.1002/hed.21505
- Monroe MM, Gross ND. Evidence-based practice: management of the clinical node-negative neck in early-stage oral cavity squamous cell carcinoma. Otolaryngol Clin North Am 2012;45:1181–93. [PubMed] DOI:10.1016/j.otc.2012.06.016
- Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med 2011;364:412–21. [PubMed] DOI:10.1056/NEJMoa1008108
- Mermod M, Bongiovanni M, Petrova TV, et al. Prediction of occult lymph node metastasis in squamous cell carcinoma of the oral cavity and the oropharynx using peritumoral Prospero homeobox protein 1 lymphatic nuclear quantification. Head Neck 2016;38:1407–15. [PubMed] DOI:10.1002/hed.24452
- Mourouzis C, Pratt C, Brennan PA. Squamous cell carcinoma of the maxillary gingiva, alveolus, and hard palate: is there a need for elective neck dissection?. Br J Oral Maxillofac Surg 2010;48:345–8. [PubMed] DOI:10.1016/j.bjoms.2009.07.012
- Abu-Ghanem S, Yehuda M, Carmel NN, et al. Elective neck dissection vs observation in early-stage squamous cell carcinoma of the oral tongue with no clinically apparent lymph node metastasis in the neck: A systematic review and Meta-analysis. JAMA Otolaryngol Head Neck Surg 2016;142:857–65. [PubMed] DOI:10.1001/jamaoto.2016.1281
- Patel TD, Vázquez A, Marchiano E, et al. Efficacy of elective neck dissection in T1/T2N0M0 oral tongue squamous cell carcinoma: A population-based analysis. Otolaryngol Head Neck Surg 2016;155:588–97. [PubMed] DOI:10.1177/0194599816643695
- Orabona GD, Bonavolontà P, Maglitto F, et al. Neck dissection versus " watchful-waiting” in early squamous cell carcinoma of the tongue our experience on 127 cases. Surg Oncol 2016;25:401–4. [PubMed] DOI:10.1016/j.suronc.2016.09.005
- Kelner N, Vartanian JG, Pinto CA, et al. Does elective neck dissection in T1/T2 carcinoma of the oral tongue and floor of the mouth influence recurrence and survival rates?. Br J Oral Maxillofac Surg 2014;52:590–7. [PubMed] DOI:10.1016/j.bjoms.2014.03.020
- Peng KA, Chu AC, Lai C, et al. Is there a role for neck dissection in T1 oral tongue squamous cell carcinoma? The UCLA experience. Am J Otolaryngol 2014;35:741–6. [PubMed] DOI:10.1016/j.amjoto.2014.06.019
- Liu KY, Durham JS, Wu J, et al. Nodal disease burden for early-stage oral cancer. JAMA Otolaryngol Head Neck Surg 2016;142:1111–9. [PubMed] DOI:10.1001/jamaoto.2016.2241
- Fakih AR, Rao RS, Borges AM, et al. Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. Am J Surg 1989;158:309–13. [PubMed] DOI:10.1016/0002-9610(89)90122-0
- Vandenbrouck C, Sancho-Garnier H, Chassagne D, et al. Elective versus therapeutic radical neck dissection in epidermoid carcinoma of the oral cavity: results of a randomized clinical trial. Cancer 1980;46:386–90. [PubMed] DOI:10.1002/1097-0142(19800715)46:2<386::AID-CNCR2820460229>3.0.CO;2-9
- Kligerman J, Lima RA, Soares JR, et al. Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. Am J Surg 1994;168:391–4. [PubMed] DOI:10.1016/S0002-9610(05)80082-0
- Yuen AP, Ho CM, Chow TL, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck 2009;31:765–72. [PubMed] DOI:10.1002/hed.21033
- D’Cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 2015;373:521–9. [PubMed] DOI:10.1056/NEJMoa1506007
- Ganly I, Patel S, Shah J. Early stage squamous cell cancer of the oral tongue — clinicopathologic features affecting outcome. Cancer 2012;118:101–11. [PubMed] DOI:10.1002/cncr.26229
- Flach GB, Tenhagen M, de Bree R, et al. Outcome of patients with early stage oral cancer managed by an observation strategy towards the N0 neck using ultrasound guided fine needle aspiration cytology: No survival difference as compared to elective neck dissection. Oral Oncol 2013;49:157–64. [PubMed] DOI:10.1016/j.oraloncology.2012.08.006
- Kim DW, Lee BD, Lim JH, et al. Elective neck dissection versus observation in early stage oral squamous cell carcinoma: recurrence and survival. J Korean Assoc Oral Maxillofac Surg 2016;42:358–64. [PubMed] DOI:10.5125/jkaoms.2016.42.6.358
- Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg 1994;120:699–702. [PubMed] DOI:10.1001/archotol.1994.01880310005001
- Okura M, Aikawa T, Sawai NY, et al. Decision analysis and treatment threshold in a management for the N0 neck of the oral cavity carcinoma. Oral Oncol 2009;45:908–11. [PubMed] DOI:10.1016/j.oraloncology.2009.03.013
- Yeh CF, Li WY, Yang MH, et al. Neck observation is appropriate in T1-2, cN0 oral squamous cell carcinoma without perineural invasion or lymphovascular invasion. Oral Oncol 2014;50:857–62. [PubMed] DOI:10.1016/j.oraloncology.2014.06.002
- Liu M, Wang SJ, Yang X, et al. Diagnostic efficacy of sentinel lymph node biopsy in early oral squamous cell carcinoma: A Meta-analysis of 66 studies. PLoS One 2017;12:e170322. [PubMed] DOI:10.1371/journal.pone.0170322
- Monroe MM, Lai SY. Sentinel lymph node biopsy for oral cancer: supporting evidence and recent novel developments. Curr Oncol Rep 2014;16:385. [PubMed] DOI:10.1007/s11912-014-0385-1
- Chaturvedi P, Datta S, Arya S, et al. Prospective study of ultrasound-guided fine-needle aspiration cytology and sentinel node biopsy in the staging of clinically negative T1 and T2 oral cancer. Head Neck 2015;37:1504–8. [PubMed] DOI:10.1002/hed.23787
- Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:122–37. [PubMed] DOI:10.3322/caac.21389
- International Consortium for Outcome Research (ICOR) in Head and Neck Cancer. Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: an international multicenter retrospective study. JAMA Otolaryngol Head Neck Surg 2014;140:1138–48. [PubMed] DOI:10.1001/jamaoto.2014.1548
