Enumeration and molecular characterization of circulating tumor cell using an in vivo capture system in squamous cell carcinoma of head and neck
Introduction
Although circulating tumor cell (CTC) has been reported almost 150 years ago, efficiency detection and molecular assay have only gained attention recently. Technology improvement makes CTC isolation possible for clinical application and more and more CTC detection methods have been reported recently (1). However, it is still a great challenge to detect CTC from peripheral blood of cancer patients. Due to rarity of CTC and high unspecific cells background, with 6×106 leukocytes, 2×108 platelets and 4×109 erythrocytes per mL of patient-derived blood sample, isolation and downstream molecular analysis of CTC are still a challenge (2). High detection rate, sufficient amounts of CTC and downstream molecular analysis are needed to satisfy clinical utility (3-5).
Squamous cell carcinoma of head and neck (SCCHN) is one of the frequently occurring and deadly diseases. CTC is a promising blood biomarker for “liquid biopsies” to improve individual treatment regimens for cancer patients (6-8), So far, several studies have shown that the sensitivity of CTC isolation is low and insufficient to satisfy clinical requirement in SCCHN (9-13). Although molecular characterization analysis could provide more clinical information for individual treatment, CTC isolation method with little unspecific white blood cells still needs to be improved (14,15).
More than 40 different methods for CTC detection have been reported (16). Various detection strategies have been developed to increase detection sensitivity and yield of CTC (2,17). To our best knowledge, a few of those methods had qualification for clinical utility (18,19). In this study, we used a new strategy to capture CTC in vivo with CellCollector (GILUPI CellCollector, GILUPI GmbH, Germany) (20). During 30 min incubation in the arm vein of cancer patients, functional domain of CellCollector was exposed to 2–3 L of blood, which significantly improved CTC detection sensitivity. CellCollector has high detection rate in lung cancer and neuroendocrine tumor (21,22). Monitoring treatment response with changes in CTC counts detected with CellCollector shows promising potential in clinic (21). This study will support the use of in vivo CTC capture as a new diagnostic principle for future clinical study testing the relevance of “liquid biopsies” to cancer treatments.
Materials and methods
Clinical information
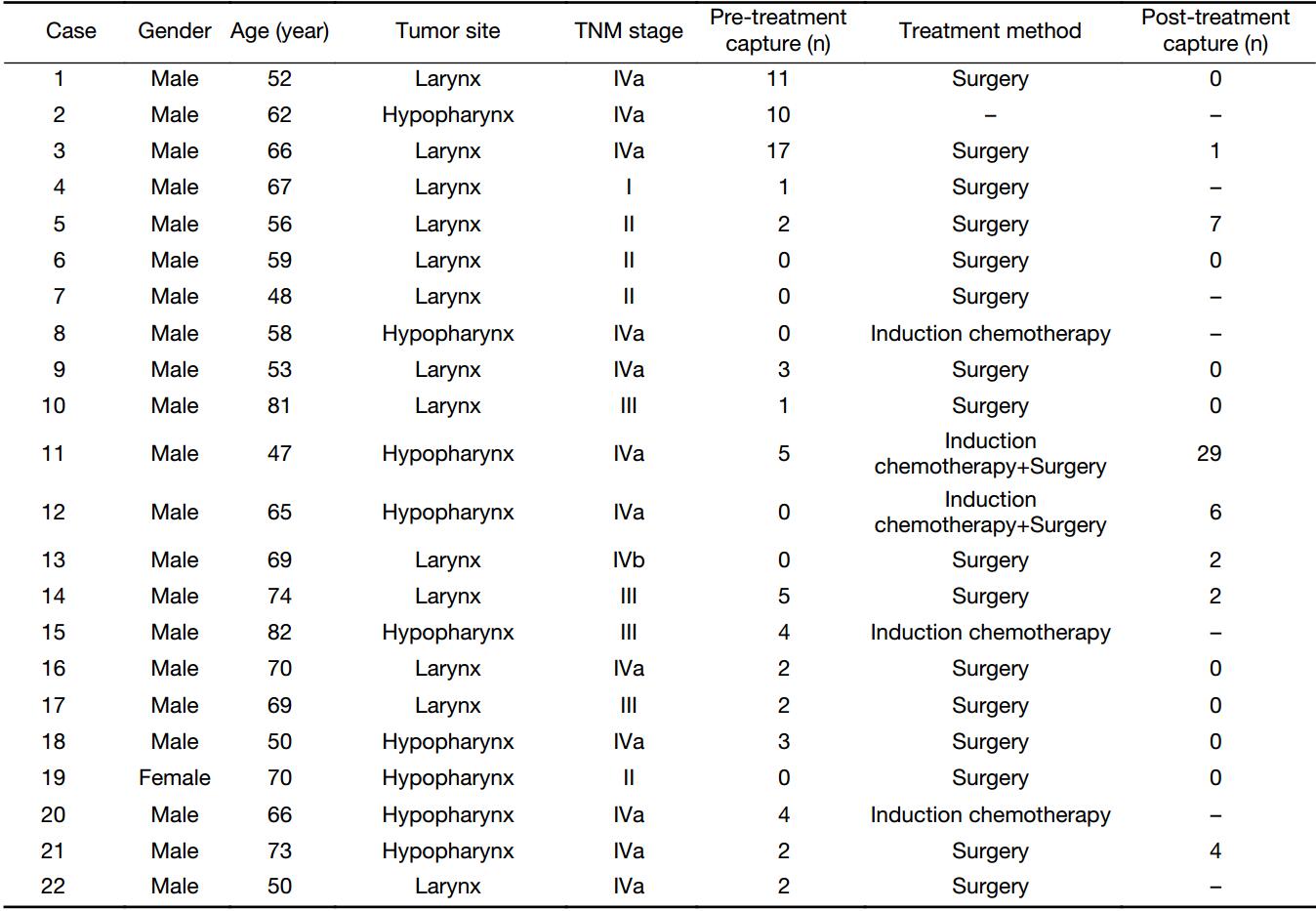
This prospective, CTC observer blinded, single-center clinical study investigated the detection and molecular characterization of CTC from the peripheral blood of SCCHN patients using the CellCollector. The study was conducted at the Nanjing Tongren Hospital in November 2016 to April 2017, and included 22 cases with 37 times of detection. All of them were newly diagnosed SCCHN. Most patients were male (21/22) aged 47–82 years old, including laryngocarcinoma (40.9%, 9/22) and hypopharyngeal carcinoma (59.1%, 13/22). All patients were received CTC detection before treatment. Three patients received induction chemotherapy after the first CTC detection. One case died of cancer-related complications before further treatment. Sixteen patients received surgical therapy, 13 of whom received postoperative CTC detection. Two patients received both induction chemotherapy and surgery treatment. Patients underwent two successive CellCollector detections 24 h before and 7 d after surgical therapy. A detailed summary of all patient data, including CTC counts, is shown in Table 1. At the same time, 9 healthy volunteers with no evidence of cancer disease were included as the control group. This study is registered in ClinicalTrials.gov (No. NCT03071900), and was approved by the Ethics Committee of Nanjing Tongren Hospital.
Full table
In vivo application of CellCollector
The GILUPI CellCollector is based on a sterile stainless steel medical wire, covered with a gold layer and a hydrogel layer at its functionalized tip. Before the application of the device, a 20 G peripheral venous catheter was placed into the median cubital vein of the patient. The CellCollector was inserted into the vein through the catheter until the tip of the device was extended 2 cm into the vein. After 30 min, the CellCollector was removed from the vein, cleaned and fixed for further examination.
CTC identification and epidermal growth factor receptor variant type III (EGFRVIII) staining
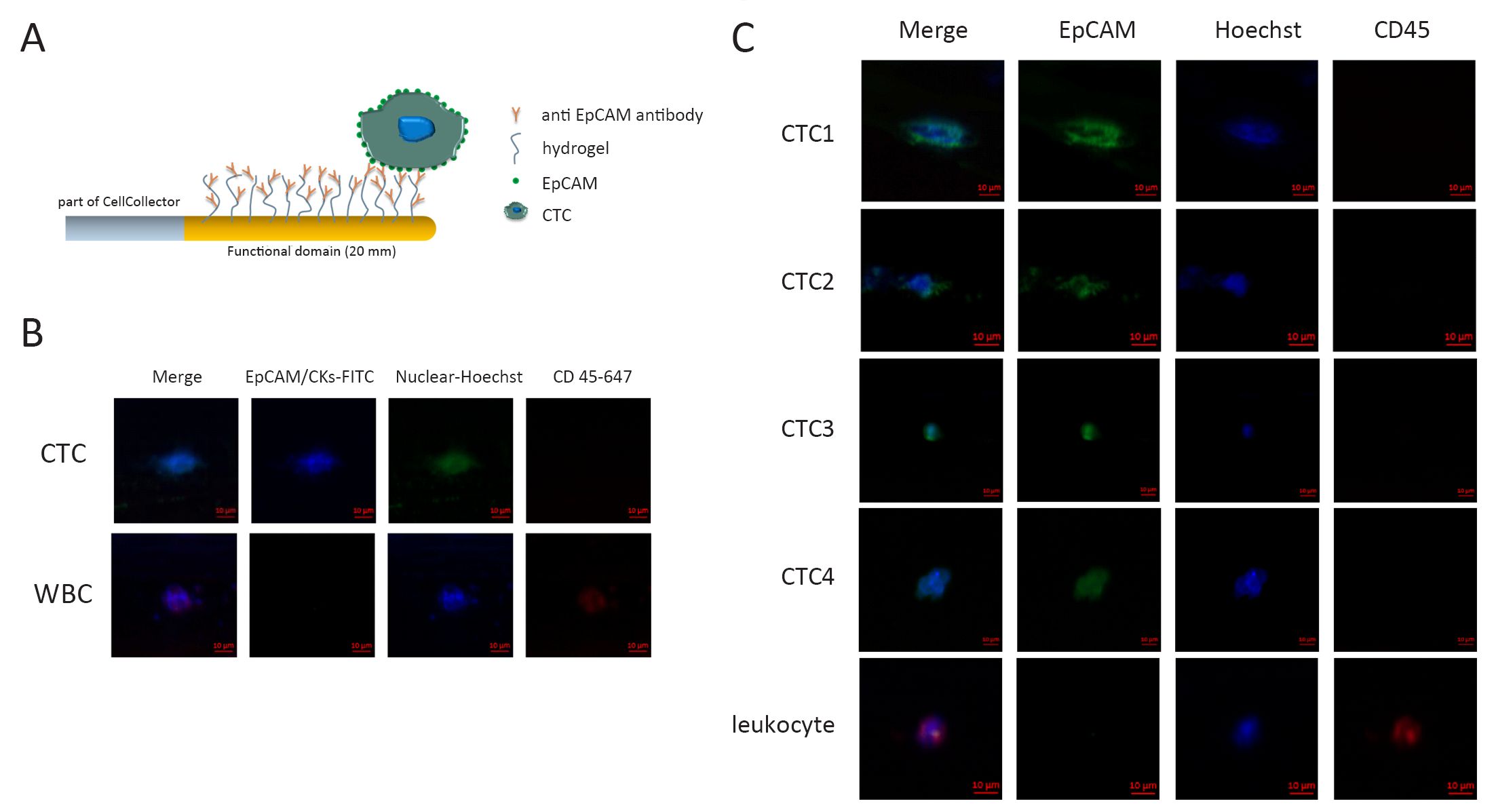
This function domain of CellCollector is covalently coupled with epithelial cell adhesion molecule (EpCAM) (humanized HEA 125, GILUPI GmbH, Potsdam, Germany) (Figure 1A). After the in vivo application, the CellCollector was washed three times and fixed with acetone for 10 min at room temperature. Captured cells were stained with EpCAM (Acris, New York, USA; clone HEA125-FITC) and pan-keratins (Exbio, Vestec, Czech Republic; clone C11-Alexa488; Millipore, Massachusetts, USA; clone LP5K-FITC; Exbio, Vestec, Czech Republic; clone A53-B/A2-Alexa488). CD45 (also called leukocyte common antigen) staining was performed to identify unspecific leucocytes (Exbio, Vestec, Czech Republic; clone MEM-28-Alexa647). Nuclear staining was performed with Hoechst33342 (Invitrogen, California, USA) at a concentration of 1 mg/mL for 2 min. A cell was counted as positive CTC when its morphological features (4 μm, intact nucleus) and staining patterns were consistent with those of an epithelial cell (Hoechst33342/pan-keratin and/or EpCAM-positive/CD45-negative; Figure 1B). Specific antibody against EGFRVIII was used in EGFRVIII staining. The EGFRVIII antibody was labeled with phycoerythrin (PE) fluorescent dye. EGFRVIII expression was analyzed by immunohistochemical (IHC) method as described in published references.
Statistical analysis
Statistical analyses were performed with SPSS Statistics (Version 17.0; SPSS Inc., Chicago, IL, USA) for Windows, and figures were generated using Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Normal distribution was analyzed. Nonparametric tests (Kolmogorov-Smirnov test) were used. CTC counts in preoperative and postoperative groups were analyzed with Wilcoxon rank sum test. P<0.05 was considered statistically significant.
Results
Patient characteristics
We enrolled 22 SCCHN patients and 9 healthy volunteers as the control group. Table 1 shows the clinicopathological characteristics of patients with laryngocarcinoma (n=9) and hypopharyngeal carcinoma (n=13). The median age of SCCHN patients was 65.5 (range, 47–82) years, and the patients were in different stages, including stage I (n=1), stage II (n=4), stage III (n=4) and stage IV (n=13).
In this study, 16 patients underwent surgical treatment, 3 patients underwent induction chemotherapy, and 2 patients received both induction chemotherapy and surgical treatments. One case died of cancer-related complications before further treatment. Fifteen patients received CTC detection before and after surgical treatment respectively.
CTC counts and correlations to clinicopathologic characteristics
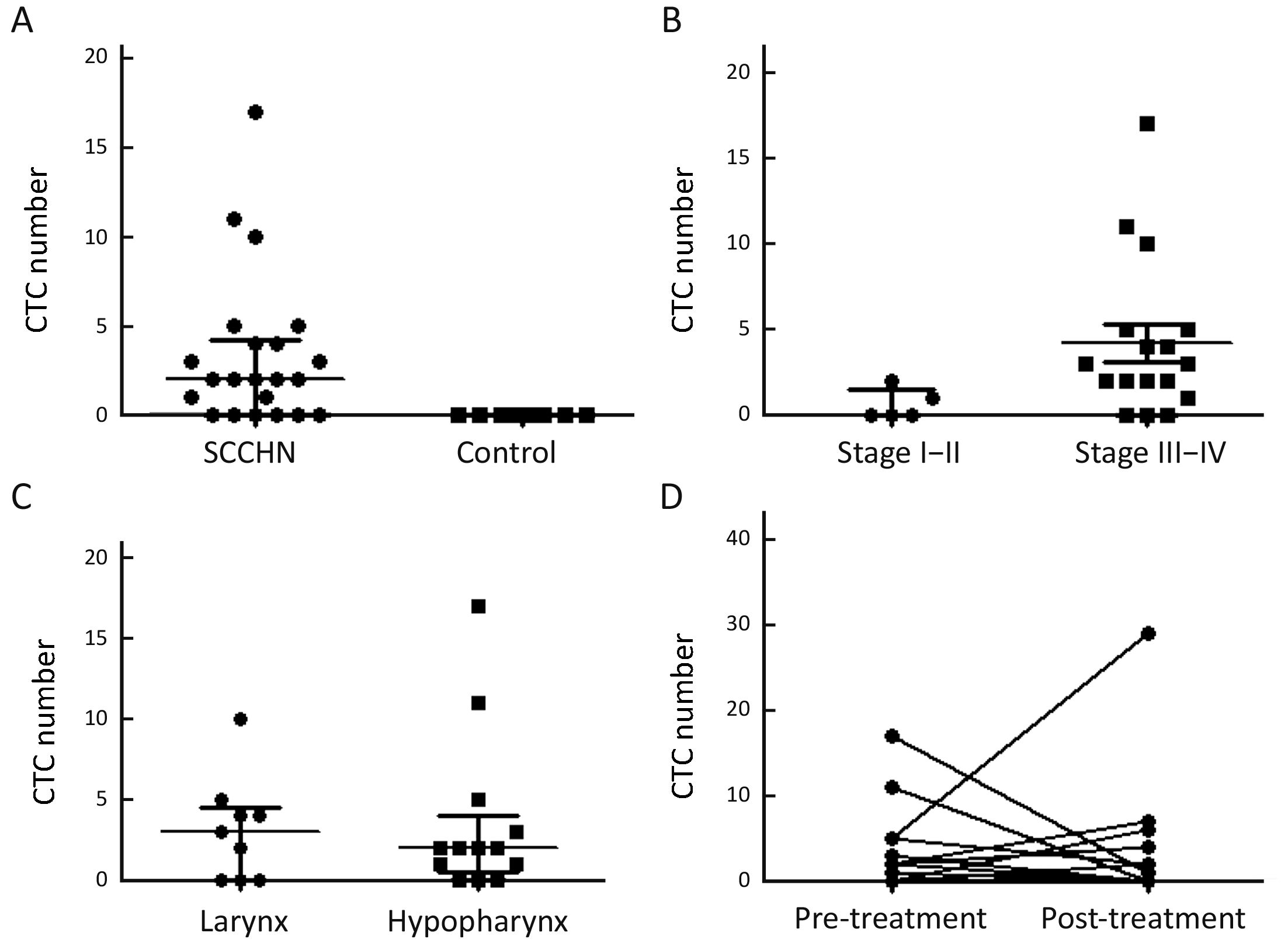
For analyzing clinical correlation to CTC enumeration, CTC detection rate and CTC counts were used in multivariate analysis. The repeatability and stabilization of CTC analysis method that we used in this study were verified before (21). Representative images of CTC from spiking experiments are show in Figure 1C. In total, 72.7% (16/22) of the patients were positive for ≥1 CTC (range, 1–17 cells; Figure 2A). Most of the analyzed cells were single CTC. However, CTC clusters (range, 2–8) were also detected in 8 of 37 observed CellCollector results. No CTC-like events were observed on the CellCollector used in control group (Figure 2A). CTC detection rate was higher in patients with late stage than in patients with early stage (P<0.05). In total, 5 patients were detected with stage I and II, two of whom had CTC-positive results, while, 82.4% (14/17) of stage III and IV patients have CTC-positive detection results, indicating CellCollector has high detection rate in SCCHN, especially in late stage (Figure 2B). CTC detection rates were not different between laryngocarcinoma and hypopharyngeal carcinoma (Figure 2C).
Monitoring of CTC during surgical therapy
To evaluate CTC number changes as a marker for analyzing surgical response in six patients with surgery, CTC detection was performed with CellCollector 24 h before and 7 d after surgical therapy. The detection rate of positive CTC decreased from 72.7% (16/22) with median CTC number of 2 before surgical treatment to 46.7% (7/15) with median CTC number of 0 after surgical treatment (P<0.05), indicating that changes of CTC counts may have potential value to show treatment effect (Figure 2D).
EGFRVIII expression assay in CTC and tissue
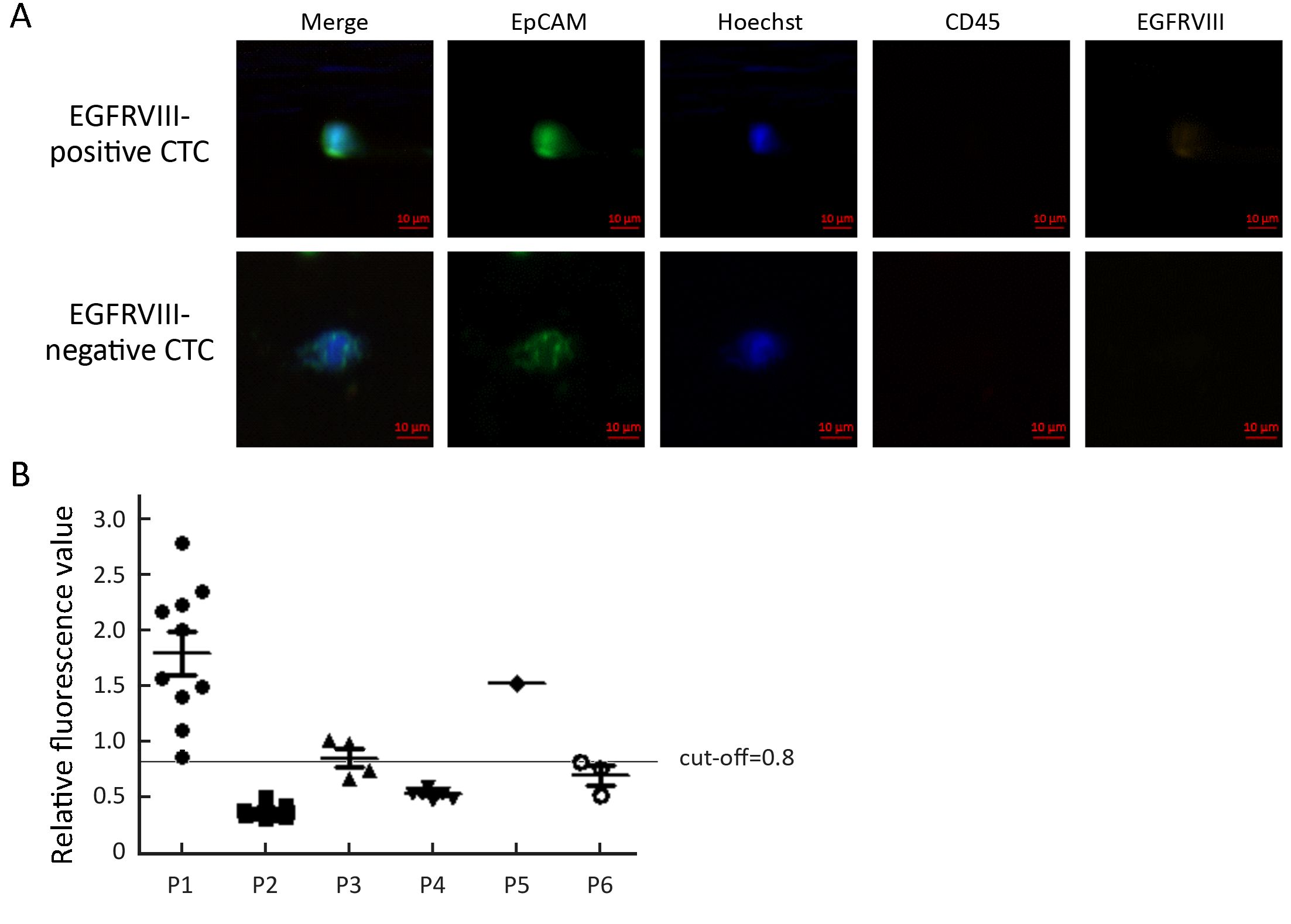
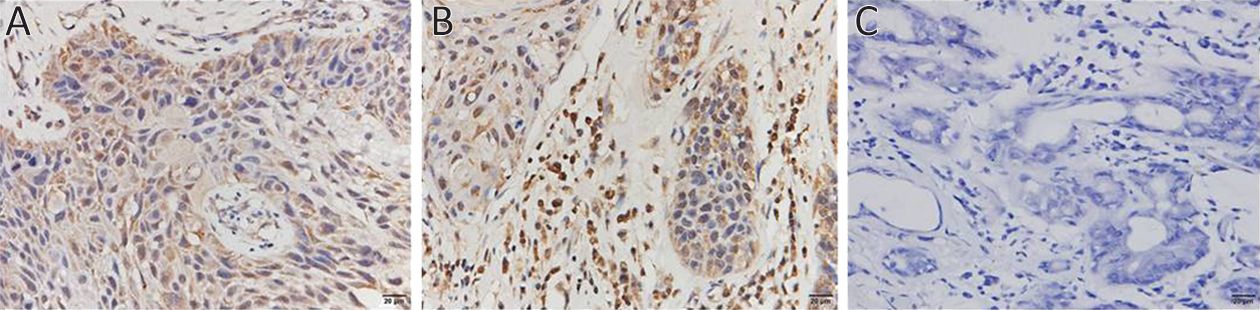
To analyze EGFRVIII expression, CTC from six patients were analyzed with EGFRVIII fluorescence antibody staining during CTC identification process. PE was covalently linked to EGFRVIII antibody to observe EGFRVIII at orange channel by fluorescence microscope. EGFRVIII positive and negative CTC are shown in Figure 3A. EGFRVIII expression level was calculated with peripheral blood mononuclear cell (PBMC) as a negative control. Cut-off value is a ratio that was calculated with PBMC fluorescence value divided by background (unstaining area) fluorescence value. The cut-off value is 0.8 here. Base on calculation results, two patients were found EGFRVIII-positive CTC, one case positive boundary, and three negative among those analyzed patients (Figure 3B). The expression of EGFRVIII was detected in the primary tumor and metastatic tumor of a patient with IHC staining (Figure 4).
Discussion
In vivo analysis
Here, we reported that in vivo detection of CTC is a promising method for clinical utilization. In vivo detection methods show high CTC isolation rate (11,23). CellCollector has been shown high detection rate in lung cancer and neuroendocrine tumor (21,22). Increasing of blood volumes may overcome ex vivo limitation to increase detection rate (3). Although CellCollector was used in an in vivo way, it is user-friendly for clinical application. The 30-min incubation in peripheral vein is acceptable for the most of tested patients. With immunofluorescence technique, which was proved by clinical evidence, CTC could be identified under a fluorescence microscope (20).
CTCEGFRVIII expression
EGFRVIII is important for growth of tumor cells and specifically expressed in tumor cells (24,25). Here, we reported EGFRVIII expression on CTC. In total, 33.3% (2/6) patients were found CTCEGFRVIII positive. Interestingly, we found that in this patient with EGFRVIII-positive CTC, EGFRVIII expression in both primary and metastatic tumor tissues was positive, indicating EGFRVIII expression level may be coordinated between CTC and tumor tissue. CTC-based EGFRVIII analysis may be important for SCCHN especially when tumor tissue is not available. The comparison of CTC and tumor tissue shows that EGFRVIII expression may have consistency, indicating CTC is a potential biomarker for analyzing EGFRVIII expression.
Molecular analysis of CellCollector
It already has been approved that gene mutations of CTC captured by CellCollector could be detected with digital polymerase chain reaction (PCR) (21). Next generation sequencing (NGS) could provide much more genetic information compared with digital PCR or amplification refractory mutation system-quality PCR (ARMS-qPCR) (26). CTC on functional domain was easy to separate from CellCollector with little unspecific cells by using a special cutter. Isolated CTC and CellCollector fragment together were collected into one Eppendorf (EP) tube to amplify the whole CTC genomic DNA for NGS analysis. Due to this isolation process, the sensitivity of NGS was greatly increased for detecting CTC genomic mutations. Next, we will focus on the work of CTC sequencing in SCCHN.
Clinical value of CellCollector in SCCHN
Base on high detection rate and high yield of CTC, monitoring of therapy effect with the changes of CTC counts before and after treatment could give more clinical information (21,27,28). Chemotherapy response of CTC counts have been reported for years (29). Additionally, CTC number sharply decreased after surgery compared with pre-operation CTC detection (30-32). In this study, two successive CTC detections have been carried out during surgical and/or induction chemotherapy process. After treatment, many patients had no CTC observed. In 5 of 15 patients, increased CTC number was found after treatment. The follow-up of those patients may be important to analyze different progression-free survival or overall survival compared with patients with decreased CTC number.
Conclusions
Low detection rate is the most important limitation for CTC study in SCCHN. In this study, CellCollector showed high sensitivity to CTC isolation from SCCHN patient’s peripheral vein blood, especially in advanced SCCHN patients. Moreover, CTC was easy to separate from CellCollector after identification with immunofluorescence staining. Molecular analysis of CTC may push “liquid biopsy” to a new level to apply and study.
Acknowledgements
We thank Dr. Jiaoteng Bai from Viroad Biotechnology Co. for technical support for this study. This study was supported by Grants from the National Nature Research Program of China (No. 81541057); Science and Technology Department Research Program of Jiangsu Province (No. BK20151080); and Science and Technology Department Research Program of Nanjing (No. 201402060).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol 2009;6:339–51. [PubMed] DOI:10.1038/nrclinonc.2009.44
- Joosse SA, Pantel K. Biologic challenges in the detection of circulating tumor cells. Cancer Res 2013;73:8–11. [PubMed] DOI:10.1158/0008-5472.CAN-12-3422
- Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer 2014;14:623–31. [PubMed] DOI:10.1038/nrc3820
- Ignatiadis M, Dawson SJ. Circulating tumor cells and circulating tumor DNA for precision medicine: dream or reality?. Ann Oncol 2014;25:2304–13. [PubMed] DOI:10.1093/annonc/mdu480
- Zhang J, Chen K, Fan ZH. Circulating tumor cell isolation and analysis. Adv Clin Chem 2016;75:1–31. [PubMed] DOI:10.1016/bs.acc.2016.03.003
- Braun S, Marth C. Circulating tumor cells in metastatic breast cancer — toward individualized treatment?. N Engl J Med 2004;351:824–6. [PubMed] DOI:10.1056/NEJMe048163
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781–91. [PubMed] DOI:10.1056/NEJMoa040766
- Vered M, Dayan D, Salo T. The role of the tumour microenvironment in the biology of head and neck cancer: lessons from mobile tongue cancer. Nat Rev Cancer 2011;11:382. [PubMed] DOI:10.1038/nrc2982-c1
- Zen H, Nakashiro K, Shintani S, et al. Detection of circulating cancer cells in human oral squamous cell carcinoma. Int J Oncol 2003;23:605–10. [PubMed] DOI:10.3892/ijo.23.3.605
- Tong X, Yang L, Lang JC, et al. Application of immunomagnetic cell enrichment in combination with RT-PCR for the detection of rare circulating head and neck tumor cells in human peripheral blood. Cytometry B Clin Cytom 2007;72:310–23. [PubMed] DOI:10.1002/cyto.b.20177
- Bozec A, Ilie M, Dassonville O, et al. Significance of circulating tumor cell detection using the CellSearch system in patients with locally advanced head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 2013;270:2745–9. [PubMed] DOI:10.1007/s00405-013-2399-y
- Tinhofer I, Konschak R, Stromberger C, et al. Detection of circulating tumor cells for prediction of recurrence after adjuvant chemoradiation in locally advanced squamous cell carcinoma of the head and neck. Ann Oncol 2014;25:2042–7. [PubMed] DOI:10.1093/annonc/mdu271
- Wu XL, Tu Q, Faure G, et al. Diagnostic and prognostic value of circulating tumor cells in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Sci Rep 2016;6:20210. [PubMed] DOI:10.1038/srep20210
- Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011;333:1157–60. [PubMed] DOI:10.1126/science.1208130
- Lianidou ES. Circulating tumor cells — new challenges ahead. Clin Chem 2012;58:805–7. [PubMed] DOI:10.1373/clinchem.2011.180646
- Lianidou ES, Markou A. Circulating tumor cells in breast cancer: detection systems, molecular characterization, and future challenges. Clin Chem 2011;57:1242–55. [PubMed] DOI:10.1373/clinchem.2011.165068
- Dotan E, Cohen SJ, Alpaugh KR, et al. Circulating tumor cells: evolving evidence and future challenges. Oncologist 2009;14:1070–82. [PubMed] DOI:10.1634/theoncologist.2009-0094
- Lianidou ES, Mavroudis D, Georgoulias V. Clinical challenges in the molecular characterization of circulating tumour cells in breast cancer. Br J Cancer 2013;108:2426–32. [PubMed] DOI:10.1038/bjc.2013.265
- Ilie M, Hofman V, Long E, et al. Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine? Ann Transl Med 2014;2:107. [PubMed] DOI:10.3978/j.issn.2305-5839.2014.08.11
- Saucedo-Zeni N, Mewes S, Niestroj R, et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire . Int J Oncol 2012;41:1241–50. [PubMed] DOI:10.3892/ijo.2012.1557
- Gorges TM, Penkalla N, Schalk T, et al. Enumeration and molecular characterization of tumor cells in lung cancer patients using a novel in vivo device for capturing circulating tumor cells . Clin Cancer Res 2016;22:2197–206. [PubMed] DOI:10.1158/1078-0432.CCR-15-1416
- Mandair D, Vesely C, Ensell L, et al. A comparison of CellCollector with CellSearch in patients with neuroendocrine tumours. Endocr Relat Cancer 2016;23:L29–32. [PubMed] DOI:10.1530/ERC-16-0201
- Nichols AC, Lowes LE, Szeto CC, et al. Detection of circulating tumor cells in advanced head and neck cancer using the CellSearch system. Head Neck 2012;34:1440–4. [PubMed] DOI:10.1002/hed.21941
- Weller M, Kaulich K, Hentschel B, et al. Assessment and prognostic significance of the epidermal growth factor receptor VIII mutation in glioblastoma patients treated with concurrent and adjuvant temozolomide radiochemotherapy. Int J Cancer 2014;134:2437–47. [PubMed] DOI:10.1002/ijc.28576
- Chen JR, Xu HZ, Yao Y, et al. Prognostic value of epidermal growth factor receptor amplification and EGFRVIII in glioblastoma: meta-analysis. Acta Neurol Scand 2015;32:310–22. [PubMed] DOI:10.1111/ane.12401
- De Luca F, Rotunno G, Salvianti F, et al. Mutational analysis of single circulating tumor cells by next generation sequencing in metastatic breast cancer. Oncotarget 2016;7:26107–19. [PubMed] DOI:10.18632/oncotarget.8431
- Camara O, Rengsberger M, Egbe A, et al. The relevance of circulating epithelial tumor cells (CETC) for therapy monitoring during neoadjuvant (primary systemic) chemotherapy in breast cancer. Ann Oncol 2007;18:1484–92. [PubMed] DOI:10.1093/annonc/mdm206
- Serrano MJ, Rovira PS, Martínez-Zubiaurre I, et al. Dynamics of circulating tumor cells in early breast cancer under neoadjuvant therapy. Exp Ther Med 2012;4:43–8. [PubMed] DOI:10.3892/etm.2012.540
- Fei F, Du Y, Di G, et al. Are changes in circulating tumor cell (CTC) count associated with the response to neoadjuvant chemotherapy in local advanced breast cancer? A meta-analysis. Oncol Res Treat 2014;37:250–4. [PubMed] DOI:10.1159/000362378
- Yamashita JI, Kurusu Y, Fujino N, et al. Detection of circulating tumor cells in patients with non-small cell lung cancer undergoing lobectomy by video-assisted thoracic surgery: a potential hazard for intraoperative hematogenous tumor cell dissemination. J Thorac Cardiovasc Surg 2000;119:899–905. [PubMed] DOI:10.1016/S0022-5223(00)70084-5
- Sandri MT, Zorzino L, Cassatella MC, et al. Changes in circulating tumor cell detection in patients with localized breast cancer before and after surgery. Ann Surg Oncol 2010;17:1539–45. [PubMed] DOI:10.1245/s10434-010-0918-2
- van Dalum G, van der Stam GJ, Tibbe AG, et al. Circulating tumor cells before and during follow-up after breast cancer surgery. Int J Oncol 2015;46:407–13. [PubMed] DOI:10.3892/ijo.2014.2694
