Serial stimulated thyroglobulin measurements are more specific for detecting distant metastatic differentiated thyroid cancer before radioiodine therapy
Introduction
Differentiated thyroid carcinoma (DTC), which accounts for almost 90% of thyroid cancer, is characterized by an indolent course with a low mortality and a favorable long-term prognosis. However, distant metastasis (DM), which occurs in 1%–23% of patients with DTC (1-3), is generally considered to be the most frequent cause of cancer-related death. As a consequence, the 10-year overall survival rate even after radioactive iodine (RAI) therapy is about 42% (4).
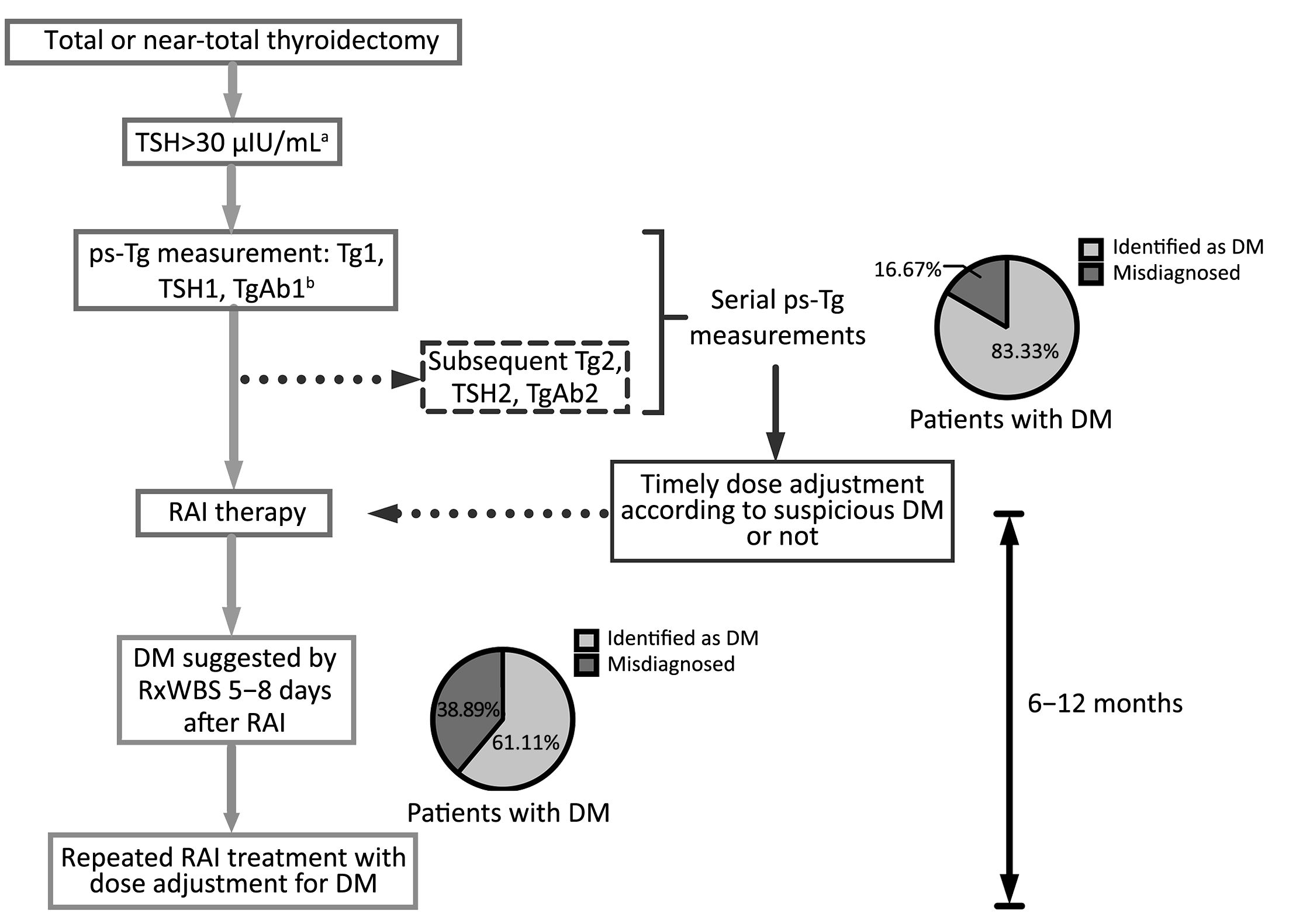
It is noteworthy that early diagnosis of functioning DM is closely related to earlier and more appropriate adjuvant therapy as well as a better prognosis (4), and some patients even benefited from a timely high dose of initial RAI treatment (5). However, in some cases, distant metastatic lesions may remain unidentified with negative pre-RAI radiographic images such as computed tomography (CT) and diagnostic whole-body RAI scan (DxWBS), and were revealed only when post-therapy whole-body RAI scan (RxWBS) or even 18F-FDG positron emission tomography (PET)/CT was performed later (Figure 1).
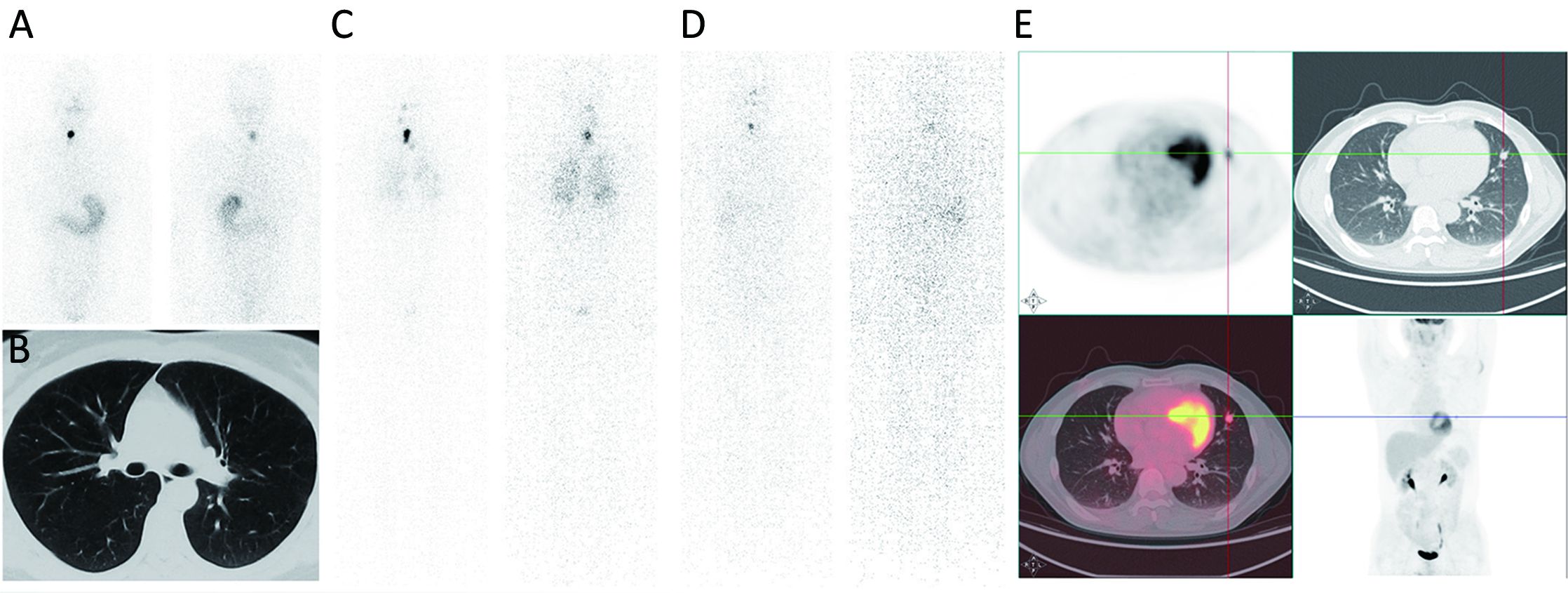
Our previous studies have revealed the potential of pre-RAI stimulated thyroglobulin (ps-Tg) levels in predicting DM-DTC, with a cut-off value of 52.75 ng/mL (6,7), which manifested its potential role in facilitating initial risk stratification and timely RAI dose adjustment to avoid inadequate therapy. Nevertheless, our recent study showed that the predictive value of ps-Tg might be affected by the corresponding thyrotropin (TSH) level (8), and that the presence of massive remnant thyroid in non-DM patients could also cause elevated ps-Tg levels, which might lead to the misdiagnosis of DM (9).
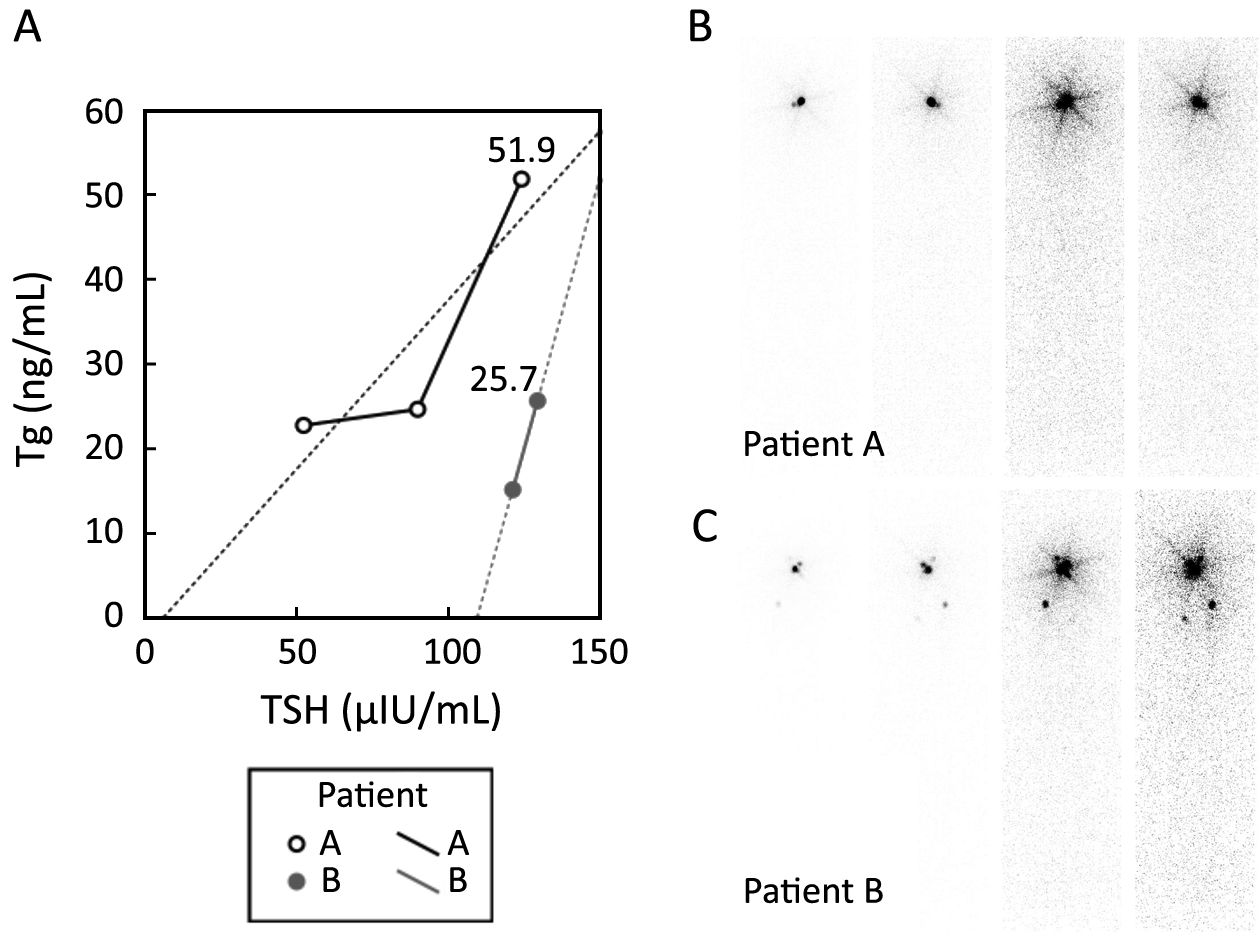
Thus, the reported cut-offs of ps-Tg for guiding decision-making regarding RAI administration were highly variable (10-13). Furthermore, even a high ps-Tg level indicating a high probability of DM-DTC may sometimes not be accurate, as shown in Figure 2, which begs the question as to whether a single ps-Tg measurement is sufficient. Thus, we retrospectively evaluated the potential value of serial ps-Tg measurements in identifying DM-DTC prior to RAI therapy.
Materials and methods
Patients
In this retrospective study, 864 potentially eligible DTC patients were reviewed, and all of them were referred to RAI therapy consecutively at Peking Union Medical College Hospital between January 2012 and October 2014, among whom, 547 patients were excluded due to at least one of the following reasons: 1) absence of serial serological data; 2) elevated TgAb (>46 IU/mL) (14); or 3) levels of serial ps-Tg or TSH all beyond the ranges of the assays, which might disenable the calculation of dynamic parameters. Finally, a total of 317 DTC patients [310 with papillary thyroid carcinoma (PTC) and 7 with follicular thyroid carcinoma (FTC)] were enrolled. All of them underwent total or near total thyroidectomy and/or cervical lymph node dissection followed by RAI therapy. Group M1 (n=72) and group M0 (n=245) were divided according to the presence of DM or not. Patients with any of the following were identified as DM-DTC: 1) distant metastatic lesions confirmed by pathology; 2) focal or diffuse uptake in distant metastatic lesions on RxWBS after excluding the contamination and physiological RAI uptake, with or without positive findings on other complementary imaging modalities (chest CT, X-rays, magnetic resonance imaging, bone scintigraphy or 18F-FDG PET/CT) or elevated Tg levels; 3) positive findings on either 18F-FDG PET/CT or bone scintigraphy after excluding other malignancies and benign diseases, with a rising Tg level, despite negative RxWBS results; or 4) negative findings on functional imaging, but structural lesions suggested by chest CT or X-ray after excluding other malignancies and benign diseases, with a rising Tg level. The Ethics Committee of Peking Union Medical College Hospital approved the study.
Study design
The following characteristics were registered and compared between M0 and M1 groups: age at diagnosis, gender, pathology, American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) TNM staging (7th edition), as well as the amount of remnant thyroid.
ps-Tg, TSH and TgAb levels were tracked serially during off-levothyroxine (LT4) therapy before RAI administration. The initial ps-Tg measured with a corresponding off-LT4 TSH level exceeding 30 μIU/mL was marked as Tg1, and the subsequent ps-Tg collected right before RAI therapy was defined as Tg2. The median interval between Tg1 and Tg2 was 8 (range: 6–13) days. ΔTg denotes the change in ps-Tg (Tg2–Tg1). The same notations were applied to TSH1, TSH2 and ΔTSH.
Initially, Tg1, Tg2, ΔTg and ΔTg/ΔTSH were tested for their diagnostic performance to serve as possible serum predictors of DM, and further compared with imaging modalities including chest CT and RxWBS.
Treatment protocol
All patients underwent RAI therapy for remnant ablation or treatment of recurrent/metastatic lesions at least once, following thyroid hormone withdrawal (THW) or with no LT4 therapy after surgery, accompanied by a strict low-iodine diet for at least 2 weeks. Doses of RAI, varying from 1.11 GBq (30 mCi) to 7.4 GBq (200 mCi), were administered to patients according to their tumor stage and recurrence risk stratification. RAI treatment for persistent or recurrent disease was repeated at an interval of 6–12 months according to the American Thyroid Association (ATA) guidelines (15). Medical examinations before RAI treatment included high-resolution chest CT, neck ultrasonography, and serial measurements of ps-Tg, TSH, and TgAb. RxWBS was routinely performed 5–8 days after therapeutic RAI administration, and LT4 therapy was initiated or resumed 3 days after RAI administration.
Image analysis and serological assays
RxWBS was performed using a dual-head γ-camera (Infinia Hawkeye 4, GE Healthcare, USA) with high-energy parallel-hole collimators. Single-photon emission computed tomography (SPECT) images of the neck and chest were obtained for further identification in patients with negative planar image findings. The amount of remnant thyroid was evaluated semiquantitatively and classified as none, moderate or overt, the latter two of which were artificially divided based on the differential cut-off value of 10% between thyroid bed and whole body using a region of interest technique. All images were analyzed separately by two experienced nuclear medicine physicians who were unaware of either the clinical findings or any other diagnostic imaging data.
Serological measurements of Tg, TgAb and TSH were performed in the same laboratory using the same kits. Tg and TgAb levels were determined using electrochemiluminescence immunoassays (Roche Diagnostics GmbH, Mannheim, Germany) and measuring ranges were 0.100–1,000 ng/mL and 10–4,000 IU/mL, respectively. TSH was determined using chemiluminescence immunoassays (Siemens Healthcare Diagnostics Inc., New York, USA), with a measuring range of 0.004–150 μIU/mL. Values above the upper limits of Tg and TSH measurements were reported as >1,000 ng/mL and >150 μIU/mL, respectively, while values below the lower limit of Tg measurement were reported as <0.1 ng/mL.
Statistical analysis
Student’s t test, χ2 test and Fisher’s exact test were applied to analyze the statistical differences in age, gender and pathology between the two groups. Categorical variables such as T1–4 status (AJCC/UICC), initial lymph node status, staging I–IV, as well as the condition of thyroid remnant were compared using the Mann-Whitney U test. Parameters such as Tg1, Tg2, ΔTg and ΔTg/ΔTSH were expressed as medians with ranges and compared using the Mann-Whitney U test. Receiver operating characteristic (ROC) curves were drawn to determine the best cut-off value for each serological marker. Comparison of ROC curves was performed using the method of Hanley & McNeil for the same set of patients (16). With the corresponding cut-off values fixed, sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) were eventually calculated to evaluate the overall diagnostic performance of these parameters as biochemical markers for DM-DTC, and then compared with each imaging modality. All tests were two-sided, and P<0.05 was considered statistically significant. Statistical analysis and graph generation were processed using IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA) and MedCalc software (Version 11.4.2.0; Mariakerke, Belglum).
Results
Clinicopathologic characteristics of patients
Of the 317 DTC patients, the mean age at diagnosis was 41.76±11.95 (range: 12–75) years, with a male-to-female ratio of 1:1.99 (M0, 1:2.1; M1, 1:1.7). The mean follow-up period was 20.9 months. According to the criteria mentioned previously, DM was identified in 72 patients (66 presented in lungs, one in bone, and five with multiple-site metastases including four in both lung and bone, and one in both lung and liver). As shown in Table 1, there were no statistical differences in age, gender, and thyroid remnant between the M1 and M0 groups. As expected, DM was significantly associated with follicular histology (P<0.001), advanced T (P<0.001) and N status (P<0.001), and a higher tumor stage (P<0.001).
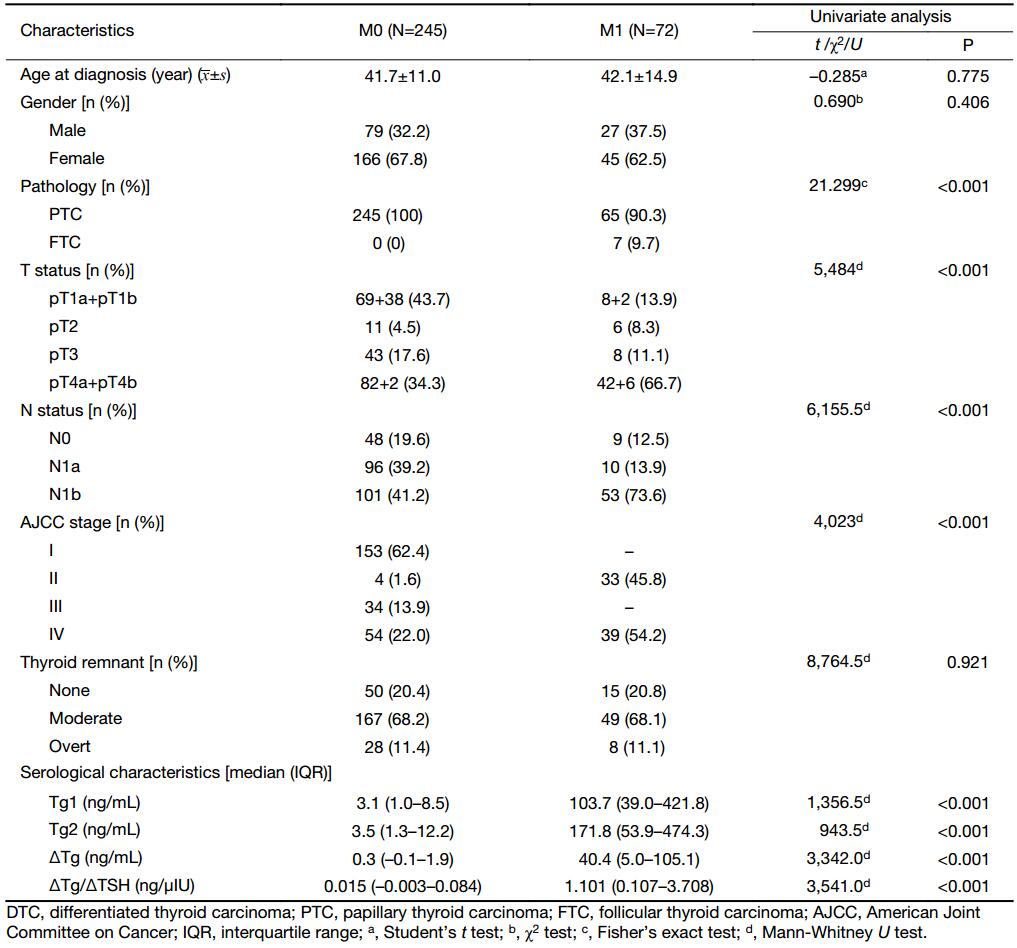
Full table
Serological characteristics before RAI treatment
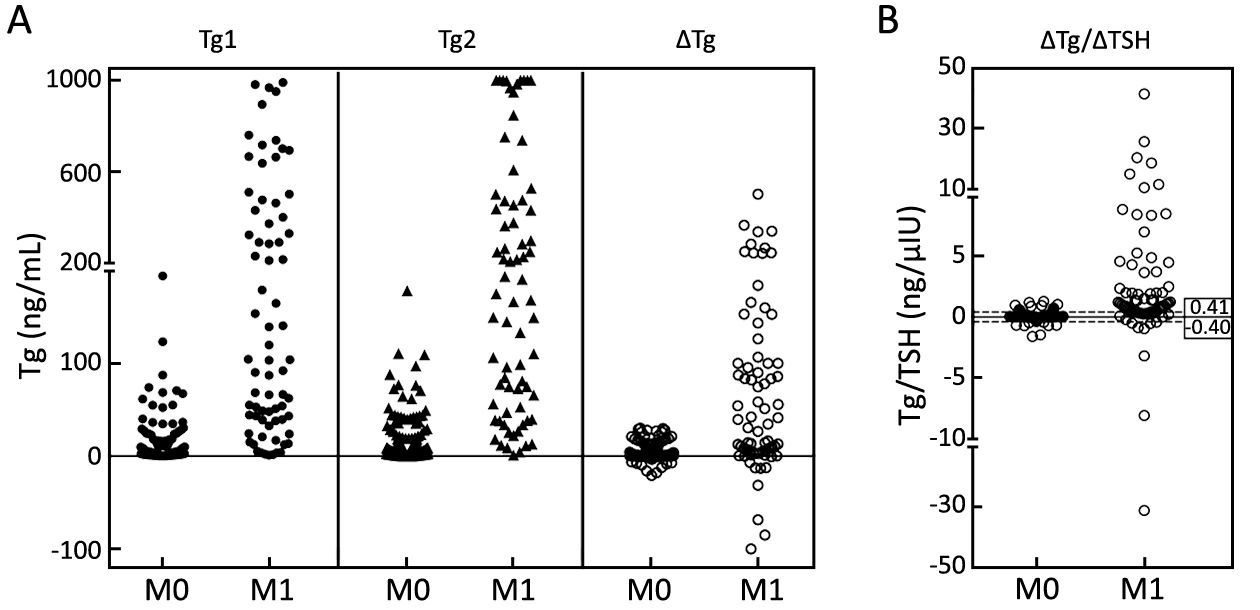
Both Tg1 and Tg2 were higher in the M1 group (P<0.001) (Table 1), while in the M0 group, ΔTg was distributed in a narrower range than either Tg1 or Tg2, which made the M0 group more discernable from the M1 group. Intriguingly, when ΔTg/ΔTSH was used as an index, the dispersed distribution in the M1 group made DM much distinguished from the extremely centralized distribution in the M0 group (P<0.01) (Figure 3).
ROC curve analysis and diagnostic performances of serial Tg parameters
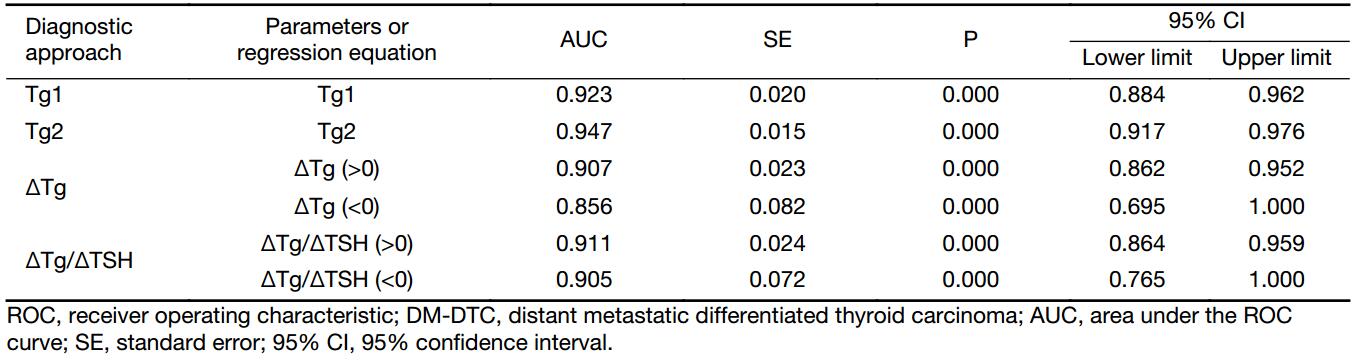
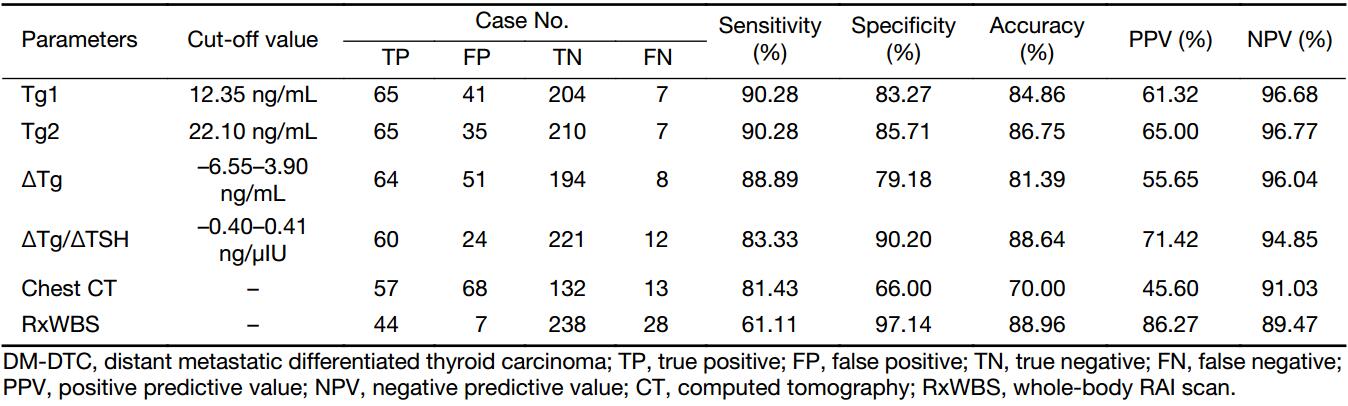
ROC curve analysis showed that the optimal cut-offs for Tg1 and Tg2 to identify DM were 12.35 ng/mL and 22.10 ng/mL, respectively. The corresponding areas under the ROC curves (AUC) to differentiate the two groups are given in Table 2, which illustrates that Tg2, with a larger AUC of 0.947 (Z=2.373, P=0.0176), showed better diagnostic performance in identifying DM-DTC than Tg1, with both higher accuracy (86.75%) and higher specificity (85.71%) (Table 3).
Full table
Full table
Considering that ΔTg and ΔTg/ΔTSH can be above or below zero, ROC curves were drawn in both conditions separately, and the two cut-offs constituted a diagnostic range, as presented in Table 3. ΔTg/ΔTSH manifested a higher accuracy (88.64%) and specificity (90.20%), as well as a lower false positive proportion (28.6%) in identifying DM-DTC than Tg1 or Tg2.
Comparison between serial ps-Tg measurements and imaging modalities
Among the 270 patients with results of pre-RAI high-resolution chest CT, 68 presented with indeterminate lesions on pre-RAI images, which made it difficult to rule out the possibility of malignancy until subsequent RxWBS, further radiographic as well as biochemical evidence were collected during follow-up, which contributed to its inferior specificity and accuracy of 66.00% and 70.00%, respectively. In contrast, the sensitivity of RxWBS was only 61.11% albeit with good specificity, which was much lower than that of serial ps-Tg measurements. Thus, with an accuracy of 88.64%, a specificity of 90.20%, and a sensitivity of 83.33% (Table 3), ΔTg/ΔTSH derived by serial ps-Tg measurements yielded better diagnostic performance than these cross-sectional imaging (Figure 4).
Discussion
In general, patients with DM-DTC are considered to have worse clinical outcomes (17). Appropriate RAI therapy has been associated with improved long-term survival and is employed as first-line treatment after surgery. Studies indicated that compared with various initial remnant RAI ablation doses, the use of high RAI treatment dose at the initial therapy, even with the presence of remnant thyroid tissue, might allow maximal uptake into those disseminated hidden metastatic lesions (18). Thus, these patients with DM-DTC can benefit from timely initial therapy for DM and result in significant structural and biochemical improvements since the initial RAI treatment (5). However, it is still a great challenge for clinicians to identify DM-DTC timely enough to tailor appropriate management prior to RAI therapy, especially in cases with no pre-RAI imaging evidence.
ps-Tg was indicated to be a predictive marker for DM-DTC in our previous studies, and a cut-off of 52.75 ng/mL was further obtained using ROC analysis (6,7). Results from other studies also documented the role of ps-Tg in identifying DM-DTC (19,20). However, only a single ps-Tg measurement was applied in the above studies, which might be affected by several factors, such as TSH levels, remnant thyroid tissue and TgAb levels, as well as others (8,9,21-23), thus leading one to question the adequacy of single static ps-Tg measurements in diagnosing DM. According to our recent study, a ps-Tg dynamic parameter, such as ΔTg/ΔTSH, might lessen the impact of TSH levels and basic Tg production from remnant thyroid tissue on ps-Tg (9). Therefore, this retrospective study was specifically aimed at further evaluating the clinical value of serial dynamic measurements of ps-Tg in identifying DM-DTC.
In this study, Tg1, Tg2, ΔTg and ΔTg/ΔTSH were found to be potential pre-RAI tumor markers for DM-DTC. Regarding thyroid remnants, no statistical difference was found between the M1 and M0 groups; hence we believe that the differences in the serological parameters between the two groups did not come from the residual thyroid tissue. However, the optimal cut-off value for Tg2 was 22.10 ng/mL, much lower than the 52.75 ng/mL that we reported previously (7). Possible explanations for this variability are: 1) less influence from residual thyroid tissue due to improvement in total thyroidectomy surgical techniques in recent years in China; 2) enrollment of some patients in this study who received a repeated RAI treatment with less or no remnant thyroid; and 3) different levels of TSH at the time of ps-Tg testing. These factors reinforced the inadequacy of a single ps-Tg measurement in identifying DM-DTC.
Derived from serial ps-Tg measurements in this study, ΔTg/ΔTSH values tended to be highly distributed in patients with DM, which manifested better diagnostic efficacy than either Tg1 or Tg2 in identifying DM-DTC with higher specificity and accuracy. As shown in Figure 2, ps-Tg, taken as a single measurement, could be stimulated to a high level in patients with either DM or massive remnant thyroid, while when we traced the change of ps-Tg over the course of increasing TSH levels, the slope represented by ΔTg/ΔTSH made DM more discernable in a much clearer manner. It seems that ΔTg/ΔTSH could uncover different patterns of ps-Tg changes between DM and residual thyroid by exposing the differential stimulative effect of increasing TSH on ps-Tg (24).
We propose that there might be a relatively delayed active reaction in DM lesions as opposed to the gradual saturated reaction in residual thyroid tissue over the course of rising TSH levels. During TSH stimulation, more ps-Tg and endogenous or tumorous exogenous thyroxine might induce a decrease in TSH by feedback inhibition, thus resulting in ΔTg/ΔTSH to be either above or below zero. It is worthy to mention that ΔTg/ΔTSH manifested higher specificity, accuracy, and lower false positives in identifying DM-DTC than either Tg1 or Tg2, thus further manifested the role of serial ps-Tg measurements and added incremental value to the identification of DM-DTC, especially in the context of significant residual thyroid tissue.
Regarding the comparison with imaging modalities, serial ps-Tg measurements also showed clear superiority in the pre-RAI identification of DM-DTC. As we know, the application of DxWBS prior to RAI therapy was somewhat limited due to its low sensitivity caused by either the low dose of RAI or possible DM masking by dominated residual thyroid uptake (25). In addition, some small DM lesions could not be detected due to the relatively low resolution of SPECT, so even after a therapeutic dose was administered, the sensitivity of RxWBS was still not good enough (61.11%).
With respect to high-resolution chest CT, the indeterminate pulmonary nodules were usually considered as positive findings prior to RAI administration with the purpose of avoiding inadequate RAI treatment, resulting in a poor specificity of 66.00% in this study, according to later serological and imaging follow-up. Hence, the overall diagnostic performance of serial ps-Tg was more advantageous than each imaging modality, which might compensate the low sensitivity of functional image, and reflect the biochemical behavior of DM lesions when compared with the non-specific anatomical image as well. Moreover, when compared with RxWBS, serial measurements of ps-Tg might be more helpful in identifying DM earlier and specifically, so that timely and appropriate dose adjustment could be made prior to initial RAI administration.
The results of this study emphasized the concept of diagnosing the presence of DM through dynamic monitoring of changes in ps-Tg rather than using a single static measurement. By applying serial ps-Tg measurements and the derived calculation, patients with DM could be identified much earlier prior to RAI administration (as demonstrated for two cases shown in Figure 1). In addition, those non-DM patients with thyroglobulinemia, who might be misdiagnosed as having DM using a single ps-Tg measurement, could be differentiated from DM patients more accurately because they present with gradually increasing or even decreasing Tg levels (such as patient A in Figure 2). Thus, because of serial ps-Tg measurements, more patients could receive an appropriate dose of RAI therapy by timely and appropriate dose adjustment.
The limitations of this study are primarily the limited number of cases and the retrospective nature of the data. Therefore, the clinical utility of serial ps-Tg measurements in identifying DM-DTC should be validated in further studies. Furthermore, upper and lower limits of our Tg and TSH assays might also influence the assessment of pre-RAI changes and thus lead to the exclusion of some potentially eligible patients.
Conclusions
The findings of this study suggest that serial ps-Tg measurements even over as short an interval as 8 days may have incremental value in the identification of DM-DTC prior to RAI treatment. With a higher specificity and accuracy than single measurements of ps-Tg, ΔTg/ΔTSH holds the promise to be a reliable indicator of DM-DTC. Serial ps-Tg measurements also hold the potential to serve as an alternative DM indicator to imaging modalities prior to RAI treatment, especially for those with negative imaging results.
Acknowledgements
We gratefully acknowledge the assistance of Prof. Guangliang Shan for guidance in statistical analysis. This work was supported by the Ministry of Health Industry Special Scientific Research Projects of China (No. 201202012) and the National Natural Science Foundation of China (No. 81571714).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sampson E, Brierley JD, Le LW, et al. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer 2007;110:1451–6. [PubMed] DOI:10.1002/cncr.22956
- Nixon IJ, Whitcher M, Palmer FL, et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid 2012;22:884–9. [PubMed] DOI:10.1089/thy.2011.0535
- Lee J, Soh EY. Differentiated thyroid carcinoma presenting with distant metastasis at initial diagnosis clinical outcomes and prognostic factors. Ann Surg 2010;251:114–9. [PubMed] DOI:10.1097/SLA.0b013e3181b7faf6
- Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–9. [PubMed] DOI:10.1210/jc.2005-2838
- Okamoto S, Shiga T, Uchiyama Y, et al. Lung uptake on I-131 therapy and short-term outcome in patients with lung metastasis from differentiated thyroid cancer. Ann Nucl Med 2014;28:81–7. [PubMed] DOI:10.1007/s12149-013-0781-x
- Lin Y, Li T, Liang J, et al. Predictive value of preablation stimulated thyroglobulin and thyroglobulin/thyroid-stimulating hormone ratio in differentiated thyroid cancer. Clin Nucl Med 2011;36:1102–5. [PubMed] DOI:10.1097/RLU.0b013e3182291c65
- Li T, Lin Y, Liang J, et al. The value of pre-ablation stimulated thyroglobulin in predicting distant metastasis of papillary thyroid cancer. Zhonghua He Yi Xue Yu Fen Zi Ying Xiang Za Zhi (in Chinese) 2012;32:189–91. DOI:10.3760/cma.j.issn.2095-2848.2012.03.007
- Zhao T, Liang J, Guo Z, et al. Serum thyrotropin level of 30 μIU/mL is inadequate for preablative thyroglobulin to serve as a prognostic marker for differentiated thyroid cancer. Endocrine 2016;53:166–73. [PubMed] DOI:10.1007/s12020-015-0842-0
- Zhao T, Liang J, Li T, et al. Value of serial preablative thyroglobulin measurements: can we address the impact of thyroid remnants?. Nucl Med Commun 2016;37:632–9. [PubMed] DOI:10.1097/MNM.0000000000000485
- Lee JI, Chung YJ, Cho BY, et al. Postoperative-stimulated serum thyroglobulin measured at the time of 131I ablation is useful for the prediction of disease status in patients with differentiated thyroid carcinoma . Surgery 2013;153:828–35. [PubMed] DOI:10.1016/j.surg.2012.12.008
- González C, Aulinas A, Colom C, et al. Thyroglobulin as early prognostic marker to predict remission at 18-24 months in differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2014;80:301–6. [PubMed] DOI:10.1111/cen.12282
- Piccardo A, Arecco F, Puntoni M, et al. Focus on high-risk DTC patients: high postoperative serum thyroglobulin level is a strong predictor of disease persistence and is associated to progression-free survival and overall survival. Clin Nucl Med 2013;38:18–24. [PubMed] DOI:10.1097/RLU.0b013e318266d4d8
- Giovanella L, Ceriani L, Suriano S, et al. Thyroglobulin measurement before rhTSH-aided 131I ablation in detecting metastases from differentiated thyroid carcinoma . Clin Endocrinol (Oxf) 2008;69:659–63. [PubMed] DOI:10.1111/j.1365-2265.2008.03244.x
- Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 1998;83:1121–7. [PubMed] DOI:10.1210/jcem.83.4.4683
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1–133. [PubMed] DOI:10.1089/thy.2015.0020
- Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839–43. [PubMed] DOI:10.1148/radiology.148.3.6878708
- Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol (Oxf) 2005;63:87–93. [PubMed] DOI:10.1111/j.1365-2265.2005.02304.x
- Lee J, Soh EY. Differentiated thyroid carcinoma presenting with distant metastasis at initial diagnosis clinical outcomes and prognostic factors. Ann Surg 2010;251:114–9. [PubMed] DOI:10.1097/SLA.0b013e3181b7faf6
- Makarewicz J, Adamczewski Z, Knapska-Kucharska M, et al. Evaluation of the diagnostic value of the first thyroglobulin determination in detecting metastases after differentiated thyroid carcinoma surgery. Exp Clin Endocrinol Diabetes 2006;114:485–9. [PubMed] DOI:10.1055/s-2006-951778
- Wu S, Wang H. Efficacy analysis of 131I therapy and predictive value of preablation stimulated thyroglobulin for lung metastases from differentiated thyroid cancer . Ann Endocrinol (Paris) 2013;74:40–4. [PubMed] DOI:10.1016/j.ando.2012.11.007
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003;13:3–126. [PubMed] DOI:10.1089/105072503321086962
- Suh I, Vriens MR, Guerrero MA, et al. Serum thyroglobulin is a poor diagnostic biomarker of malignancy in follicular and Hurthle-cell neoplasms of the thyroid. Am J Surg 2010;200:41–6. [PubMed] DOI:10.1016/j.amjsurg.2009.08.030
- Phan HT, Jager PL, van der Wal JE, et al. The follow-up of patients with differentiated thyroid cancer and undetectable thyroglobulin (Tg) and Tg antibodies during ablation. Eur J Endocrinol 2008;158:77–83. [PubMed] DOI:10.1530/EJE-07-0399
- Spencer CA, LoPresti JS, Fatemi S, et al. Detection of residual and recurrent differentiated thyroid carcinoma by serum thyroglobulin measurement. Thyroid 1999;9:435–41. [PubMed] DOI:10.1089/thy.1999.9.435
- Carril JM, Quirce R, Serrano J, et al. Total-body scintigraphy with thallium-201 and iodine-131 in the follow-up of differentiated thyroid cancer. J Nucl Med 1997;38:686–92. [PubMed]
