Role of tumor microenvironment in triple-negative breast cancer and its prognostic significance
Introduction
Breast cancer is a heterogeneous disease that encompasses several distinct entities with remarkably different biological characteristics and clinical behavior (1). Based on the expression of hormone receptors (HRs) (estrogen and progesterone) and human epidermal growth factor receptor (HER2), breast cancer can be classified into four subtypes: Luminal A, Luminal B, HER2-enriched, and triple-negative (2). The definition of triple-negative breast cancer (TNBC) is now commonly used to describe breast cancer subtypes that are estrogen receptor (ER) negative, progesterone receptor (PR) negative, and HER2 negative according to the clinically available immunohistochemical (IHC) staining methods for these biomarkers (3). Notably, TNBC is often, but not always, a basal-like breast cancer (4). Specifically, the triple-negative and basal-like phenotypes are similar (5) because the majority of basal-like cancers are also TNBCs and most TNBCs (approximately 80%) are also basal-like breast cancers (6).
Although TNBCs comprise 10%–15% of all breast cancers, patients with triple-negative tumors have a relatively poor outcome and cannot be treated with endocrine therapy or therapies that target HER2 (4). Because the tumor microenvironment is increasingly recognized as a major regulator of carcinogenesis (7), a growing number of studies have focused on the tumor environment to explore the complex mechanisms underlying tumorigenesis and disease progression, identify new biomarkers or target in stromal components to predict clinical outcome and guide therapy in TNBCs (8-10). In addition to neoplastic cells, the breast microenvironment consists of extracellular matrix (ECM) and numerous stromal cell types, including endothelial cells, immune cells, fibroblasts, and adipocytes (11,12). Moreover, the cells of the tumor microenvironment communicate via soluble mediators or intercellular receptor-ligand interactions.
The different subtypes of breast cancer may require distinct contributions from the microenvironment to undergo malignant progression (10), and we believe that cell-cell interactions investigated in co-culture conditions can conveniently and directly elucidate this contribution. Therefore, understanding the unique expression profiles of cell types, the differential expression of genes, and the role of tumor stroma and internal cells that distinguish TNBC from other subtypes of breast cancer, such as luminal subtype cancer, will help elucidate how the tumor microenvironment may contribute to faster progression and poorer prognosis of TNBC and even enable treatments to be more effectively tailored to patients (Table 1).
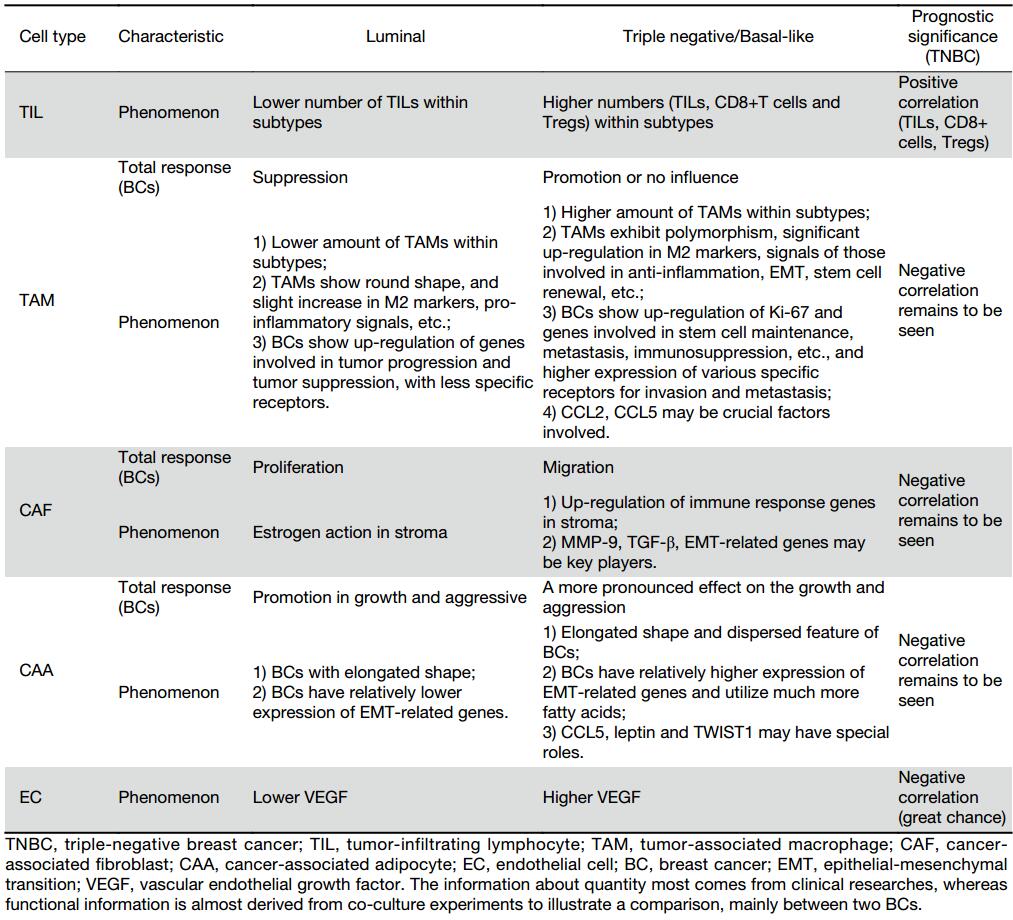
Full table
Tumor-infiltrating lymphocytes (TILs)
Characteristics of TILs in TNBC
TILs are increasingly recognized as critical players in the breast tumor microenvironment (13). These TILs consist primarily of T cells and are frequently observed in breast cancers (14). All tumor subtypes generally exhibit a more pronounced infiltration at the invasive margin than in the tumor center (15). Most importantly, large variations were observed within each breast tumor subtype, especially in the quantity rather than the functionality of T cells (15).
As one of the most aggressive breast cancer phenotypes, TNBC exhibits a higher rate of TIL infiltration within the tumor environment (16), possibly as a result of somatic mutations that lead to the emergence of neo-antigens, which exacerbate the immune response (17). High-TIL tumors are more likely to be ER negative (18), and conspicuous T cell infiltration common among triple-negative and/or basal-like cancers (19). This feature may suggest that the underlying biological properties of various tumor subtypes be reflected by the density of infiltrated immune cells. TNBCs are also more likely to have >50% lymphocytic infiltrate, termed lymphocyte-predominant breast cancer (LPBC) (16), almost three times that observed in HR+ disease (20). When comparing luminal and non-luminal tumors, the latter has a significantly lower ratio in the tumor center but a higher ratio at the invasive margin (15).
The population of tumor-infiltrating T cells mainly composes of CD8+ cytotoxic T cells, CD4+ helper T cells and CD4+ regulatory T cells (Tregs). CD8+ T cells are the key effector cell population that mediate effective anti-tumor immunity, which improves clinical outcomes (21). The total number of CD8+ cells inversely correlates with ER and PR expression (22). Moreover, the density of CD8+ TILs is apparently higher in TNBCs (20).
Tregs are thought to protect the host and maintain systemic immune homeostasis to prevent autoimmune disease by suppressing self-reactive cells (23). In the tumor environment, T cells mediate the most important immunological response in tumor growth in the early stages of cancer but change to Tregs after chronic stimulation and interactions with tumor cells, which block anti-tumor responses (23) and promote, rather than inhibit, cancer development and progression (24). FoxP3 is a marker of immunosuppressive CD25+CD4+ regulatory T cells (25). The median number of FoxP3+T cells also directly correlates with prognosis (26). Specifically, TNBCs tend to have a higher average number of FoxP3-positive cells than other subtypes (20,26,27), and the ratio of FoxP3+ to CD8+ cells is the highest in basal-like tumors and the lowest in luminal A tumors both in the tumor and at the invasive margin (15). Moreover, luminal tumors generally have lower ratios than their non-luminal counterparts, especially at the tumor center (15).
The proportion of immune cells may also differ by sites: breast tumors tend to have a higher ratio of FoxP3+ cells to CD8+ cells in the tumor center, which corroborates other reports showing that tumors can recruit regulatory T-cells as part of their immune escape mechanism and are better adapted to the tumor microenvironment (15). Adaptive immunity seems to be much stronger in TNBCs. Since incidence of chromosomal instability is significantly higher in TNBC (28), a greater number of mutations increase the possibility that mutated protein sequences will be expressed and potentially recognized as novel antigens by the immune system, thereby inducing a stronger immune response to fight the tumor (16).
Prognostic value of TILs in TNBC
Studies of TILs in breast cancer have come to inconsistent conclusions about prognosis and outcome, which may be due to the following: 1) differences in defining, measuring and analyzing TILs; 2) differences in the examination of intratumoral infiltrating lymphocytes (iTILs), stromal infiltrating lymphocytes (sTILs) and total infiltrating lymphocytes (tTILs); or 3) examining only total T lymphocytes (which might include larger numbers of regulatory T cells that could to some extent reflect immune suppression) without distinguishing cell subtypes. Thus, the extent to which TILs contribute to tumor progression and clinical outcome in breast cancer has remained controversial, possibly because the effect is limited to certain subgroups of patients, such as those with TNBC. In the context of neoadjuvant chemotherapy (NAC), the results are different if other subtypes are included. However, higher TILs in TNBCs predicted a higher pathologic complete response (pCR) rate (29). Measuring TILs before NAC, after NAC and after post-NAC surgery all seem to have a favorable prognostic impact on TNBC, suggesting that TILs may represent a surrogate marker for survival and measuring treatment efficacy (30,31).
In the adjuvant chemotherapy setting for TNBC, increasing lymphocytic infiltration has been associated with excellent prognosis (32). More specifically, each 10% increase in intratumoral and stromal lymphocytic infiltration was associated with 17% and 15% reduced recurrence-free survival (RFS), respectively, and 27% and 17% reduced overall survival (OS) in TNBCs, and these reductions were significant irrespective of chemotherapy type (32). The following year, stromal, but not intratumoral lymphocytic infiltration, was confirmed to constitute a robust prognostic factor in TNBC in Eastern Cooperative Oncology Group (ECOG) 2197 and ECOG 1199, which obtained similar figures as BIG 02-98 (24). In a multivariate analysis, Dieci et al. (18) added further evidence to support the strong prognostic role of TILs (both iTIL and sTIL) for OS in TNBC. Notably, the TNBC low-TIL (<10%) group tended to relapse earlier (30).
Recently, based on scoring methods recommended by the International TIL Working Group 2014, sTILs and iTILs did not significantly correlate with RFS and OS (all P>0.05) (33), which may have been due to the small sample size. Using the same guidelines, one of two studies demonstrated that TILs were a powerful prognostic marker in patients with TNBC, independent of traditional clinicopathological characteristics (34). Another study showed that sTILs were associated with the prognosis of patients with TNBC and could serve as an independent prognostic biomarker in TNBC because the number of sTILs was directly correlated with prognosis (35). Furthermore, a pooled analysis of twenty-five published studies examining 22,964 patients (36) suggested that TILs are prognostic markers for both DFS and OS only in TNBC patients (36).
Based on these data, sTILs have been related to improved outcome. However, the results regarding iTILs have been mixed and are consequently unreliable, which may be due to a variety of factors, including the use of various TIL markers, variable cut-offs to define low or high TIL quantity or the varying definitions of the location of TILs, with peritumoral TILs often considered to be intratumoral (37).
Moreover, CD8+ T lymphocytes are an independent prognostic factor associated with better survival in only basal-like breast cancer but not in non-basal TNBCs, as demonstrated in a cohort of 3,403 patients with breast cancer (38). In a previous study of 1,334 pan-breast tumors, total CD8+ T cell counts were significantly associated with better breast cancer-specific survival (BCSS) in the basal phenotype but not the ER-positive counterparts (22). In a study of 4 cohorts consisting of more than 12,000 patients, the presence of stromal and intratumoral CD8+ TILs was independently associated with a reduced risk of death from breast cancer in ER-negative patients, whereas in the benefit of intratumoral CD8+ TILs was limited in ER-positive breast cancers (39).
Because CD4+ T cells change their dominant subsets from Th1 in the early stages to Treg and Th17 cells in the late stages of cancer progression (21), their roles are very complex and difficult to confirm (36). Little is known about the dynamic and functional alterations of these CD4+ T cell subsets in the immune-editing processes during breast cancer progression (21). Based on IHC staining for CD4 and CD8, high levels of CD8+ TILs and CD4+ TILs as well as the combination of high CD4+/high CD8+ TILs are good prognostic indicators in TNBC (37). Interestingly, the functions of Tregs in TNBC are mixed. Although Tregs have been reported to be negative regulators in the tumor microenvironment, two recent studies reported different results: Lee et al. (40) showed that FoxP3 expression was a favorable prognostic indicator for patients with TNBC in their study. Specifically, the OS of patients with TNBC ≥15 FoxP3-positive cells per 10 high-power fields was significantly better than that of patients with TNBC ≤15 FoxP3-positive cells. Moreover, West et al. discovered that the number of FoxP3+ T cells level directly correlated with prolonged RFS in TNBC, particularly among basal-like breast cancer, for which the FoxP3 status is an independent prognostic factor (41). Therefore, anti-tumor immunity may be sufficiently strong in TNBC, despite the existence of Tregs, which may partly explain the contradictory results. Of note, the association of FoxP3+ TILs with good outcome depended on the presence of large numbers of CD8+ TILs (41). Additionally, although the CD8+/FoxP3+ ratio has been used to evaluate the prognosis of patients with breast cancer (26), this ratio may not be an appropriate parameter for prognosis due to two reasons: 1) the presence of Tregs actually represents a natural secondary consequence of an active immune response (42), and 2) persistent high-level PD-1 expression on antigen-experienced CD8+ T cells leads to “T cell exhaustion”, namely, a CD8+ T cell phenotype characterized by impaired effector function and the persistent expression of inhibitory receptors (43).
Considering the limited data and corresponding mixed results, more prospective or retrospective studies of larger patient populations are warranted to delineate the prognostic value of TILs in TNBC. To this end, better methods and techniques will be needed to analyze TILs in TNBC.
Tumor-associated macrophages (TAMs)
Characteristics of TAMs in TNBC
TAMs originate from blood monocytes and are recruited to the tumor site by factors secreted by the tumor or stromal cells, such as CCL2 (44). In general, macrophages can be schematically identified as M1 (classically activated) and M2 (alternatively activated) (23,45).
TAMs have been suggested to be biased towards an M2 phenotype but contain elements of both M1 and M2 (23). Breast cancer cells secrete factors that drive macrophages toward M2 differentiation, and this effect was most pronounced for MDA-MB-231 cells, the most aggressive breast cancer cell type (46,47). The polarization toward M2 is mainly mediated by Th2 cytokines, such as IL-4, and anti-inflammatory cytokines, such as IL-10, transforming growth factor-β (TGF-β) and M-CSF (48). Compared to other subtypes, TNBCs secrete more G-CSF, thereby priming M1 cells to promote tumor growth by skewing them to an anti-inflammatory phenotype (M2) (49).
M1-type macrophages are commonly known to facilitate tumor destruction via pro-inflammatory effects and promote anti-tumor immune responses. However, macrophages in the tumor microenvironment largely exhibit the M2 phenotype and express high levels of cytokines, growth factors and proteases, and their immunophenotype is distinct from that of other tissue macrophages (45). They also stimulate angiogenesis, contribute to matrix remodeling, enhance tumor cell migration and invasion, and promote immunosuppression (50,51). In other words, they mediate so-called pro-oncogenic functions, such as cell division, metastasis, and the survival of tumor cells. However, both the complexity of macrophage polarization and the impact of macrophage localization within the tumor microenvironment lead the contributions of macrophages to breast cancer growth and progression, which are likely quite intricate (52). Moreover, distinct patterns of cytokine secretion and stimulated macrophage migration were observed in basal-like breast cancer (53). Previous studies examining CCL2 expression in mammary tumors showed that CCL2 was expressed in macrophages and that this expression correlated with macrophage recruitment (44,54). However, one study indicated that CCL2 likely is not the only and/or the most crucial factor involved in macrophage attraction to the breast tumor site (55). Recently, a study showed that in vivo CCL2 gene silencing inhibited MDA-MB-231 primary tumor growth and metastasis, which was associated with a reduction in cancer stem cell renewal and the recruitment of M2 macrophages (56).
With respect to cell density, Medrek et al. (47) stained M2 macrophages for CD163 to show that CD163+ cells were in particularly abundant in triple-negative/basal-like breast cancer. They also showed that in tumor stroma, but not in the tumor nest, M2 macrophages positively correlated with several parameters, including ER negativity and triple-negative/basal-like breast cancer, whereas they inversely correlated with Luminal A breast cancer. This result is partly explained in a co-culture experiment described below (57). Another study confirmed that triple-negative/basal-like breast tumor stroma contained more CD163+ and CD68+ cells than the stroma of luminal A tumors, and the proportion of CD163+ cells was higher than that of CD68+ cells (46). However, employing CD163 as a marker might not be the best way to ensure pure M2. It would be necessary to perform immunostaining for better markers to identify both macrophage subtypes separately and distinguish their expression patterns from a pan-macrophage marker (58). Additionally, the number of TAMs and the expression levels of the cytokine IL-6 and chemotactic factor CCL5 increased in patients with TNBC after the surgery (59).
In the breast tumor microenvironment, the diverse interactions between macrophages and cancer cells gradually shed light on the microenvironment. Because breast cancer is a disease of high heterogeneity, the cross talk between these kinds of cells may vary. A significant difference in the effects of these two distinct breast tumor cells on TAMs was observed when macrophages co-culturing with the HR+ breast tumor cell lines T47D and TNBC, which is counterpart of MDA-MB-231 respectively (57): 1) morphology and macrophage mannose receptor (MMR); 2) gene expression; 3) activated biological pathways that modulate pro- or anti-inflammatory responses; and 4) the secretion of pro-inflammatory factors. More specifically, more aggressive performance are inclined to appear in macrophages co-cultured with MDA-MB-231, including apparent change in morphology, significantly higher level of MMR and other M2 markers, pro-inflammatory factors, especially CCL2 (57). Conversely, ER+ and TNBC breast cancer cell lines were also distinctly influenced by the presence of TAMs, which influenced tumor progression, Ki-67 expression, and the expression of several other genes (57). Compared with luminal cell lines, basal-like breast cancer cell lines preferentially expressed a broader range of receptors that are mostly associated with tumor invasion and metastasis in response to macrophage-derived cytokines, including hepatocyte growth factor receptor (HGFR, also known as MET), CD44, epithelial growth factor receptor (EGFR), oncostatin M receptor (OSMR) and transforming growth factor receptor 2 (TGFBR2) (60).
Prognostic value of TAMs in TNBC
Although CD163+ macrophages in the tumor stroma correlated with triple-negative/basal-like breast cancer, they did not have prognostic value (47). However, their prognostic value was proved in luminal A. Medrek et al. thought that the small size of the group and their worse overall prognosis impeded the significant value. Two studies showed that high levels infiltrating TAMs are an unfavorable prognostic factor for patients with TNBC (61), especially in the high infiltrated group (59,61), but both studies used CD68 as a marker. Moreover, patients with high levels of infiltrating CD68+ TAMs express higher levels of IL-6 and CCL5 (59), which are well known to correlate with poor prognosis.
Recently, a signature consisting of the metastasis suppressor Raf Kinase Inhibitory Protein (RKIP) was identified to promote the recruitment of TAMs via chemokines, such as CCL5, and pro-metastatic TAM factors were able to predict survival in patients with TNBC (62). Additionally, analyzing the localization rather than only the presence of TAMs is important (47). Specifically, the prognostic value of CD163+ macrophages in a larger triple-negative/basal-like cohort remains to be seen, and the location and presence of TAMs in TNBC need to be correlated with clinical prognosis.
Cancer-associated fibroblasts (CAFs)
Characteristics of CAFs in TNBC
CAFs, the most common component of tumor stroma, especially in breast cancers, have been found to play a critical role in the breast tumor environment (50,63). The origin of CAFs is complex and debated. For example, CAFs may derive from resident fibroblasts, bone marrow-derived mesenchymal stem cells, hematopoietic stem cells, epithelial cells (epithelial-mesenchymal transition), and endothelial cells (endothelial-mesenchymal transition) (64). They not only promote cancer initiation, progression, invasion, and metastasis (50) but are also involved in series of microenvironmental events, including angiogenesis, ECM remodeling, the deposition of basement membrane components, cancer-associated inflammation and the regulation of differentiation events in associated epithelial cells, which are all known to be associated with cancer pathogenesis (65,66). A specific marker is necessary to detect CAFs in the tumor, and the most widely used such marker is α-smooth muscle actin (α-SMA) (64).
Recently, a study showed that CAFs may enhance TNBC progression by activating TGF-β (67). CAFs have also been proposed to engage in crosstalk to influence other cells in the breast tumor microenvironment. Moreover, co-culture experiments showed that interactions between basal-like cancer cells and fibroblasts induced the expression of numerous interleukins and chemokines, including IL-6, IL-8, CXCL1, CXCL3, and TGF-β (68). Moreover, CXCL16 expressed by myeloid cells activated CAFs to recruit more myeloid cells and fibroblasts in TNBC (69). Podoplanin expression in stromal breast cancer-associated fibroblasts was associated with higher grade and TNBC (70). Furthermore, galectin-1, whose expression is higher in CAFs than in normal counterparts, regulates CAF activation and promotes MDA-MB-231 metastasis by up-regulating matrix metalloproteinase 9 (MMP-9) expression (71). In vivo, the expression of syndecan-1 by stromal fibroblasts enhanced MDA-MB-231 breast cancer cell growth and angiogenesis (72). Metabolically, both basal-like breast cancer cells and co-cultured CAFs were proven to interact and shown to exhibit higher glucose up-take, glucose oxidation and glycogen synthesis than luminal cells (73).
Tchou et al. (74) were the first to show that CAFs derived from HER2+ breast cancers significantly augmented the invasive properties of the tumor cells via pathways associated with cancer cell migration, and these cells were more invasive than those from TNBC and ER-positive type cancers. Similar results were recently reported: high levels of all CAF-related proteins, such as platelet-derived growth factor receptor alpha (PDGFRα), PDGFRβ and fibroblast activation protein alpha (FAPα) were reportedly associated with tumor invasiveness and more likely to be found in the HER-2 subtype than in TNBC (75).
Prognostic value of CAFs in TNBC
The subtypes of breast tumor stroma may be able to predict outcome (76), which suggests that fibroblast interactions specifically influence TNBC. In adjacent stroma, TNBCs were associated with the up-regulation of genes related to the immune response, whereas luminal breast cancers were more commonly associated with estrogen-response pathways (10). In vitro, under the influence of fibroblasts, luminal lines were more likely to exhibit altered proliferation, whereas basal-like breast cancer cell lines exhibited increased migration (68). Up-regulation of genes like TGFB1, TWIST and epithelial-mesenchymal transition (EMT) related pathways were detected (68). These unique interactions between fibroblasts and cancer cells are likely to be characteristic of the general microenvironmental of TNBC, which markedly worsens the prognosis of patients with TNBC. Moreover, these interactions can partly account for the risk of loco-regional recurrence and provide novel insights into the progression of TNBC. Interestingly, patients with TNBC harboring stroma-rich tumors (≥50% stroma) were found to have a poorer outcome than patients harboring tumors with small amounts of stroma (77). Opposite result was observed in ER positive breast cancers (78).
A previous study showed that myofibroblasts are an important predictor of unfavorable prognosis for patients with invasive breast cancer (79). Accordingly, co-culturing MDA-MB-231 cells with CAFs sharply increased the amount of α-SMA-positive stromal elements, indicating the acquisition of a myofibroblast-like phenotype, whereas MCF-7 breast cancer cells were not able to influence the α-SMA expression of the tumor-associated fibroblast population (80). Moreover, Witkiewicz et al. revealed that patients with TN and high-levels of stromal Cav-1 had a good clinical outcome, suggesting that the stromal Cav-1 levels had prognostic value (81). Genes that encode membrane protein and factors secreted from CAFs are also likely associated with chemotherapy resistance. For example, 6 candidate genes expressed by CAFs co-cultured with MDA-MB-231 cells may correlate with chemotherapy resistance (82).
MMPs are predominantly synthesized by fibroblasts (12) and primarily mediate ECM degradation and remodeling (83). These proteins shape the tumor microenvironment and drive cancer progression and metastasis (84) by targeting and cleaving a wide range of substrates in the ECM (85). In addition to these functions, MMPs can contribute to tumor progression by increasing tumor cell growth, inflammation and promoting angiogenesis (86). The activity of stromal cell-derived MMPs is often increased in breast cancer, and the stratification of MMP types may serve as a prognostic factor (87). Although MMP1 and MMP2 expression were significantly up-regulated in tumors known to exhibit more aggressive metastatic behavior, their expression was the highest in luminal B tumors (88). Of note, MMP-9 was produced mainly by the tumor cells, and its expression was the highest in human basal-like and triple-negative tumors (89,90) and negatively correlated with prognosis (91).
Thus, CAFs may aid the clinical diagnosis and prognosis of breast cancer. CAF-derived molecular markers and bio-products as well as gene expression signature are widely used to evaluate clinical outcome. Because the complex biological mechanism of CAFs is not well studied within breast tumor subtypes, further research on detection and evaluation of their unique markers may hopefully provide information for the prognosis of breast cancer and elucidate the distinct interaction between CAFs and specific subtypes of tumors.
Cancer-associated adipocytes (CAAs)
In breast cancer, stromal cells also include resident adipocytes, but these cells have not been extensively studied. Nevertheless, adipocytes can produce hormones, growth factors, cytokines, and so-called adipokines (92). A previous study demonstrated that invasive breast cancer cells at least partly impacted surrounding adipocytes, which exhibited a modified phenotype and specific biological features, and these changes were sufficient to name these cells CAA (93). In return, CAAs increased the aggressiveness of cancer cells (94). However, the molecular mechanism by which adipocytes promote tumorigenesis remains largely unknown (93), especially in various subtypes.
CAA-conditioned medium promoted the migration of breast cancer cells via IL-6 (94,95) and CCL2 (95), and CAAs were shown to promote breast tumor radioresistance (96). Furthermore, co-cultured with adipocytes induced elongated shape and dispersed feature, pronounced advance in aggressiveness, and higher expression of EMT-related genes such as Twist related protein 1 (TWIST1) in MDA-MD-231 cells (97). Adipocytes also enhanced MDA-MB-231 cancer cell invasiveness, and this effect was induced, at least in part, by CCL5, which negatively correlated with OS (98). Besides, leptin, an adipokine, maintained cancer stem cell-like properties in TNBC cells and mediated tumor recurrence and metastasis (99). Metabolically, breast cancer cells can stimulate release of fatty acids from adipocytes, and co-culture with adipocytes promoted the growth and migration of both MCF-7 and MDA-MB-231 cells. However, adipocyte-released fatty acids were more readily transferred to and utilized by MDA-MB-231 cells than MCF-7 cells (100). Thus, adiposity may contribute to the production of inflammation-related factors, which may worsen the prognosis (101).
Endothelial cells
Endothelial cells are well investigated in breast cancer. For example, increased endothelial cell retraction is known to be tightly associated with the enhanced adhesion of tumor cells and their invasion into the endothelial monolayer, and tumor cells can also induce the contraction of endothelial cells (102). Vascular endothelial growth factor (VEGF) is the most important pro-angiogenesis factor and highly dysregulated in TNBC (103). Specifically, VEGF binds to its receptor on the surface of endothelial cells to affect the tumor. VEGF can induce the adhesion and migration of MDA-MB-231 cells when co-cultured with endothelial cells, whereas MDA-MB-231 cells alone were not responsive to VEGF in an invasion assay (102). Additionally, TNBCs can express endothelial markers and acquire the ability to form vascular-like channels via endothelial cell differentiation both in vitro and in vivo, generating blood lacunae surrounded by tumor cells (104). Of note, the intratumoral levels of VEGF were significantly higher in TNBC than in non-TNBC tumors (105), i.e., 3 and 1.5 times higher in TNBC than in the ER/PR-positive group and the HER2-positive group, respectively. However, the prognostic significance of VEGF in TNBC seems to be established (106,107), and microvessel density may be a better surrogate.
ECM
The ECM plays a multifaceted role in both normal breast tissue homeostasis and the breast tumor microenvironment due to its diverse nature, composition and cellular changes (85). Specifically, the ECM is a complex network of various proteins with structural and regulatory function (108), and the protein composition and physical properties of the ECM govern cell destiny via biochemical and biomechanical mechanisms (109). The ECM was shown to consist of three main types of proteins with distinct roles: structural proteins (e.g., collagen and elastin), specialized glycoproteins (e.g., fibronectin) and proteoglycans (85,110). Collagen, which is the main component of the ECM (111), provides tissues with strength and resilience, and specialized glycoproteins are important for proper cell-ECM adhesion, whereas the passage of many cytokines and growth factors between cells is controlled by proteoglycans (85). Interactions between each kind of protein also widely facilitate a favorable microenvironment for tumor growth (83). Type I collagen is the main structural protein and acts as a physical barrier in the interstitial ECM, whereas type IV collagen is a key component of the basement membrane (BM) and essential for tissue polarity (112). In patient-derived xenograft models, TNBC tumors exhibited collagen accumulation (67), and type I collagen was shown to induce apoptotic cell death in luminal-like breast carcinoma cells but not in basal-like breast carcinoma cells (113). In MDA-MB-231 breast cancer cells, type I and type IV collagen may augment the aggressive characteristics of cancer cells (114,115).
EMT, which induced by a series of factors (e.g., Snail), is characterized by a loss of epithelial cell polarity and the development of a migratory and invasive mesenchymal phenotype (116,117). Studies of TNBC showed that a loss of membranous E-cadherin and Snail2 expression is significantly associated with high-grade TNBCs (118). Furthermore, c-Met signaling pathways are known to initiate EMT, and c-Met expression is elevated in 8% of breast cancers (119). However, c-Met is over-expressed in 52% of TNBC patient-derived samples, and the OS and RFS of these patients are shorter (120).
Studies of biomechanical factors, such as the stiffness of the ECM, have attracted increasing attention. Specifically, the biomechanical properties of the ECM change under pathological conditions. For example, the ECM contributes to mammary gland stiffening as cells transition from normal to invasive carcinoma (121), and TNBCs showed a significant surge of myeloid cells and an increase in the number of fibroblasts, which drive ECM remodeling by increasing matrix stiffness (122). Moreover, the stroma of the more aggressive basal-like and HER2 tumor subtypes was significantly associated with collagen deposition and matrix stiffness compared to the less aggressive Luminal A and B subtypes (123). In TNBC, stromal stiffening positively correlated with not only TGF-β signaling, which stimulated the production of collagen but also TAMs infiltration (123). Levental et al. (124) demonstrated that the overexpression of lysyl oxidase (LOX) can increase ECM stiffness to ultimately promote tumor cell invasion and progression. Therefore, the increase in tissue stiffness can be partly attributed to excess LOX activity, which cross-links collagen fibers and other ECM components (110). Previously, a tougher ECM was thought to more efficiently block crosstalk between cells and the passage of soluble factors, but recent data suggest that more aggressive breast cancer subtypes likely have a stiff stroma. Thus, increased ECM rigidity may alter mechanosignaling, vascular distribution and pro-tumorigenic immune infiltration, which may all facilitate the transition to an invasive phenotype (122). This novel aspect warrants further investigation.
In breast cancer, many ECM proteins are significantly deregulated, and specific matrix components promote tumor progression and metastatic spread (109). Because metastasis is the main cause of death in TNBC, the aberrant ECM may be a therapeutic target to alleviate migration and assess treatment efficacy in this area of breast cancer (122).
Metastatic microenvironment
The “seed and soil” hypothesis (125) suggests that metastatic tumor cells (seeds) spread to certain organs (soils) because of their specific microenvironment, which results in organ-preference patterns in tumor metastasis (126), and the metastatic patterns of breast cancer subtypes may differ significantly. For instance, previous studies showed that Luminal A subtypes have a propensity for bone metastases, whereas basal-like breast cancers are more prone to metastasize to the brain and lung than other subtypes (127-130). Thus, the section below focuses on brain metastasis.
Patients with brain metastases usually have the least favorable outcomes, and brain metastases are associated the expression of breast cancer-related proteins nestin, prominin-1 (CD133) and CK-5 (127) by the primary tumor. Nestin is considered as a marker of neural stem cells (131), and CD133+ cells were reported to correlate with brain tumors (e.g., glioblastoma) (132). Breast cancer cells that express these markers may share characteristics with neural stem cells, and the brain microenvironment may consequently favor the development of brain metastases (127). Thus, the interaction between breast cancer cells and cells abundant in the brain, such as astrocytes and microglia, needs to be explored. VEGF may contribute to the formation of brain metastases by MDA-MB-231 cells by enhancing the transendothelial migration of tumor cells via the up-regulation of endothelial permeability (102). After arriving in the brain microenvironment, astrocytes release cytokines that facilitate colonization by stimulating the growth of tumor cells (133). Accordingly, co-culture with astrocytes increased the expression of survival genes, including GSTA5, BCL2L1, and TWIST1, in MDA-MB-231 cells and activated pathways related to chemotherapy resistance (134). However, continuous contact was required for this effect (134). Moreover, astrocyte-conditioned tumor cells become highly migratory and invasive, which promotes the metastasis of MDA-MB-231 cells to the brain (135), and this effect is mediated by astrocyte-secreted MMP-2 and MMP-9 (135). These data suggest that astrocytes directly influence tumor growth and metastasis.
In addition to characterizing the metastatic microenvironment, differences in the features of TILs in the microenvironment between primary tumors and paired metastatic tumors need to be identified in TNBCs. Unfortunately, metastatic biopsy samples are very rare, which has hindered research progress in this area. Therefore, most studies in this field refer to various first metastatic sites and all breast cancer subtypes. Specifically, metastatic sites were shown to contain significantly fewer TILs than the primary tumor (136-138), suggesting that tumor progression and metastasis may increase the immune response. Interestingly, the composition of the metastatic TIL population and the subsets of these in the primary site remained almost unaltered in their paired metastatic tumor, irrespective of the metastatic location, which suggests that the primary tumor plays an intrinsic role in influencing immune composition (136). In a breast cancer subtype analysis, primary TNBCs contained greater numbers of all types of TILs than their paired metastases (137), whereas metastatic TNBCs contained fewer TILs than luminal MBCs (137).
Thus, the prognostic value of lymphocytic infiltration in metastatic tumors warrants exploration. For example, a study of ER-negative breast cancers demonstrated that a higher percentage of TILs in metastatic breast tumors indicated a better prognosis (138), whereas another study suggested that lower ratios of CD8+/FoxP3+ T cells in MBCs might be associated with improved survival after the development of the metastasis (137).
Conclusions
The tumor microenvironment of TNBC significantly not only influences the malignant behavior and growth of mammary cancer cells but also re-programs the surrounding cells. Unique cell-cell interactions in TNBC distinguish this disease from other subtypes, and the phenotypes and gene expression changes invoked by cell-cell interactions, which may be associated with ER, PR and HER2 status, maintain the microenvironment. TNBC tumors more effectively re-program surrounding cells, which normally counteract the progression of cancer cells, to develop unique signaling pathways that may sometimes form a positive feedback loop. Thus, understanding the characterization of this tumor microenvironment may provide the accurate prediction of prognosis, and studying the unique characteristics of TNBC may shed light on the heterogeneity of this tumor.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ibrahim E, Al-Gahmi AM, Zeenelin AA, et al. Basal vs. luminal A breast cancer subtypes: a matched case-control study using estrogen receptor, progesterone receptor, and HER-2 as surrogate markers. Med Oncol 2009;26:372–8. [PubMed] DOI:10.1007/s12032-008-9131-6
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61–70. [PubMed] DOI:10.1038/nature11412
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429–34. [PubMed] DOI:10.1158/1078-0432.CCR-06-3045
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363:1938–48. [PubMed] DOI:10.1056/NEJMra1001389
- Kreike B, van Kouwenhove M, Horlings H, et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res 2007;9:R65. [PubMed] DOI:10.1186/bcr1771
- Weigelt B, Baehner FL, Reis-Filho JS. The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: a retrospective of the last decade. J Pathol 2010;220:263–80. [PubMed] DOI:10.1002/path.2648
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [PubMed] DOI:10.1016/j.cell.2011.02.013
- Matsumoto H, Koo SL, Dent R, et al. Role of inflammatory infiltrates in triple negative breast cancer. J Clin Pathol 2015;68:506–10. [PubMed] DOI:10.1136/jclinpath-2015-202944
- García-Teijido P, Cabal ML, Fernández IP, et al. Tumor-infiltrating lymphocytes in triple negative breast cancer: the future of immune targeting. Clin Med Insights Oncol 2016;10 (Suppl 1):31-9.
- Casbas-Hernandez P, Sun X, Roman-Perez E, et al. Tumor intrinsic subtype is reflected in cancer-adjacent tissue. Cancer Epidemiol Biomarkers Prev 2015;24:406–14. [PubMed] DOI:10.1158/1055-9965.EPI-14-0934
- Cichon MA, Degnim AC, Visscher DW, et al. Microenvironmental influences that drive progression from benign breast disease to invasive breast cancer. J Mammary Gland Biol Neoplasia 2010;15:389–97. [PubMed] DOI:10.1007/s10911-010-9195-8
- Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res 2011;13:227. [PubMed] DOI:10.1186/bcr2912
- de la Cruz-Merino L, Barco-Sánchez A, Henao Carrasco F, et al. New insights into the role of the immune microenvironment in breast carcinoma. Clin Dev Immunol 2013;2013:785317. [PubMed] DOI:10.1155/2013/785317
- Matkowski R, Gisterek I, Halon A, et al. The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer Res 2009;29:2445–51. [PubMed]
- Miyan M, Schmidt-Mende J, Kiessling R, et al. Differential tumor infiltration by T-cells characterizes intrinsic molecular subtypes in breast cancer. J Transl Med 2016;14:227. [PubMed] DOI:10.1186/s12967-016-0983-9
- Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer 2016;4:59. [PubMed] DOI:10.1186/s40425-016-0165-6
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565–70. [PubMed] DOI:10.1126/science.1203486
- Dieci MV, Mathieu MC, Guarneri V, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol 2015;26:1698–704. [PubMed] DOI:10.1093/annonc/mdv239
- Rakha EA, Ellis IO. Triple-negative/basal-like breast cancer: review. Pathology 2009;41:40–7. [PubMed] DOI:10.1080/00313020802563510
- Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol 2016;2:1354–60. [PubMed] DOI:10.1001/jamaoncol.2016.1061
- Huang Y, Ma C, Zhang Q, et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015;6:17462–78. [PubMed] DOI:10.18632/oncotarget.3958
- Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29:1949–55. [PubMed] DOI:10.1200/JCO.2010.30.5037
- Allen M, Louise Jones J. Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J Pathol 2011;223:162–76. [PubMed] DOI:10.1002/path.2803
- Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959–66. [PubMed] DOI:10.1200/JCO.2013.55.0491
- Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest 2003;112:1437–43. [PubMed] DOI:10.1172/JCI19441
- Liu F, Lang R, Zhao J, et al. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes . Breast Cancer Res Treat 2011;130:645–55. [PubMed] DOI:10.1007/s10549-011-1647-3
- Bohling SD, Allison KH. Immunosuppressive regulatory T cells are associated with aggressive breast cancer phenotypes: a potential therapeutic target. Mod Pathol 2008;21:1527–32. [PubMed] DOI:10.1038/modpathol.2008.160
- Smid M, Hoes M, Sieuwerts AM, et al. Patterns and incidence of chromosomal instability and their prognostic relevance in breast cancer subtypes. Breast Cancer Res Treat 2011;128:23–30. [PubMed] DOI:10.1007/s10549-010-1026-5
- Ono M, Tsuda H, Shimizu C, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat 2012;132:793–805. [PubMed] DOI:10.1007/s10549-011-1554-7
- Hida AI, Sagara Y, Yotsumoto D, et al. Prognostic and predictive impacts of tumor-infiltrating lymphocytes differ between Triple-negative and HER2-positive breast cancers treated with standard systemic therapies. Breast Cancer Res Treat 2016;158:1–9. [PubMed] DOI:10.1007/s10549-016-3848-2
- Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 2014;25:611–8. [PubMed] DOI:10.1093/annonc/mdt556
- Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013;31:860–7. [PubMed] DOI:10.1200/JCO.2011.41.0902
- Park HS, Heo I, Kim JY, et al. No effect of tumor-infiltrating lymphocytes (TILs) on prognosis in patients with early triple-negative breast cancer: Validation of recommendations by the International TILs Working Group 2014. J Surg Oncol 2016;114:17–21. [PubMed] DOI:10.1002/jso.24275
- Pruneri G, Vingiani A, Bagnardi V, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol 2016;27:249–56. [PubMed] DOI:10.1093/annonc/mdv571
- Tian T, Ruan M, Yang W, et al. Evaluation of the prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers. Oncotarget 2016;7:44395–405. [PubMed] DOI:10.18632/oncotarget.10054
- Mao Y, Qu Q, Chen X, et al. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and Meta-analysis. PLoS One 2016;11:e0152500. [PubMed] DOI:10.1371/journal.pone.0152500
- Matsumoto H, Thike AA, Li H, et al. Increased CD4 and CD8-positive T cell infiltrate signifies good prognosis in a subset of triple-negative breast cancer. Breast Cancer Res Treat 2016;156:237–47. [PubMed] DOI:10.1007/s10549-016-3743-x
- Liu S, Lachapelle J, Leung S, et al. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res 2012;14:R48. [PubMed] DOI:10.1186/bcr3148
- Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 2014;25:1536–43. [PubMed] DOI:10.1093/annonc/mdu191
- Lee S, Cho EY, Park YH, et al. Prognostic impact of FOXP3 expression in triple-negative breast cancer. Acta Oncol 2013;52:73–81. [PubMed] DOI:10.3109/0284186X.2012.731520
- West NR, Kost SE, Martin SD, et al. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer 2013;108:155–62. [PubMed] DOI:10.1038/bjc.2012.524
- Burugu S, Asleh-Aburaya K, Nielsen TO. Immune infiltrates in the breast cancer microenvironment: detection, characterization and clinical implication. Breast Cancer 2017;24:3–15. [PubMed] DOI:10.1007/s12282-016-0698-z
- Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014;146:15–24. [PubMed] DOI:10.1007/s10549-014-2988-5
- Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011;475:222–5. [PubMed] DOI:10.1038/nature10138
- Solinas G, Germano G, Mantovani A, et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009;86:1065–73. [PubMed] DOI:10.1189/jlb.0609385
- Sousa S, Brion R, Lintunen M, et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res 2015;17:101. [PubMed] DOI:10.1186/s13058-015-0621-0
- Medrek C, Pontén F, Jirström K, et al. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012;12:306. [PubMed] DOI:10.1186/1471-2407-12-306
- Laoui D, Movahedi K, Van Overmeire E, et al. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol 2011;55:861–7. [PubMed] DOI:10.1387/ijdb.113371dl
- Hollmén M, Karaman S, Schwager S, et al. G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Oncoimmunology 2015;5:e1115177. [PubMed] DOI:10.1080/2162402X.2015.1115177
- Mao Y, Keller ET, Garfield DH, et al. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev 2013;32:303–15. [PubMed] DOI:10.1007/s10555-012-9415-3
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39–51. [PubMed] DOI:10.1016/j.cell.2010.03.014
- Brady NJ, Chuntova P, Schwertfeger KL. Macrophages: regulators of the inflammatory microenvironment during mammary fland development and breast cancer. Mediators Inflamm 2016;2016:4549676. [PubMed] DOI:10.1155/2016/4549676
- Stewart DA, Yang Y, Makowski L, et al. Basal-like breast cancer cells induce phenotypic and genomic changes in macrophages. Mol Cancer Res 2012;10:727–38. [PubMed] DOI:10.1158/1541-7786.MCR-11-0604
- Fujimoto H, Sangai T, Ishii G, et al. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer 2009;125:1276–84. [PubMed] DOI:10.1002/ijc.24378
- Valković T, Fuckar D, Stifter S, et al. Macrophage level is not affected by monocyte chemotactic protein-1 in invasive ductal breast carcinoma. J Cancer Res Clin Oncol 2005;131:453–8. [PubMed] DOI:10.1007/s00432-004-0667-3
- Fang WB, Yao M, Brummer G, et al. Targeted gene silencing of CCL2 inhibits triple negative breast cancer progression by blocking cancer stem cell renewal and M2 macrophage recruitment. Oncotarget 2016;7:49349–67. [PubMed] DOI:10.18632/oncotarget.9885
- Hollmén M, Roudnicky F, Karaman S, et al. Characterization of macrophage -- cancer cell crosstalk in estrogen receptor positive and triple-negative breast cancer. Sci Rep 2015;5:9188. [PubMed] DOI:10.1038/srep09188
- Yao M, Yu E, Staggs V, et al. Elevated expression of chemokine C-C ligand 2 in stroma is associated with recurrent basal-like breast cancers. Mod Pathol 2016;29:810–23. [PubMed] DOI:10.1038/modpathol.2016.78
- Wang J, Chen H, Chen X, et al. Expression of tumor-related macrophages and cytokines after surgery of triple-negative breast cancer patients and its implications. Med Sci Monit 2016;22:115–20. [PubMed]
- Levano KS, Jung EH, Kenny PA. Breast cancer subtypes express distinct receptor repertoires for tumor-associated macrophage derived cytokines. Biochem Biophys Res Commun 2011;411:107–10. [PubMed] DOI:10.1016/j.bbrc.2011.06.102
- Yuan ZY, Luo RZ, Peng RJ, et al. High infiltration of tumor-associated macrophages in triple-negative breast cancer is associated with a higher risk of distant metastasis. Onco Targets Ther 2014;7:1475–80. [PubMed] DOI:10.2147/OTT.S61838
- Frankenberger C, Rabe D, Bainer R, et al. Metastasis suppressors regulate the tumor microenvironment by blocking recruitment of prometastatic tumor-associated macrophages. Cancer Res 2015;75:4063–73. [PubMed] DOI:10.1158/0008-5472.CAN-14-3394
- Buchsbaum RJ, Oh SY. Breast cancer-associated fibroblasts: where we are and where we need to go. Cancers (Basel) 2016;8. pii: E19.
- Shiga K, Hara M, Nagasaki T, et al. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel) 2015;7:2443–58. [PubMed] DOI:10.3390/cancers7040902
- Luo H, Tu G, Liu Z, Liu M. Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression. Cancer Lett 2015;361:155–63. [PubMed] DOI:10.1016/j.canlet.2015.02.018
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006;6:392–401. [PubMed] DOI:10.1038/nrc1877
- Takai K, Le A, Weaver VM, et al. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget 2016;7:82889–901. [PubMed] DOI:10.18632/oncotarget.12658
- Camp JT, Elloumi F, Roman-Perez E, et al. Interactions with fibroblasts are distinct in Basal-like and luminal breast cancers. Mol Cancer Res 2010;9:3–13. [PubMed] DOI:10.1158/1541-7786.MCR-10-0372
- Allaoui R, Bergenfelz C, Mohlin S, et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat Commun 2016;7:13050. [PubMed] DOI:10.1038/ncomms13050
- Niemiec JA, Adamczyk A, Ambicka A, et al. Triple-negative, basal marker-expressing, and high-grade breast carcinomas are characterized by high lymphatic vessel density and the expression of podoplanin in stromal fibroblasts. Appl Immunohistochem Mol Morphol 2014;22:10–6. [PubMed] DOI:10.1097/PAI.0b013e318286030d
- Zhu X, Wang K, Zhang K, et al. Galectin-1 knockdown in carcinoma-associated fibroblasts inhibits migration and invasion of human MDA-MB-231 breast cancer cells by modulating MMP-9 expression. Acta Biochim Biophys Sin (Shanghai) 2016;48:462–7. [PubMed] DOI:10.1093/abbs/gmw019
- Maeda T, Desouky J, Friedl A. Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis . Oncogene 2006;25:1408–12. [PubMed] DOI:10.1038/sj.onc.1209168
- Brauer HA, Makowski L, Hoadley KA, et al. Impact of tumor microenvironment and epithelial phenotypes on metabolism in breast cancer. Clin Cancer Res 2013;19:571–85. [PubMed] DOI:10.1158/1078-0432.CCR-12-2123
- Tchou J, Kossenkov AV, Chang L, et al. Human breast cancer associated fibroblasts exhibit subtype specific gene expression profiles. BMC Med Genomics 2012;5:39. [PubMed] DOI:10.1186/1755-8794-5-39
- Park SY, Kim HM, Koo JS. Differential expression of cancer-associated fibroblast-related proteins according to molecular subtype and stromal histology in breast cancer. Breast Cancer Res Treat 2015;149:727–41. [PubMed] DOI:10.1007/s10549-015-3291-9
- Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 2008;14:518–27. [PubMed] DOI:10.1038/nm1764
- Moorman AM, Vink R, Heijmans HJ, et al. The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur J Surg Oncol 2012;38:307–13. [PubMed] DOI:10.1016/j.ejso.2012.01.002
- Downey CL, Simpkins SA, White J, et al. The prognostic significance of tumour-stroma ratio in oestrogen receptor-positive breast cancer. Br J Cancer 2014;110:1744–7. [PubMed] DOI:10.1038/bjc.2014.69
- Surowiak P, Murawa D, Materna V, et al. Occurence of stromal myofibroblasts in the invasive ductal breast cancer tissue is an unfavourable prognostic factor. Anticancer Research 2007;27:2917–24. [PubMed]
- Angelucci C, Maulucci G, Lama G, et al. Epithelial-stromal interactions in human breast cancer: effects on adhesion, plasma membrane fluidity and migration speed and directness. PLoS One 2012;7:e50804. [PubMed] DOI:10.1371/journal.pone.0050804
- Witkiewicz AK, Dasgupta A, Sammons S, et al. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol Ther 2010;10:135–43. [PubMed]
- Rong G, Kang H, Wang Y, et al. Candidate markers that associate with chemotherapy resistance in breast cancer through the study on Taxotere-induced damage to tumor microenvironment and gene expression profiling of carcinoma-associated fibroblasts (CAFs). PLoS One 2013;8:e70960. [PubMed] DOI:10.1371/journal.pone.0070960
- Wang K, Wu F, Seo BR, et al. Breast cancer cells alter the dynamics of stromal fibronectin-collagen interactions. Matrix Biol 2017;60-61:86–95. [PubMed] DOI:10.1016/j.matbio.2016.08.001
- Radisky ES, Radisky DC. Matrix metalloproteinases as breast cancer drivers and therapeutic targets. Front Biosci (Landmark Ed) 2015;20:1144–63. [PubMed]
- Roy DM, Walsh LA. Candidate prognostic markers in breast cancer: focus on extracellular proteases and their inhibitors. Breast Cancer (Dove Med Press) 2014;6:81–91. [PubMed] DOI:10.2147/BCTT.S46020
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010;141:52–67. [PubMed] DOI:10.1016/j.cell.2010.03.015
- Ren F, Tang R, Zhang X, et al. Overexpression of MMP family members functions as prognostic biomarker for breast cancer patients: a systematic review and Meta-analysis. PLoS One 2015;10:e0135544. [PubMed] DOI:10.1371/journal.pone.0135544
- Catteau X, Simon P, Noël JC. Stromal expression of matrix metalloproteinase 2 in cancer-associated fibroblasts is strongly related to human epidermal growth factor receptor 2 status in invasive breast carcinoma. Mol Clin Oncol 2016;4:375–8. [PubMed] DOI:10.3892/mco.2015.721
- Yousef EM, Tahir MR, St-pierre Y, et al. MMP-9 expression varies according to molecular subtypes of breast cancer. BMC Cancer 2014;14:609. [PubMed] DOI:10.1186/1471-2407-14-609
- Mehner C, Hockla A, Miller E, et al. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014;5:2736–49. [PubMed] DOI:10.18632/oncotarget.1932
- Zhao S, Ma W, Zhang M, et al. High expression of CD147 and MMP-9 is correlated with poor prognosis of triple-negative breast cancer (TNBC) patients. Med Oncol 2013;30:335. [PubMed] DOI:10.1007/s12032-012-0335-4
- Wang YY, Lehuédé C, Laurent V, et al. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett 2012;324:142–51. [PubMed] DOI:10.1016/j.canlet.2012.05.019
- Tan J, Buache E, Chenard MP, et al. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int J Dev Biol 2011;55:851–9. [PubMed] DOI:10.1387/ijdb.113365jt
- Dirat B, Bochet L, Dabek M, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 2011;71:2455–65. [PubMed] DOI:10.1158/0008-5472.CAN-10-3323
- Fujisaki K, Fujimoto H, Sangai T, et al. Cancer-mediated adipose reversion promotes cancer cell migration via IL-6 and MCP-1. Breast Cancer Res Treat 2015;150:255–63. [PubMed] DOI:10.1007/s10549-015-3318-2
- Bochet L, Meulle A, Imbert S, et al. Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem Biophys Res Commun 2011;411:102–6. [PubMed] DOI:10.1016/j.bbrc.2011.06.101
- Lee Y, Jung WH, Koo JS. Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res Treat 2015;153:323–35. [PubMed] DOI:10.1007/s10549-015-3550-9
- D’Esposito V, Liguoro D, Ambrosio MR, et al. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget 2016;7:24495–509. [PubMed] DOI:10.18632/oncotarget.8336
- Zheng Q, Banaszak L, Fracci S, et al. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer 2013;20:797–808. [PubMed] DOI:10.1530/ERC-13-0329
- Balaban S, Shearer RF, Lee LS, et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab 2017;5:1. [PubMed] DOI:10.1186/s40170-016-0163-7
- Dirat B, Bochet L, Escourrou G, et al. Unraveling the obesity and breast cancer links: a role for cancer-associated adipocytes?. Endocr Dev 2010;19:45–52. [PubMed] DOI:10.1159/000316896
- Lee TH, Avraham HK, Jiang S, et al. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem 2003;278:5277–84. [PubMed] DOI:10.1074/jbc.M210063200
- Bender RJ, Mac Gabhann F. Expression of VEGF and semaphorin genes define subgroups of triple negative breast cancer. PLoS One 2013;8:e61788. [PubMed] DOI:10.1371/journal.pone.0061788
- Plantamura I, Casalini P, Dugnani E, et al. PDGFRβ and FGFR2 mediate endothelial cell differentiation capability of triple negative breast carcinoma cells. Mol Oncol 2014;8:968–81. [PubMed] DOI:10.1016/j.molonc.2014.03.015
- Linderholm BK, Hellborg H, Johansson U, et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol 2009;20:1639–46. [PubMed] DOI:10.1093/annonc/mdp062
- Sa-Nguanraksa D, Chuangsuwanich T, Pongpruttipan T, et al. High vascular endothelial growth factor gene expression predicts poor outcome in patients with non-luminal A breast cancer. Mol Clin Oncol 2015;3:1103–8. [PubMed] DOI:10.3892/mco.2015.574
- Schneider BP, Gray RJ, Radovich M, et al. Prognostic and predictive value of tumor vascular endothelial growth factor gene amplification in metastatic breast cancer treated with paclitaxel with and without bevacizumab; results from ECOG 2100 trial. Clin Cancer Res 2013;19:1281–9. [PubMed] DOI:10.1158/1078-0432.CCR-12-3029
- Chong HC, Tan CK, Huang RL, et al. Matricellular proteins: a sticky affair with cancers. J Oncol 2012;2012:351089. [PubMed] DOI:10.1155/2012/351089
- Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast 2013;22 Suppl 2:S66-72.
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012;196:395–406. [PubMed] DOI:10.1083/jcb.201102147
- Brabrand A, Kariuki II, Engstrøm MJ, et al. Alterations in collagen fibre patterns in breast cancer. A premise for tumour invasiveness? APMIS 2015;123:1–8. [PubMed] DOI:10.1111/apm.12298
- Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol 2010;22:697–706. [PubMed] DOI:10.1016/j.ceb.2010.08.015
- Maquoi E, Assent D, Detilleux J, et al. MT1-MMP protects breast carcinoma cells against type I collagen-induced apoptosis. Oncogene 2012;31:480–93. [PubMed] DOI:10.1038/onc.2011.249
- Kim SH, Lee HY, Jung SP, et al. Role of secreted type I collagen derived from stromal cells in two breast cancer cell lines. Oncol Lett 2014;8:507–12. [PubMed] DOI:10.3892/ol.2014.2199
- Castro-Sanchez L, Soto-Guzman A, Navarro-Tito N, et al. Native type IV collagen induces cell migration through a CD9 and DDR1-dependent pathway in MDA-MB-231 breast cancer cells. Eur J Cell Biol 2010;89:843–52. [PubMed] DOI:10.1016/j.ejcb.2010.07.004
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871–90. [PubMed] DOI:10.1016/j.cell.2009.11.007
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 2009;119:1438–49. [PubMed] DOI:10.1172/JCI38019
- Cheung SY, Boey YJ, Koh VC, et al. Role of epithelial-mesenchymal transition markers in triple-negative breast cancer. Breast Cancer Res Treat 2015;152:489–98. [PubMed] DOI:10.1007/s10549-015-3485-1
- Gonzalez-Angulo AM, Chen H, Karuturi MS, et al. Frequency of mesenchymal-epithelial transition factor gene (MET) and the catalytic subunit of phosphoinositide-3-kinase (PIK3CA) copy number elevation and correlation with outcome in patients with early stage breast cancer. Cancer 2013;119:7–15. [PubMed] DOI:10.1002/cncr.27608
- Zagouri F, Bago-Horvath Z, Rossler F, et al. High MET expression is an adverse prognostic factor in patients with triple-negative breast cancer. Br J Cancer 2013;108:1100–5. [PubMed] DOI:10.1038/bjc.2013.31
- Lopez JI, Kang I, You WK, et al. In situ force mapping of mammary gland transformation . Integr Biol (Camb) 2011;3:910–21. [PubMed] DOI:10.1039/c1ib00043h
- Kaushik S, Pickup MW, Weaver VM. From transformation to metastasis: deconstructing the extracellular matrix in breast cancer. Cancer Metastasis Rev 2016;35:655–67. [PubMed] DOI:10.1007/s10555-016-9650-0
- Acerbi I, Cassereau L, Dean I, et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 2015;7:1120–34. [PubMed] DOI:10.1039/c5ib00040h
- Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009;139:891–906. [PubMed] DOI:10.1016/j.cell.2009.10.027
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989;8:98–101. [PubMed]
- Langley RR, Fidler IJ. The seed and soil hypothesis revisited -- the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 2011;128:2527–35. [PubMed] DOI:10.1002/ijc.26031
- Sihto H, Lundin J, Lundin M, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res 2011;13:R87. [PubMed] DOI:10.1186/bcr2944
- Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res 2008;68:3108–14. [PubMed] DOI:10.1158/0008-5472.CAN-07-5644
- Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271–7. [PubMed] DOI:10.1200/JCO.2009.25.9820
- Soni A, Ren Z, Hameed O, et al. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol 2015;143:471–8. [PubMed] DOI:10.1309/AJCPYO5FSV3UPEXS
- Neradil J, Veselska R. Nestin as a marker of cancer stem cells. Cancer Sci 2015;106:803–11. [PubMed] DOI:10.1111/cas.12691
- Pavon LF, Sibov TT, de Oliveira DM, et al. Mesenchymal stem cell-like properties of CD133+ glioblastoma initiating cells. Oncotarget 2016;7:40546–57. [PubMed] DOI:10.18632/oncotarget.9658
- Sierra A, Price JE, García-Ramirez M, et al. Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Lab Invest 1997;77:357–68. [PubMed]
- Kim SJ, Kim JS, Park ES, et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia 2011;13:286–98. [PubMed]
- Wang L, Cossette SM, Rarick KR, et al. Astrocytes directly influence tumor cell invasion and metastasis in vivo. PLoS One 2013;8:e80933. [PubMed] DOI:10.1371/journal.pone.0080933
- Sobottka B, Pestalozzi B, Fink D, et al. Similar lymphocytic infiltration pattern in primary breast cancer and their corresponding distant metastases. Oncoimmunology 2016;5:e1153208. [PubMed] DOI:10.1080/2162402X.2016.1153208
- Cimino-Mathews A, Ye X, Meeker A, et al. Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol 2013;44:2055–63. [PubMed] DOI:10.1016/j.humpath.2013.03.010
- Ogiya R, Niikura N, Kumaki N, et al. Comparison of tumour-infiltrating lymphocytes between primary and metastatic tumours in breast cancer patients. Cancer Sci 2016;107:1730–5. [PubMed] DOI:10.1111/cas.13101
