Effect of contrast-enhanced ultrasound on differential diagnosis of intrahepatic cholangiocarcinoma and arterial phase enhanced hepatic inflammatory lesions
Introduction
Intrahepatic cholangiocarcinoma (ICC) originates from intrahepatic secondary and smaller peripheral bile ducts, which accounts for about 10% of primary liver tumors (1,2). No specific indications for ICC are present either in clinical or laboratory examinations. Imaging technology plays an important role in the diagnosis of ICC. Under conventional two-dimensional ultrasound, ICCs are hypoechoic space-occupying lesions with poorly defined boundaries, making the differential diagnosis from other intrahepatic space-occupying lesions difficult, especially when patients were without any disease history, such as liver cirrhosis.
Hepatic focal inflammatory lesions are uncommon, benign disease entities that occur in patients with internal malignancies, parasitic infections or hypereosinophilic syndrome etc. The pathogens could be phlegmonous, granulomas or tuberculosis.
It was reported that 56.83% of ICC patients were with HBsAg positive (3) that triggered several studies focused on the differential diagnosis of ICC and primary hepatocellular carcinoma (HCC) in patients with cirrhosis (4-6). On the other hand, patients without viral hepatitis were easily misdiagnosed as other intrahepatic space-occupying lesions. Few contrast-enhanced ultrasonography (CEUS) researches discussed the differential diagnosis of ICC and other liver lesions without cirrhosis, including hepatic inflammatory lesions that had the quite different treatment strategy from ICCs. It is important to differentially diagnose ICCs and hepatic inflammatory lesions before the patient receiving surgery.
Real-time CEUS has been widely used in the diagnosis of hepatic space-occupying lesions (7,8). It has been reported that ICCs showed different perfusion characters during CEUS (4,9-11). A study showed that the diagnosis rate for two-dimensional ultrasound and CEUS was 36.1% and 58.4% (12), indicating a low qualitative diagnosis rate.
While hepatic inflammatory lesion has variable and sometimes confusing CEUS appearances, which change as they evolve and depend on the stages, early lesions being hyper-enhancing, which may be difficult or impossible to differentiate from malignancies (13,14). Zhao et al. analyzed the CEUS results of 11 patients with ICC and found that 45% of the patients were misdiagnosed as inflammation (15). In the present study, we retrospectively analyzed the CEUS characteristics of ICC patients without liver cirrhosis, and compared the findings with those patients who had hepatic lesions and a pathological diagnosis of inflammation. We aimed to identify the differences in CEUS for these two groups of patients and thereby improving the diagnosis rate.
Materials and methods
Patients
This study was approved by the Institutional Review Board of the Peking University Cancer Hospital, and written informed consent was obtained from all patients before their CEUS examinations.
Between September 2013 and October 2016, a total of 7,619 patients were referred to the Department of Ultrasound, Peking University Cancer Hospital for CEUS. Among the patients with focal liver lesions, 46 had ICC and 39 had intrahepatic inflammatory lesions. In total, 40 patients were ultimately included in the study based on the inclusion and exclusion criteria. Patients were included if they met the following inclusion criteria: 1) the arterial phase of CEUS examination procedure lasted at least 90 s; 2) the lesion could be observed during the late phase of CEUS examination (the guideline suggested that the late phase began from 120 s and lasted until 4–6 min after contrast agent injection, so we selected 3 min as the point to observe lesions at late phase) (16); and 3) the patient was diagnosed by biopsy or operation. Patients were excluded if they 1) had a history of hepatitis, liver cirrhosis, or alcohol abuse; or 2) the time intensity curve (TIC) could not be drawn due to the poor quality of CEUS images, such as the patients breathed hard during arterial or late phase.
Instruments and CEUS
LOGIQ E9 (GE Healthcare) with Probe C5-1 was used. The frequency was 2.0–4.0 MHz. The ultrasound contrast agent we used was SonoVue (Bracco, Milan, Italy). The microbubbles were sulfur hexafluoride (SF6) with a mean diameter of 2.5 μm and a pH of 4.5–7.5. Freeze-dried ultrasound contrast agent was dissolved in normal saline before use. After shock mixing, 1.5 mL (concentration 5 mg/mL, SF6 active ingredient 12 mg/person) of the ultrasound contrast agent was used for each imaging by bolus injection (within 2–3 s) into the elbow superficial vein.
Imaging
Two-dimensional ultrasound of the liver was conducted to observe the location (s), size (s), and number of lesion (s), as well as the presence of bile duct dilatation and the echogenicity. Color Doppler was initiated to observe the blood supply of the lesion (s) and an initial diagnosis was made before conducting CEUS. According to the two-dimensional ultrasonography results, the best scanning section of a typical lesion was selected as the observation object for the CEUS. Low mechanical index (MI<0.1) was achieved by adjusting the sound power output based on the depth of the lesion and the condition (i.e., thin or fat) of the patient. The built-in timer was started as soon as the contrast agent was injected. Contrast agent perfusion characters and the changes in echo intensity of the focal areas were observed continuously in real-time. In addition to obtaining a complete record of each phase for the target areas (i.e., the main lesions), the perfusion of the adjacent areas was observed and recorded. After obtaining a diagnostic real-time image of the parenchymal phase, the whole liver was scanned rapidly.
Image analysis
Using the images stored in the ultrasound instrument, the perfusion patterns of the lesions were first observed by the naked eyes, including the pattern of the arterial phase enhancement, the starting washout time. The TIC analysis software installed in the GE LOGIQ E9 was used for quantitative analysis. Regions of interest (ROIs) with the same size were selected including the lesions and the liver parenchyma adjacent to liver lesions. The TICs of the contrast agents were drawn. The perfusion parameters of the contrast agent we selected for comparison were the arrival time, peak intensity (PI) in the lesions, the starting time for washout, and the intensity difference at 3 min (ΔI3) between the lesion and the liver parenchyma.
Statistical analysis
The averaged values were used for the continuous data. In the analysis of patient characteristics and imaging features, the data were expressed as x±s. Differences in proportions between the two diseases were analyzed using the Chi-square test and Fisher’s exact test. Parametric data were compared using Student’s t-test, and nonparametric data were analyzed with the Mann-Whitney U test. All P values were derived from two-tailed tests, and a level of less than 0.05 was accepted as statistically significant. IBM SPSS Statistics (Version 21.0; IBM Corp., New York, USA) was used for the statistical analysis.
Results
General information of patients
A total of 40 patients were included. There were 25 patients with ICC (15 males and 10 females) and the mean age was 62.0±8.9 years. Fifteen patients had intrahepatic inflammatory lesions (8 males and 7 females) and the mean age was 53.3±8.7 years. Of these patients, 22 were diagnosed by surgical pathological diagnosis, and the pathological diagnosis of 18 patients was made by ultrasound guided liver biopsy. The median size of the lesions was 4.4 (range: 1.8–11.8) cm.
Two-dimensional ultrasonography
In this study, two-dimensional ultrasonography of the ICC patients was mostly hyperechoic. Most lesions were on the liver left lobe, with unclear borders. Bile duct dilatation was also seen in 17 patients (68.0%). The majority of the inflammatory lesions were hypoechoic, and only 2 patients (13.3%) had bile duct dilatation. There was no significant difference in lesion size between the two groups (Table 1).
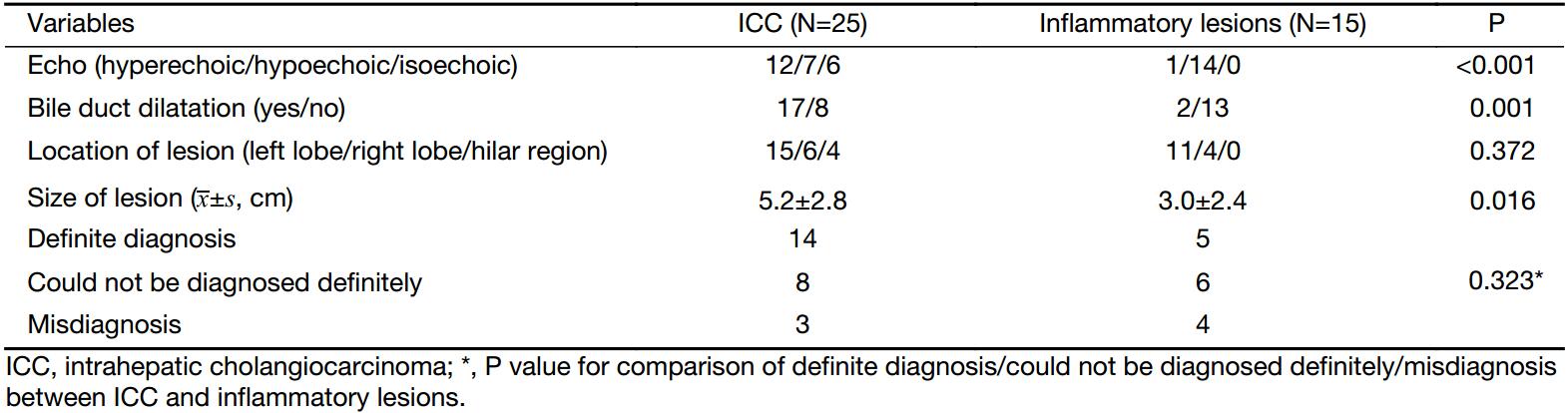
Full table
Definite initial diagnosis could not be made based on the two-dimensional ultrasound findings for 8 patients with ICC and 6 patients with inflammatory lesions. Three patients with ICC were misdiagnosed as having inflammatory lesions. For these 3 patients, the lesions were located in the left lobe, and no obvious bile duct dilatation was present. Four patients with inflammatory lesions were misdiagnosed as having malignant lesions (Table 1).
CEUS
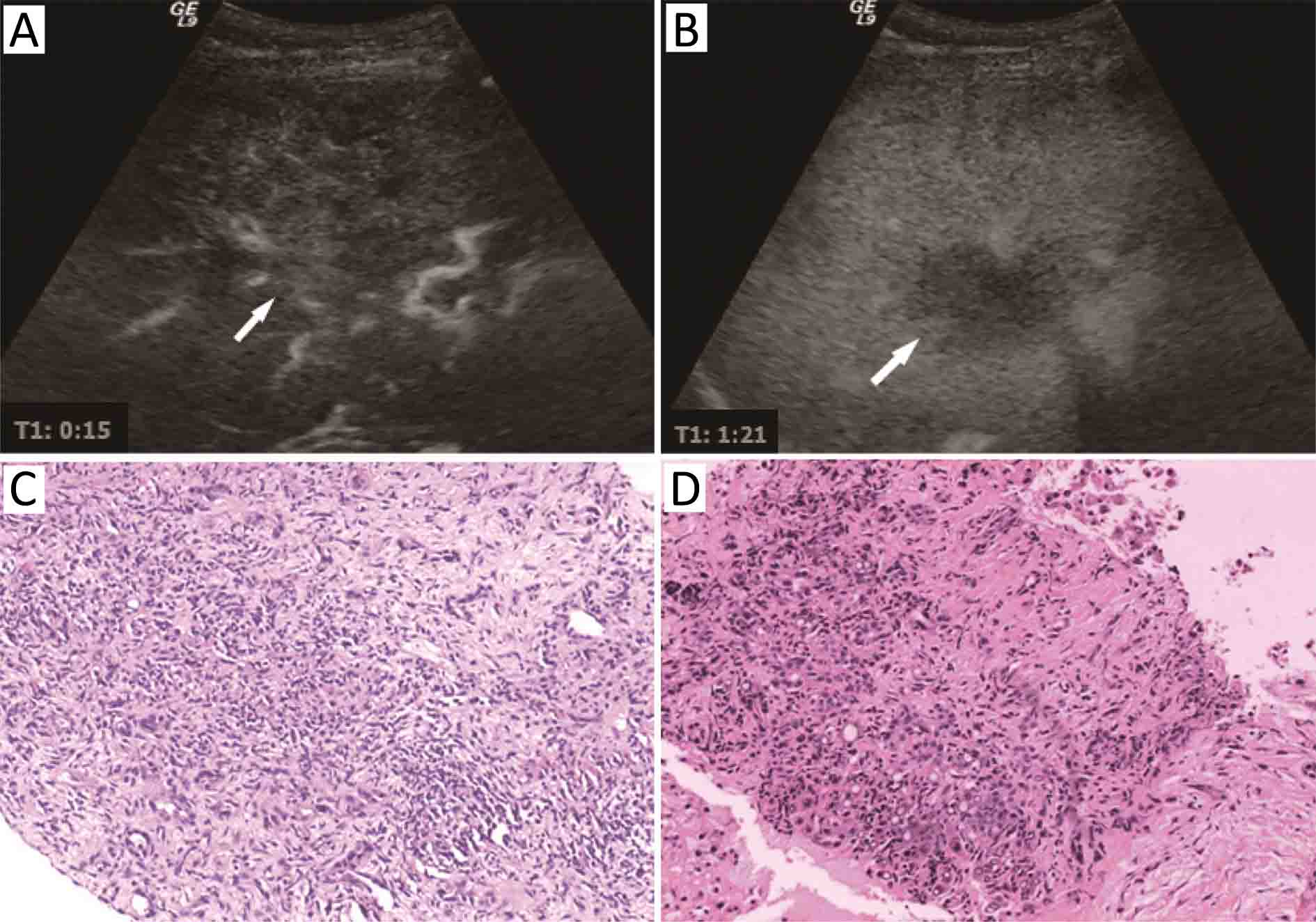
Twelve cases (48%) of ICC showed peripheral rim-like hyper-enhancement, while only 2 cases (13.3%) of inflammatory lesions showed arterial phase peripheral rim-like hyper-enhancement. Twelve cases (48%) of ICC and 13 cases (86.7%) of inflammatory lesions showed flaky heterogeneous hyper-enhancement. Only one (4%) ICC case showed homogeneous hyper-enhancement. Three ICC cases (12%) were misdiagnosed as inflammatory lesions, and all of them showed flaky heterogeneous hyper-enhancement with poorly defined boundaries at the arterial phase and hypo-enhancement at the portal venous phase (Table 2). For one of the three patients, the pathological diagnosis after the first liver biopsy was inflammatory lesion, while ICC was diagnosed in the second liver biopsy carried out one month later (Figure 1). Three inflammatory cases were misdiagnosed as malignant because of the early starting washout time and the hypo-enhancement at late phase (Figure 2, 3).
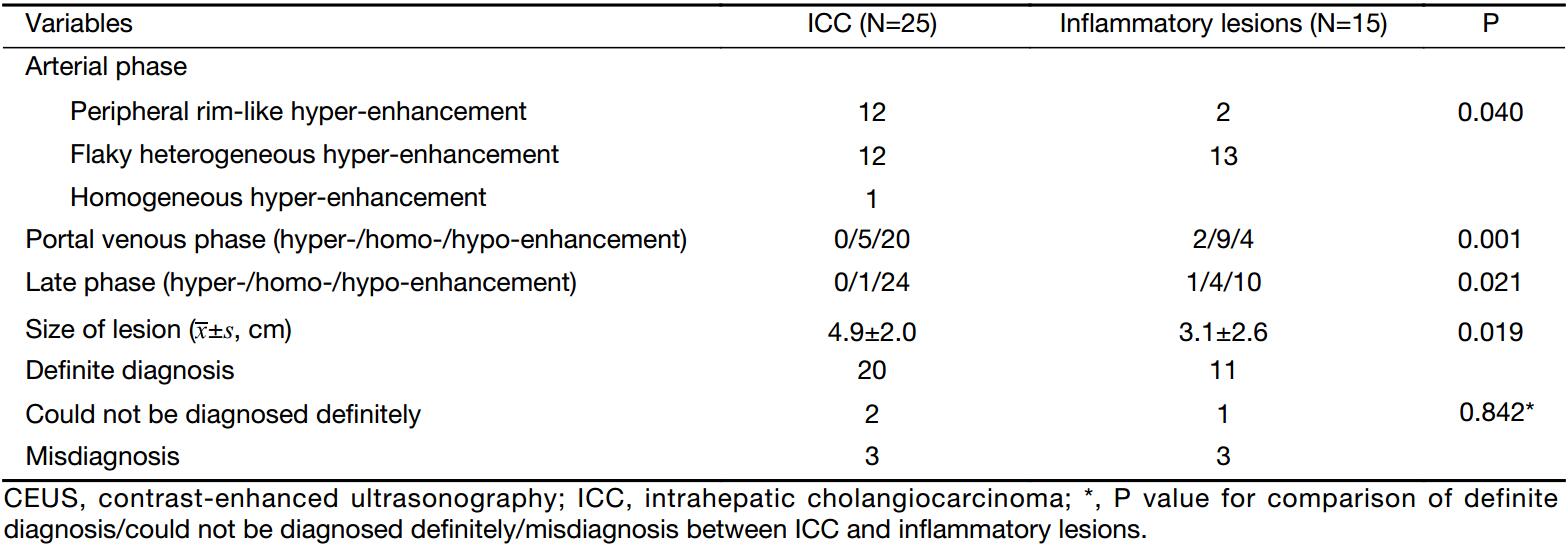
Full table


TIC
TIC analysis showed that between ICC and inflammatory lesions there was no significant difference in the starting time of enhancement. For ICC, the starting time of washout was earlier compared with inflammatory lesions (P<0.001), while the ΔI3 between lesion and liver parenchyma was more obvious (P<0.001). It indicated that washout in ICC started early, and a more obvious washout was observed (Table 3).
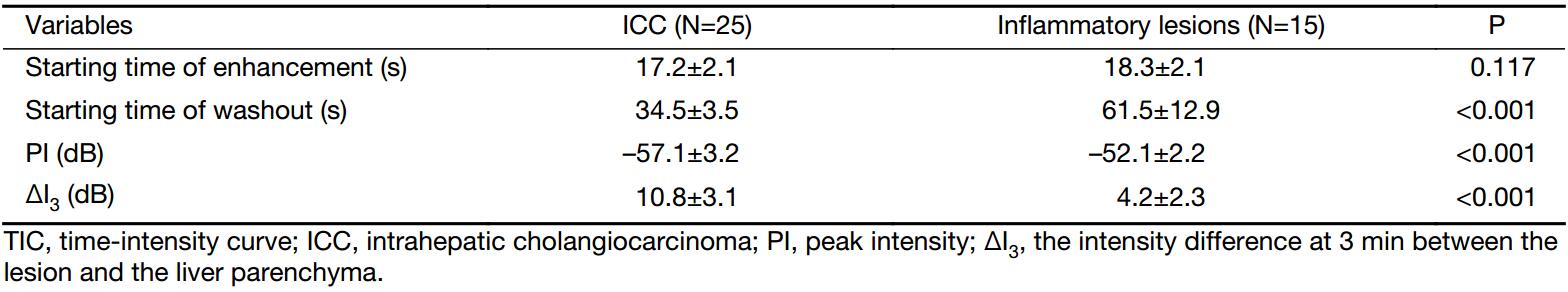
Full table
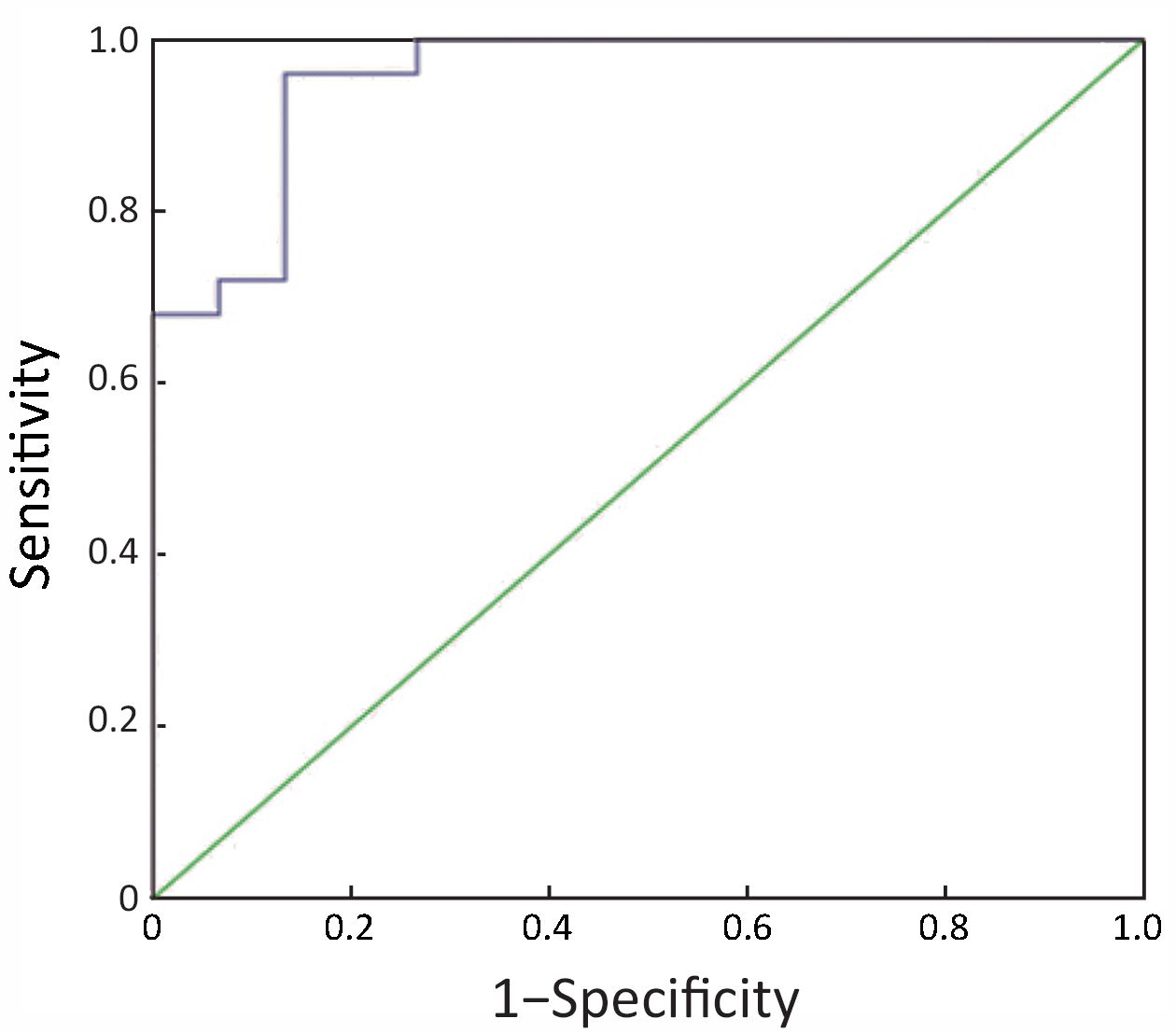
The receiver-operating characteristic (ROC) curve analysis showed that if the cut-off for intensity difference was 7.7 dB, the sensitivity and specificity for the differentiation of ICC and inflammatory lesions were 76% and 87%, respectively; and the area under the curve (AUC) was 0.955 (Figure 4).
Discussion
SonoVue is a blood pool agent used in CEUS. It allows real-time dynamic observation of the microvascular perfusion within the lesions and has been widely used in the diagnosis and differential diagnosis of liver lesions. Different perfusion patterns have been reported for ICC. For the arterial phase, four perfusion patterns were seen in the literature, including peripheral rim-like hyper-enhancement (50%), heterogeneous hyper-enhancement (24%), heterogeneous hypo-enhancement (20%), and homogeneous hyper-enhancement (6%) (5,6,8). These differences in arterial phase enhancement may be due to the amount of tumor cells and the fiber distribution within the lesions (6). Recently, some authors (17) reported that the arterial phase had a “petal-like” or “honeycomb-like” appearance and hypothesized that the single rim-like sign was an typical angiographic manifestation for ICC, while others suggested that an “internal feathery-like” appearance should be the characteristic feature for ICC (18,19). In the present study, we found that it was difficult to define hyper-enhancement and hypo-enhancement clearly in ICC, the arterial phase perfusion patterns we summarized as the following three types: peripheral rim-like enhancement (48%), heterogeneous flaky enhancement (48%), and homogeneous hyper-enhancement (4%), among which the proportion of patients with peripheral rim-like enhancement was similar to that of previous studies.
A hepatic inflammatory lesion is a benign disease of the liver caused by acute or chronic inflammation that is commonly associated with bacterial infection, immunologic reactions, parasites, drug-related hepatitis, tuberculosis and industrial pollution (20). Histologic findings of the lesion include hyperplasic degenerated fibrotic tissue with various degrees of inflammatory infiltration by plasma, lymph and fibrotic cells and formation of inflammatory granulomas with hyperplasic capillaries (21). Conventional gray-scale ultrasound has several limitations in the detection and characterization of bacterial infectious diseases in liver. In the early stage, tissue alterations may be so subtle that the lesion could present as isoechoic and was difficult to be detected by conventional ultrasound. While on CEUS, the inflammatory lesion has variable and sometimes confusing appearances, which change as they evolve, early lesions being hyper-enhancing, mature lesions develop hypo-enhancing foci as liquefaction progresses on the other hand. Because of the various perfusion patterns of the inflammatory lesion, especially the hyper-enhanced lesion during arterial phase on CEUS, the differential diagnosis from the malignant lesion was quite difficult. In clinical practice we found that it was difficult to differentiate ICC from inflammatory lesions when liver cirrhosis was not present, which triggered the current study to retrospectively analyze such cases.
We retrospectively analyzed 25 patients with ICC who did not have liver cirrhosis and found that with two-dimensional ultrasonography, three patients were misdiagnosed and 8 patients could not be diagnosed definitely; with CEUS, the number of patients who misdiagnosed and could not be diagnosed definitely was two and three, respectively. Although CEUS reduced the number of patients without a definite diagnosis, the rate of misdiagnosis could not be negligible.
It has been reported that the minor differences in two-dimensional ultrasonography between inflammatory lesions and ICC are due to the fact that there are more fibrous connective tissues within ICC that lead to significant echo attenuation at the background and cause poorly defined rear boundary. In addition, the contraction of the fibrous tissues within the tumor can pull the surrounding bile ducts towards the tumor (22), causing the dilated bile ducts to form a “dead branch” appearance around the lesions of some ICC cases. The presence or absence of bile duct dilatation is significant in differentiating ICC from inflammatory lesions. The results of this study suggest that 68.0% of the patients with ICC had bile duct dilatation, whereas only 13.3% inflammatory cases with bile duct dilatation. Peripheral ICC originates from the bile duct epithelium of the secondary or smaller intrahepatic bile ducts which could not result in peripheral bile duct dilatation. In the current analysis, neither of the two patients with ICC who were misdiagnosed as inflammatory lesions showed obvious bile duct dilatation in two-dimensional ultrasonography which may be one of the reasons to make them misdiagnosis.
All three misdiagnosed ICC cases showed heterogeneous flaky hyper-enhancement, while 86.7% (13/15) of inflammatory lesions showed heterogeneous flaky hyper-enhancement during the CEUS arterial phase. CEUS appearance of ICC made it not easy to be differentiated from the arterial phase of inflammatory lesions where similar flaky enhancement was observed. Although CEUS can facilitate continuous real-time observation of the morphology and blood flow changes of liver lesions at different phases, the diagnosis remains subjective to various degrees if it is made solely on the perfusion characteristics.
European guidelines for CEUS in the liver stated that ICC showed rapid washout at the portal venous phase with hypo-enhancement; and they also stated that inflammatory lesions could have rim-like enhancement at the arterial phase and washout at the portal venous phase and the late phase, making the differentiation diagnosis between inflammatory lesions and malignant lesions difficult (16). In this study, TIC analysis, which can assess quantitative parameters of the tumor blood flow, was employed to achieve a fairly accurate quantitative analysis. Overall, the starting of washout in ICC was earlier than that in inflammatory lesions, which showed a “fast out” pattern; but for the 5 patients who were misdiagnosed or whose diagnosis was not definite, a later washout starting time (all later than 60 s) was seen and hypo-enhancement was shown in the portal venous phase. TICs reflect the relative echo intensity of the lesion. In addition, this study being a retrospective analysis, the pre-set instrument parameters (total gain and MI), the dose of the contrast agent, and the injection speed were all different; while the peak density and other parameters had fairly large individual differences. Therefore, the fact that we did not find significant difference in the peak density between ICC and inflammatory lesions may be related to the limitations of this study. However, to avoid the impact of the machine set up and any other individual difference, we also used a relative value measuring the difference between the density of the lesion and the density of liver parenchyma near the lesion (lesion density minus parenchymal density) at 3 min. Results showed that at 3 min, the difference in density between the lesion and the surrounding liver parenchyma was significant (10.8±3.1 dB vs. 4.2±2.3 dB), indicating an early and obvious washout in the malignant lesions of ICC. It is speculated that for inflammatory lesions without the formation of liquefied necrosis, washout starts earlier in the lesions than in the normal liver parenchyma as a result of vasodilation caused by inflammation. While for ICC, the central area of the lesion has fewer tumor cells and abundant fibrous tissue, the distribution of blood vessels is sparse with most of the vessels buried in interstitial fibers, and the blood flow is not the normal portal vein blood supply. During late phase, this may lead to a more obvious washout in the lesions than in the surrounding liver parenchyma receiving portal vein blood supply. ROC analysis showed that using 7.7 dB as the cut-off for intensity difference, the sensitivity and specificity for the differentiation of ICC and inflammatory lesions were 76% and 87%; and the AUC was 0.955.
One limitation of this study was its retrospective design, which resulted in the inconsistency of the pre-set instrument parameters before CEUS that may have the effect on results of TIC. Another limitation was the small sample size. Thus, the use of TIC parameters for the differential diagnosis of ICCs and hepatic inflammatory lesions requires further prospective studies with large sample sizes.
Conclusions
This study suggests that for patients with ICC who cannot be differential diagnosed from inflammatory lesions by two-dimensional ultrasonography, CEUS can provide more diagnostic information. Combined with TIC analysis, and particularly with the characteristic of the early-starting and obvious washout in ICC, CEUS can be effectively used in differential diagnosis between inflammatory lesions and ICC.
Acknowledgements
This study was supported by Beijing Municipal Science & Technology Commission (No. Z151100004015186).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vogel A, Saborowski A. Cholangiocellular Carcinoma. Digestion 2017;95:181–5. [PubMed] DOI:10.1159/000454763
- Wirth TC, Vogel A. Surveillance in cholangiocellular carcinoma. Best Pract Res Clin Gastroenterol 2016;30:987–99. [PubMed] DOI:10.1016/j.bpg.2016.11.001
- Li Y, Wang H, Li D, et al. Occult hepatitis B virus infection in Chinese cryptogenic intrahepatic cholangio-carcinoma patient population. J Clin Gastroenterol 2014;48:878–82. [PubMed] DOI:10.1097/MCG.0000000000000058
- Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display vascular pattern similar to hepato-cellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010;51:2020–9. [PubMed] DOI:10.1002/hep.23600
- Li R, Cai P, Ma KS, et al. Dynamic enhancement patterns of intrahepatic cholangiocarcinoma in cirrhosis on contrast-enhanced computed tomo-graphy: risk of misdiagnosis as hepatocellular carcinoma. Sci Rep 2016;6:26772. [PubMed] DOI:10.1038/srep26772
- Guo LH, Xu HX. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: Controversy over the ASSLD Guideline. Biomed Res Int 2015;2015:349172. [PubMed] DOI:10.1155/2015/349172
- Wu W, Chen M, Yan K, et al. Evaluation of contrast-enhanced ultrasound for diagnosis of dysplastic nodules with a focus of hepatocellular carcinoma in liver cirrhosis patients. Chin J Cancer Res 2015;27:83–9. [PubMed] DOI:10.3978/j.issn.1000-9604.2015.02.06
- Schellhaas B, Wildner D, Pfeifer L, et al. LI-RADS-CEUS-proposal for a contrast-enhanced ultrasound algorithm for the diagnosis of hepatocellular carcinoma in high-risk populations. Ultraschall Med 2016;37:627–34. [PubMed] DOI:10.1055/s-0042-112221
- Chen LD, Xu HX, Xie XY, et al. Enhancement patterns of intrahepatic cholangiocarcinoma: comparison between contrast-enhanced ultrasound and contrast-enhanced CT. Br J Radiol 2008;81:881–9. [PubMed] DOI:10.1259/bjr/22318475
- Xu HX, Chen LD, Liu LN, et al. Contrast-enhanced ultrasound of intrahepatic cholangiocarcinoma: correlation with pathological examination. Br J Radiol 2012;85:1029–37. [PubMed] DOI:10.1259/bjr/21653786
- Kong WT, Wang WP, Zhang WW, et al. Contribution of contrast-enhanced sonography in the detection of intrahepatic cholangiocarcinoma. J Ultrasound Med 2014;33:215–20. [PubMed] DOI:10.7863/ultra.33.2.215
- Lyu MD, Xie XY, Xu ZF, et al. Evaluation of the usefulness of conventional ultrasound and contrast-enhanced ultrasound in characterization of focal liver lesions. Zhongguo Chao Sheng Yi Xue Za Zhi (in Chinese) 2005;21:924–6.
- Cao BS, Li XL, Li N, et al. The nodular form of hepatic tuberculosis: contrast-enhanced ultrasono-graphic findings with pathologic correlation. J Ultrasound Med 2010;29:881–8. [PubMed]
- Liu GJ, Lu MD, Xie XY et al, et al. Real-time contrast-enhanced ultrasound imaging of infected focal liver lesions. J Ultrasound Med 2008;27:657–66. [PubMed]
- Zhao HJ, Dong BW, Yu XL, et al. Contrast-enhanced ultrasound features and pathology foundation of intrahepatic cholangiocarcinoma. Zhongguo Chao Sheng Yi Xue Za Zhi (in Chinese) 2008;24:162–4.
- Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS) in the liver — update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 2013;39:187–210. [PubMed] DOI:10.1016/j.ultrasmedbio.2012.09.002
- Jiang LP, Yu Q, Yuan XC, et al. Value of contrast-enhanced ultrasonography in the differential diagnosis of intrahepatic cholangiocarcinoma and bacterial liver abscess. Shi Yong Yi Xue Za Zhi (in Chinese) 2014:2976-8.
- Song HS, Ding HY, Shen J, et al. Ultrasonography and pathological study of intrahepatic cholangio-carcinoma without bile duct dilation. Jiang Su Yi Yao (in Chinese) 2014;40:2882–3.
- Wu LL, Su ZZ, Wu T, et al. Application of contrast-enhanced ultrasound in differential diagnosis of cholangiocarcinoma and early stage liver abscess. Lin Chuang Chao Sheng Yi Xue Za Zhi (in Chinese) 2013;15:235–7.
- Ralls PW. Focal inflammatory disease of the liver. Radiol Clin North Am 1998;36:377–89. [PubMed]
- Wu ZB, Yang GH. Chinese surgical pathology. Beijing: People’s Medical Publishing House, 2006.
- Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology 2008;48:308–21. [PubMed] DOI:10.1002/hep.22310
