Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor
Introduction
In recent years, rapid progress has been achieved in the field of diagnosis, treatment and research on gastrointestinal stromal tumors (GIST). Some of the newly emerged evidence will have important implications for GIST clinical practice. In order to further promote the standardization of diagnosis and treatment of GIST in China, the members of Chinese Society of Clinical Oncology (CSCO) Expert Committee on GIST thoroughly discussed the key contents of the consensus guidelines, and voted on the controversial issue. In final, the Chinese consensus guidelines for the diagnosis and management of GIST (2017 edition) was formed on the basis of 2013 edition consensus guidelines, which is hereby announced.
Principles of pathologic diagnosis
Definition of GIST
GIST is the most common mesenchymal tumor in the gastrointestinal tract. The biological behavior of GIST varies from benign to malignant. CD117 and DOG1 are usually positive in immunohistochemical (IHC) staining, and thus are useful to confirm the diagnosis. Most GISTs show differentiation towards interstitial cells of Cajal (ICC) and have an activating mutation in gene encoding KIT or platelet-derived growth factor receptor alpha (PDGFRA) receptor tyrosine kinase (1). In few cases where the mutation of KIT or PDGFRA is absent, changes of other molecules, including SDHX, BRAF, NF1, K/N-RAS and PIK3CA genes, may be involved (2).
Requirements for specimens
The specimens should be fixed in time. In detail, the specimens should be sent to the pathology section within 30 min after excision. Fixation is completed by immersion of the specimens in sufficiently neutral 10% formalin solution (at least 3 times the volume of the specimens). Tumors which are larger than 2 cm in diameter must be sliced every 1 cm to ensure fully fixation. The fixation time should be 12–48 h, so as to ensure the feasibility and accuracy of subsequent IHC and molecular biology examination. If possible, the remaining fresh tissue should be kept frozen in case of further genetic tests.
Pathological diagnosis and ancillary examination
General diagnosis
Histologically, according to the morphology of tumor cells, GIST can be divided into 3 subtypes: spindle cell type (70%), epithelioid cell type (20%) and mixed, spindle-epithelioid type (10%). Even within the same subtype, the morphology of GIST varies greatly among cases. In addition to the typical morphology, GIST may have some special appearances, including pleomorphic cells that may be observed in limited cases, especially in epithelioid GIST. The mesenchyme may be sclerosing and accompanied by calcification, especially in small GIST. Sometimes myxoid degeneration may also be observed. In addition, the nodules with eosinophilic filament appearances (Skeinoid fiber) are often detected in small intestinal GIST, which have some suggestive significance for the diagnosis (3,4).
Pathological diagnosis after targeted therapy
After targeted therapy, necrosis and/or cystic degeneration can occur in GIST. In some cases, the cell density decreases significantly. The tumor cell component is sparse and is accompanied by extensive interstitial collagenzation and by more or less inflammatory cell infiltration and histiocytic reaction. In recent years, there are increasing numbers of GIST specimen retrieved from surgical resection after targeted therapy. To have histological evaluation on the response to targeted therapy, the following evaluation criteria are recommended: slight effective, 0–10%; low effective, >10% and <50%; moderately effective, ≥50% and ≤90%; and highly effective, >90%. However, the correlation between efficacy in histological evaluation and prognosis of GIST remains unclear and more cases are needed.
IHC examination
IHC staining of GIST is recommended using a panel with five markers including CD117, DOG1, CD34, succinate dehydrogenase B (SDHB) and Ki67. SDHA markers could be added as appropriate. Positive control is suggested when detecting CD117 and DOG1.
Molecular assays
Molecular assays should be conducted in qualified laboratories. Polymerase chain reaction (PCR) amplification and direct sequencing methods are recommended to ensure the accuracy and consistency of the results. The molecular detection of GIST is very important, which is helpful for the diagnosis of some difficult cases, the prediction of the therapeutic effect of molecular targeted drugs and the guidance of clinical treatment.
The expert committee recommended molecular detection should be carried out on the cases with the following circumstances: performing c-kit or PDGFRA mutational analysis to identify the diagnosis of GIST in difficult cases; intending to use molecular targeted therapy preoperatively; all the cases initially diagnosed as recurrent or metastatic GIST and the molecular targeted therapy is considered; the risk of recurrence of the primary resected GIST is moderate or high and the molecular targeted therapy is considered; to identify wild-type GIST; for differential diagnosis of synchronous and metachronous multiple GIST; or when the secondary resistance occurs with available specimens.
Detection of gene mutations should include at least exon 9, 11, 13, and 17 of c-kit gene and exon 12 and 18 of PDGFRA gene. For patients with secondary resistance, exon 14 and 18 of c-kit gene should also be included. The primary mutation of c-kit gene includes a variety of types of mutations and deletion mutation accounts for approximately 50%, in which codon 557–558 mutation owns worse biological behavior than non-deletion mutation. It also demonstrates shorter effective imatinib treatment period. Identifying which types of c-kit exon 11 mutation provides values on the evaluation of the tumor biological behavior and therapeutic strategies decision of the tumor (5). Therefore, description on each type of exon 11 mutations of a GIST should be included in the molecular examination report.
The BRAF mutation can be detected in a few wild-type GIST, which suggests the presence of a special subgroup of wild-type GIST. Given the fact that BRAF serving as a treatment target is reported in limited cases and the overall frequency of mutation is low (6), BRAF is not recommended as a routine examination on gene mutation.
At present, the next generation sequencing (NGS) and liquid biopsy in GIST are reported in low volume. But several studies found that these methods have the potential application of detecting rare mutations and early detection of secondary mutations (7,8). In view of the reliability and clinical value of NGS and liquid biopsy remains to be further evaluated, these two kinds of modality still cannot replace the role of direct sequencing in genotyping of primary GIST. But for advanced GIST, especially secondary resistant cases, NGS and liquid biopsy are recommended as parts of exploratory research.
Diagnostic algorithm
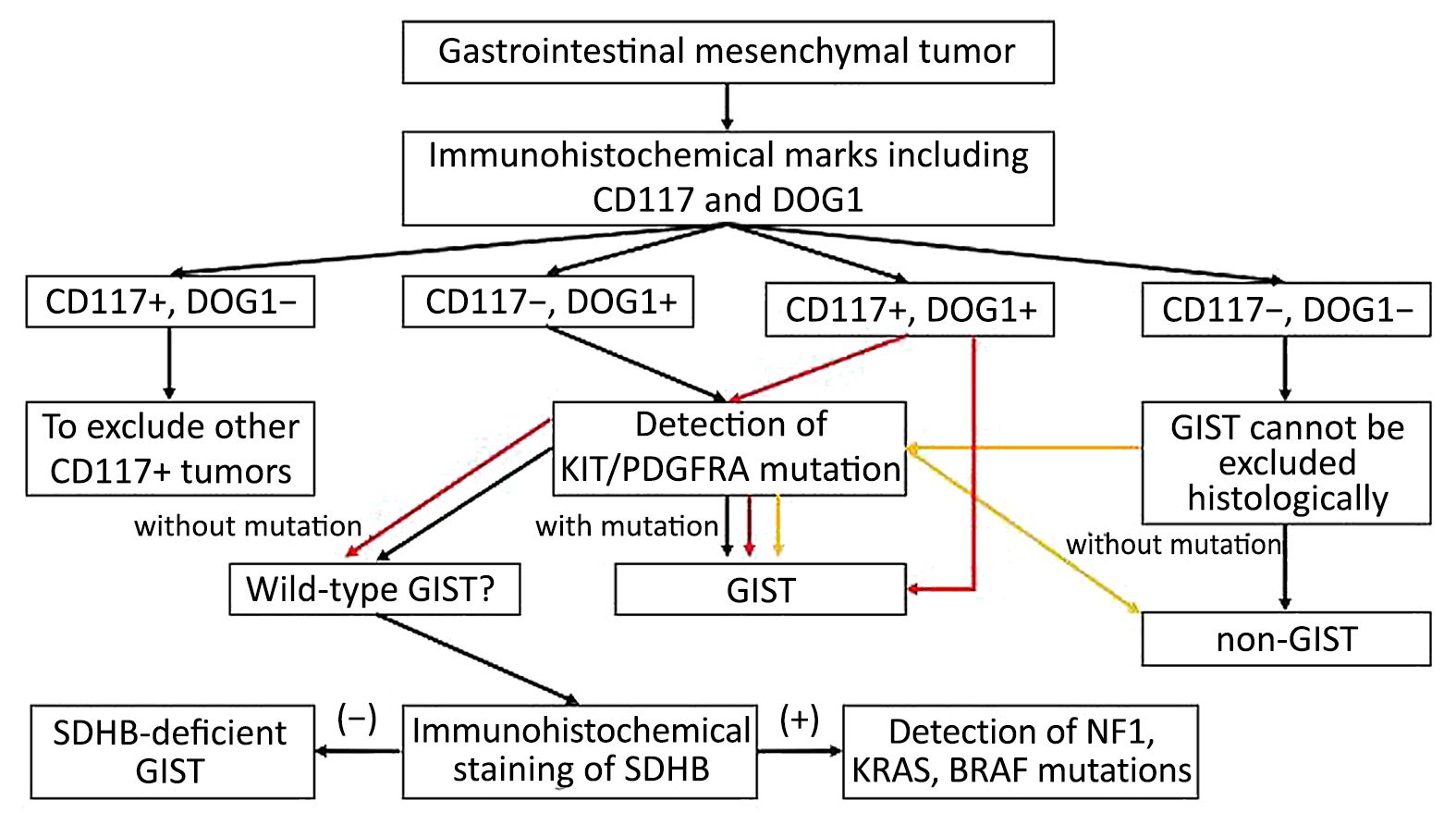
Pathologists who engage in GIST pathological diagnosis should not only be familiar with various morphological manifestations of GIST, but also be aware of various tumors easily misdiagnosed as GIST. IHC detection emphasizes on combined use of CD117 and DOG1 markers: when histological morphology accords with GIST and both markers diffuse positive, the diagnosis can be made of GIST; when morphology shows epithelioid but CD117 negative or weak positive while DOG1 positive, genotyping is needed to determine whether there is PDGFRA gene mutation, especially D842V mutation; for cases showing CD117 positive while DOG1 negative, other tumors with CD117 positive need to be ruled out first before the decision making. Genotyping should be carried out when necessary for differential diagnosis; when either histological morphology or IHC marks is in line with GIST but the genotyping lacks of c-kit nor PDGFRA mutation, the possibility of wild-type GIST needs to be considered and additional SDHB marker should be examined. Loss of SDHB expression should be taken into account toward the diagnosis of SDHB-deficient GIST. When SDHB expression is positive, the possibility of other kinds of wild-type GIST should be considered and respective molecular screening is recommended; if the both markers are negative, the diagnosis is usually non-GIST. After exclusion of other types of tumor, genotyping is necessary when GIST is suspected. The pathological diagnostic algorithm of GIST is shown in Figure 1.
Wild-type GIST
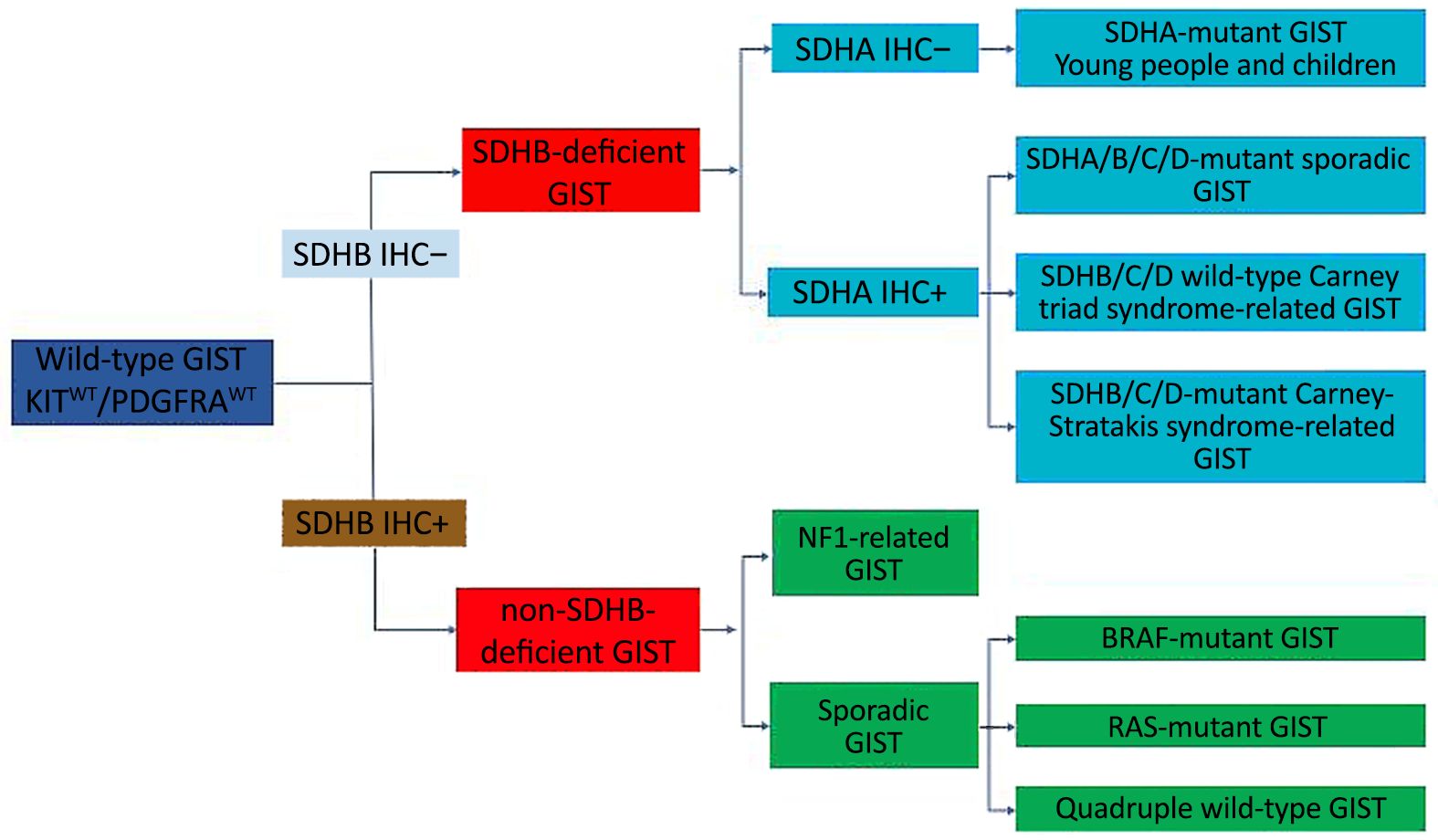
Wild-type GIST refers to the case which is consistent with GIST in pathological diagnosis, but there is no c-kit/PDGFRA mutation in molecular detection. Approximately 85% of children with GIST and 10%–15% of adults with GIST are wild-type GIST. According to whether there is a loss of SDHB expression, wild-type GIST can be roughly divided into two categories: the SDH-deficient GIST, including SDHA-mutant GIST, sporadic GIST, Carney triad syndrome-related GIST, and Carney-Stratakis syndrome-related GIST; non-SDH-deficient GIST, including BRAF-mutant GIST, NF1-related GIST, K/N-RAS mutation-associated GIST, and quadruple wild-type GIST (9-11). The categories of wild-type GIST are shown in Figure 2.
SDH-deficient GIST
SDH-deficient GIST accounts for about half of wild-type GIST (9), including sporadic, non-syndrome-associated, SDH-deficient GIST, Carney triad syndrome-associated GIST, and Carney-Stratakis syndrome-associated GIST. Sporadic SDH-deficient GIST mainly occurs in children and young people, and is predominant in women. The tumor occurs in the stomach and usually appears multinodular or with a plexiform growth pattern under the microscope. The tumor cells are epithelioid or mixed type. Lymphatic tumor thrombus can be observed in about 50% of the cases and about 10% cases can be diagnosed with regional lymph node metastasis. The tumor cells can express CD117 and DOG1 by IHC staining but the expression of SDHB is lost. Molecular detection shows that about half of the SDH subunits, SDHA, SDHB, SDHC or SDHD, own loss-of-function germline mutations, among which, about 30% for SDHA mutation, is mostly germline mutation, with loss of SDHA expression under IHC staining, and 20% for SDHB, C or D mutation. The other half of the cases may have SDHC promoter methylation or epigenetic silencing on SDH complex and usually with overexpression of insulin-like growth factor 1 receptor (IGF1R). There is no familial inheritance in Carney triad syndrome-associated GIST, which may be accompanied by pulmonary chondroma, often multifocal, and extra adrenal paraganglioma at the same time. Only about 22% of the cases have all the three tumors, 53% with simultaneous GIST and pulmonary chondroma, and 24% with simultaneous GIST and paraganglioma. Other synchronous neoplasms associated with this disease include pheochromocytoma, adrenal adenoma, and esophageal leiomyoma. Carney triad syndrome-associated GIST is considered to be caused by SDHC methylation and is SDHB-negative and SDHA-positive under IHC staining. Carney-Stratakis syndrome associated GIST is an autosomal genetic disease with incomplete dominance. Germline inactivation of SDHB (10%), SDHC (80%) and SDHD (10%) resulted in loss of protein expression and negative for SDHB in IHC assay (12-16).
BRAF-mutant GIST
BRAF-mutant GIST accounts for about 3%–7% of wild-type GIST. It mainly occurs in small intestine (56%), followed by stomach (22%). The histological morphology is mostly spindle cell type, and CD117 and DOG1 are both positive by IHC. BRAF gene exon 15 mutation (p. V600E) can be observed in molecular detection. In a few cases, it serves as secondary imatinib resistance mutation (17-19).
NF1-related GIST
The incidence of GIST in NF1 patients is 7%. Patients with NF1-related GIST are relatively younger, with tumors mostly located in jejunum and ileum, often multinodular and are accompanied by ICC hyperplasia. Molecular assays reveal loss-of-function germline mutations in NF1 but without hot spots. The mutation types may be insertion, deletion, intra-frame shift, or missense mutation (20,21).
K/N-RAS-mutant GIST
RAS mutation may occur in either primary drug-resistant GIST or KIT/PDGFRA-mutant GIST (22).
Quadruple wild-type GIST
Quadruple wild-type GIST is very rare. The involved molecules include CALCRL and COL22A1; NTRK6, a tyrosine kinase; CDK6, a cyclin dependent kinase, and ERG, an ETS transcription factor, etc (23). The molecular mechanism of quadruple wild-type GIST remains to be studied.
Other wild-type GISTs
Genotypes of other wild-type GISTs include PIK3CA mutations and ETV6-NTRK3 fusion genes (24,25).
Small GIST and micro-GIST
GIST less than 2 cm in diameter is referred to as small GIST. Among which, GIST less than 1 cm in diameter is referred to as micro-GIST. Most of small GISTs were discovered incidentally. Although most small GIST or micro-GIST show benign or indolent clinical course, there are still very few cases showing aggressive behavior, especially those tumors with high mitotic counts (26).
Risk assessment of primary GIST after complete resection
The risk assessment is applied to the primary GIST after complete resection. The following conditions are not candidates for risk assessment: all types of biopsy specimens, including fine needle aspiration biopsy, core needle biopsy, and endoscopic biopsy; recurrent and/or metastatic GIST; GIST that has undergone targeted therapy.
The recurrence risk assessment system for the primary GIST after complete resection includes modified National Institutes of Health (NIH) classification system (2008), World Health Organization (WHO) TNM classification system (2013), American Forces Institute of Pathology (AFIP) criteria, and National Comprehensive Cancer Network (NCCN) Guideline biological behavior predictor system (2016 second version), prognostic contour map, and nomogram (1,27-31). In view of the simplicity in application, the CSCO Expert Committee recommends the modified NIH classification, which may be more suitable for Asian population. While there is still no perfect evaluation system, each center should combine the risk assessment with different circumstances. In the case of mitotic count, the existing evaluation system uses 50 high power fields (HPF) but the microscope eyepiece used by each center is different. The Expert Committee recommends the use of 5 mm2 instead of the 50 HPF. Corresponding to the microscope with 22 mm eyepiece, which is used by most units, the actual count fields is 21 HPF (10 mm2 is equal to 42 HPF). In addition, the risk assessment of GIST can be inconsistent with the clinical and pathological findings. Clinicians participate in GIST targeted therapy should analyze the clinical, imaging and pathological data to make the decision.
It should be noted that SDH-deficient GIST is different from common GIST, and mitotic count cannot be used as risk assessment indicators. Cases with low mitotic counts may develop liver metastasis, while cases with high mitotic counts may not. Another characteristic is that it takes longer time to develop metastasis, so long-term follow-up is necessary.
Standardized GIST pathological diagnosis report
The pathological report should be standardized. It must indicate the primary site, tumor size, mitotic counts, and presence of tumor rupture accurately. The IHC results are indispensable. Results of molecular pathology test should be attached if necessary. The recommended standardized GIST pathology report is attached at the end of this article.
Principle of surgical treatment
Biopsy
Principle of biopsy
Surgical resection can be performed when complete removal can be achieved without affecting the function of the relevant organ. Routine biopsy is not recommended for most patients with resectable GIST. If preoperative medication is necessary, biopsy should be performed. The biopsy should be carried out very carefully because inappropriate biopsy may cause tumor rupture, bleeding, and increase risk of tumor dissemination (32).
Indications of biopsy
The indications of biopsy are as followings: for cases needing multiple organ resection or the surgery may significantly affect the relevant organ function, preoperative biopsy should be considered to establish the pathological diagnosis for treatment decision making; for cases with unresectable GIST or R0 resection of the lesion is estimated difficult to obtain and preoperative medication is considered, biopsy should first be conducted; for initially suspected GIST, preoperative biopsy is needed to exclude other neoplasms, e.g., lymphoma; for clinically suspected recurrent and metastatic GIST, biopsy is needed to confirm the diagnosis before drug treatment.
Methods of biopsy
The methods of biopsy include: endoscopic ultrasonography guided fine needle aspiration (EUS-FNA), core needle biopsy (CNB), endoscopic biopsy, transrectal or transvaginal needle aspiration biopsy, and intraoperative frozen biopsy. EUS-FNA is the first choice because of the low probability of intraluminal implantation. However, it is limited to the lumen of the digestive tract where endoscopic ultrasonography is available. Another limitation is that the diagnosis is sometimes difficult because of the small amount of tissue obtained. CNB can be performed as percutaneous puncture under the guidance of ultrasound or computed tomography (CT). The consistency with the surgical specimens in term of IHC can be up to 90% and the diagnostic accuracy can also reach more than 90%. However, because of the risk of tumor rupture and intraperitoneal implantation, this method is often used for metastatic lesions. Endoscopic biopsy is suitable for the cases with mucosa involvement but sometimes can cause serious bleeding of tumor. Transrectal or transvaginal needle aspiration biopsy can be used for rectal, rectovaginal septum, or pelvic masses. Intraoperative frozen biopsy is not recommended routinely unless lymph node metastasis is suspected or other malignancies could not be ruled out during the operation (32).
Surgery
Principle of surgery
Surgical resection is the preferred treatment for localized and potentially resectable GIST. The objective of operation is to achieve R0 resection as far as possible. For the case of R1 resection, positive microscopic margin, adjuvant targeted therapy is recommended as there is no evidence that re-operation has prognostic benefit. Given the fact that GIST rarely has lymph node metastasis, routine dissection of regional lymph nodes is not needed. If enlarged lymph nodes were observed and lymphatic metastasis was suspected during operation, SDH-deficient GIST should be considered and the enlarged lymph nodes should be dissected (16). Tumor rupture should be avoided as far as possible and the pseudocapsule should be kept intact. The causes of tumor rupture include spontaneous tumor rupture and bleeding which is less frequent, and improper grasp of the tumor. Therefore, careful manipulation should be taken during the operation.
Indications of surgery
Localized GIST can be removed by surgery without preoperative medication. When the tumor is assessed as unresectable or potentially resectable but the surgery is with significant risk or may seriously affect the function of organs, preoperative molecular targeted drug therapy should be carried out to shrink the tumors before surgery. For suspected GIST located on stomach with less than 2 cm in diameter and without symptom, the indication of surgery shall be determined according to the risk classification of endoscopic ultrasound, the adverse factors include irregular border, ulcer, strong echo and heterogeneity. When the adverse factors are present, surgery should be considered. When adverse factors are absent, periodical endoscopic ultrasonography follow-up with a time interval of 6–12 months is reasonable. GIST locating in other sites owns relatively high malignancy, surgical resection should be considered once it is detected. For GIST locating in special anatomic sites, such as rectum, gastroesophageal junction, and duodenum, considering that the operation difficulty, for example, anus or cardiac preserving and multiviseral resection, will increase significantly once the tumor increases in size, surgery should be performed as early as possible. After an initially unresectable GIST receiving imatinib therapy to reach the remission and the lesion is then assessed resectable, surgery should be conducted as soon as possible.
For recurrent or metastatic GIST, surgical consideration varies based on the following circumstances: prior to molecular targeted drug therapy, if complete removal of the tumor is estimated feasible with minor risk, surgery combined with subsequent drug therapy can be considered; for cases that molecular targeted drug therapy is effective and the tumor maintains stable, if all lesions are estimated as resectable, surgical removal of all the lesions is also indicated; for recurrent/metastatic cases with limited progression, if disease remains stable by molecular targeted therapy except for only one or a few lesions in progression, patients with good general condition can be chosen carefully for surgical resection. The goal of surgery is to remove the progressive lesion and more metastases as far as possible and achieve a satisfactory tumor reduction surgery; for the cases show general progression under molecular targeted drug therapy, surgery is not recommended; palliative tumor reduction surgery is limited to the cases that patients can tolerate surgery and surgery is predicted to improve the quality of life (33).
The indications of emergency operation include: complete intestinal obstruction or perforation of digestive tract caused by the tumor, gastrointestinal hemorrhage during conservative treatment, and intraperitoneal hemorrhage caused by spontaneous rupture of tumor.
Operation methods
Open surgery is still a commonly used method of surgery in GIST. In most cases, wedge or segmental resection is enough for radical resection of the tumor. The objective of the surgical resection is to achieve minimal complications and avoid complicated operations (e.g. total gastrectomy, abdominal-perineal resection, etc.) or multiple organ resections (e.g. pancreatoduodenectomy). In cases where GIST locating in special locations, function preservation or organ-sparing procedures, such as sphincter-sparing surgery for lower rectum and esophagus-sparing surgery for gastroesophageal junction, is recommended respectively (27). For cases involving repeated surgery or organ function preserving, multidisciplinary consultation is recommended for whether preoperative imatinib is necessary. For GIST locating in rectum or rectovaginal septum, local resection under lithotomy position or jackknife position should be considered.
The indications for laparoscopic surgery have been expanding in recent years. Laparoscopic resection can be performed according to the location and size of the tumor in an experienced medical center. Lesions less than 5 cm in diameter locating in favorable anatomic sites, such as the greater curvature or anterior wall of gastric body and fundus, can be considered by laparoscopic method. The significance of laparoscopic operation for jejunum and ileum GIST is mainly to explore and locate the lesion. In addition, small GIST in the upper rectum can also be considered with laparoscopic resection. If the tumor in large size needs a larger abdominal incision to complete the removal, laparoscopic surgery is not recommended. Since tumor rupture is an independent adverse prognostic factor, surgery should follow the principle of “no touch, less compression” and must use “extract bag” to avoid tumor rupture and spillage (34-36).
Endoscopic resection
Since most GISTs originate from the muscularis propria and have varying growth patterns, the boundary between tumor and adjacent muscle tissue is not very clear; it is not easy to radically resect the lesion under endoscopy. In addition, the incidence of complications, such as hemorrhage, perforation, tumor cell implantation is relatively high. Presently, there are still insufficient studies concerning long-term safety of endoscopic resection in GIST. Thus, the endoscopic method is not recommended as a routine treatment for GIST.
Principles of molecular targeted drug therapy
Preoperative targeted therapy
Significance of preoperative targeted therapy
The significance of preoperative molecular targeted therapy is as followings: to reduce the tumor volume and thereby down stage; to minimize the extent of operation; to avoid unnecessary multiple organ resection; to reduce the risk of surgery while to increase the chance of radical resection; to protect the structure and function of important organs for tumor locating in special sites; and to reduce the risk of iatrogenic dissemination.
Indications of preoperative targeted therapy
The indications of preoperative targeted therapy include: R0 resection assessed as difficult to achieve preoperatively; huge tumor (>10 cm in diameter) and with significant risk of intraoperative rupture or hemorrhage leading to iatrogenic dissemination; tumor locating in special anatomic sites, such as gastroesophageal junction, duodenum and lower rectum, and injury to major organs function is estimated to be inevitable; potentially resectable tumor but with significant operation risk, high recurrence rate and high mortality; tumor is estimated for multiple organ resection surgery; patients with recurrent, metastasis or difficulty in resection, preoperative targeted therapy is also feasible for tumor shrinkage before cytoreduction surgery (37-39).
Duration of preoperative targeted therapy
During the molecular targeted drug treatment, the response to treatment should be evaluated at regular intervals (every 2–3 months). The evaluation should refer to Choi criteria (40) or Response Evaluation Criteria in Solid Tumors (RECIST) (41). With regard to the duration of preoperative treatment, it is generally accepted that 6 to 12 months’ preoperative treatment of imatinib is reasonable (38,42). Prolonged treatment may lead to secondary resistance.
Before molecular targeted drug treatment, genotyping is recommended to optimize the initial dose of imatinib. For progression develops under imatinib treatment, comprehensive assessment of the disease should be taken. If the progression lesion is potentially resectable, discontinuation of the drug and surgical intervention should be considered. For cases that surgery is not feasible, the patients should be treated with second-line treatment in accordance with the recurrence/metastasis cases.
Withholding medication and postoperative treatment
It is suggested that the molecular targeted drugs are withheld 1–2 weeks before operation and the operation can be carried out until the basic condition of the patient reaches the requirement for operation. In principle, as long as the patient’s gastrointestinal function is restored and the medicine can be tolerated, drug treatment should be carried out as soon as possible. For R0 resection patients, the postoperative medication duration can refer to the principle of adjuvant therapy. The recurrence risk classification should be assessed based on the risk factors before drug treatment. For palliative resection of recurrent or metastatic disease, regardless of whether R0 resection is achieved, the postoperative treatment of molecular targeted therapy should refer to the principle of recurrent or metastatic disease without surgery.
Adjuvant therapy
Indication of adjuvant therapy
Risk classification is the main criteria for evaluation of adjuvant therapy. NIH 2008 classification modified by Chinese consensus is recommended to evaluate the recurrent risk. Patients with GIST of intermediate to high risk are the candidates of adjuvant therapy (43-47).
GISTs with PDGFRA exon 18 D842V mutation show primary resistance to imatinib and fail to benefit from adjuvant therapy, thus imatinib adjuvant therapy is not recommended. Whether GIST with c-kit exon 9 mutation and wild-type GIST could benefit from adjuvant therapy is controversial, there are still insufficient evidences because the relevant studies are based on small sample size, which cannot be used to evaluate treatment indications. Further clinical study is suggested (43,46).
Duration and dosage of adjuvant therapy
Disregarding the genotype of GIST, the recommended adjuvant imatinib dosage is 400 mg/d. Studies demonstrate that it is controversial whether patients with c-kit exon 9-mutant GIST can benefit from imatinib with 400 mg/d. At present, there is not enough evidence showing the benefit of increasing the dosage to 600 mg/d or 800 mg/d for exon 9 mutation patients.
For intermediate-risk GIST, non-gastric origin GIST, small intestinal and colorectal GIST, show higher risk of recurrence compared with gastric GIST. Therefore 3-year adjuvant imatinib therapy is recommended for non-gastric GISTs (48), whereas 1-year adjuvant imatinib therapy is recommended for gastric intermediate-risk GIST.
At least 3 years of adjuvant therapy is recommended for patients with high-risk GIST (45,46). Extended duration of adjuvant therapy should be considered for patients with tumor rupture.
That recurrence or metastasis occurs during imatinib adjuvant therapy suggests imatinib resistance, and such cases should be managed according to the principle of progression disease.
There is a lack of high level evidence-based proofs to guide the treatment of recurrence or metastasis after the adjuvant therapy course. Prospective clinical trials are recommended.
Management of recurrent/metastatic/unresectable GIST
First line treatment with imatinib
Imatinib is the first-line therapy for recurrent/metastatic/unresectable GIST with the standard dose of 400 mg/d (49). In western countries, the initial dose of imatinib is suggested to elevate to 800 mg/d for GIST harboring c-kit exon 9 mutation (50). Since only a few Chinese patients can tolerate imatinib treatment at 800 mg/d (51), it is recommended that initial 600 mg/d imatinib treatment could be used for patients with c-kit exon 9-mutant GIST. Patients with good physical scores can be directly treated with imatinib at 800 mg/d.
For recurrent/metastatic/unresectable GIST, first-line imatinib treatment should be continued until the disease progresses or intolerable toxicity emerges.
The common adverse reactions of imatinib include: edema, gastrointestinal reactions, leukopenia, anemia, skin rash, muscle cramps, diarrhea, etc (49). Most adverse reactions are mild to moderate and only symptomatic and supportive treatment is needed.
Treatment options for progression disease
If disease progression develops during imatinib treatment, it should first to confirm whether patients strictly follow the doctor’s advice and take medication in the right dose. After excluding the compliant factors, patients should be directed in accordance with the following principles.
Limited progression is defined as progression of some lesions during imatinib treatment, while other lesions remained stable or even partial remission.
For limited progression cases, surgery is recommended if the progressive foci can be completely removed. According to the condition assessment, postoperative treatment options include imatinib at original dose, shift to sunitinib, or dose escalation of imatinib. If complete resection is not obtained, the follow-up and subsequent treatment should comply with the principles of GIST with generalized progression and surgery is not recommended.
For patients with liver metastasis who is not candidate of surgery, arterial embolization and radiofrequency ablation can also be considered as part of palliative treatment (52). When above local interventions are not feasible, second-line sunitinib treatment or imatinib dose escalation is recommended.
For generalized progression disease under standard dose imatinib therapy, it is recommended to shift to sunitinib or escalate the dose of imatinib (53,54). Both continuous 37.5 mg/d and 50.0 mg/d (4/2) programs are available for sunitinib therapy. Despite the lack of randomized controlled trials, sunitinib 37.5 mg/d may have better efficacy and better tolerability. Data from Chinese studies shows that the benefit of sunitinib therapy in Chinese patients is better than that of western patients, and the adverse reactions can be relieved by symptomatic treatment. With regard to imatinib dose escalation, taking into account therapeutic tolerance, it is recommended to increase the imatinib dose to 600 mg/d first (51). The adverse effects of imatinib dose escalation can be relieved by symptomatic treatment.
Regorafenib is indicated to treat metastatic/unresectable GIST after failure of imatinib and sunitinib. It can significantly prolong the overall survival of patients and is recommended as third-line treatment. The common adverse reactions were fatigue, hypertension, hand foot syndrome, oral mucositis, anemia, and granulocyte depletion (55). If the patient cannot benefit from regorafenib, the patient are encouraged to participate in clinical research on new drugs, or are considered giving drugs that were effective and well tolerated previously for maintenance treatment.
Correlation between c-kit/PDGFRA gene mutation and therapeutic efficacy of molecular targeted therapy
The type of c-kit/PDGFRA mutation can predict the efficacy of molecular targeted drugs. In first-line therapy, patients with GIST harboring c-kit exon 11 mutation respond to imatinib therapy best (49). In second-line therapy, the patients with GIST harboring c-kit exon 9 mutation or with wild-type GIST have better survival benefit under sunitinib therapy compared with patients with c-kit exon 11-mutant GIST. With regard to secondary mutations, patients with GIST harboring c-kit exon 13 and exon 14 mutations have better response to sunitinib than patients with c-kit exon 17 and exon 18-mutant GIST (56,57). In third-line therapy, GIST patients with secondary mutations in c-kit exon 17 achieve good therapeutic effect under regorafenib treatment. While GIST with PDGFRA D816V and D842V mutation may show primary resistance to all the three tyrosine kinase inhibitors (58).
Therapeutic drug monitoring
If possible, it is recommended to monitor the imatinib plasma concentration on the following patients: patients who developed progression under first-line imatinib therapy at 400 mg/d dose; patients with severe drug adverse reactions which are caused by excessive imatinib plasma concentration. It is reasonable to reduce imatinib dose with minimal effective blood concentration; for incompliant patients who did not take regular medication, if the plasma imatinib concentration is lower than 1,100 ng/mL, the clinical efficacy will decrease and the disease will progress rapidly (59,60).
Imaging in response evaluation
Primary resistance and secondary resistance
Primary resistance to imatinib is defined as the evidence of clinical progression developing within 6 months of first-line imatinib therapy. Secondary resistance is seen in patients receiving imatinib treatment for more than 6 months with initial response of remission or stable followed by progression. Defining the nature of primary and secondary drug resistance is helpful in assessing the biological behavior and resistance mechanisms of GIST, and is of great significance for the rational formulation of a subsequent treatment strategy.
Response assessment criteria
In the past, RECIST criteria were the standard of efficacy evaluation of cytotoxic drugs and there was obvious defect that only the volume of tumor focus was taken into account. Choi et al. (40) proposed a new standard based on combining Hu value in CT with tumor diameter. Some studies have shown that its evaluation capacity may be superior to RECIST criteria. The Expert committee recommends that for the early evaluation of response, when the tumor size reduction is not obvious or even increased, the Hu value in CT should be supplemented and evaluated according to the Choi criteria.
Application of CT, magnetic resonance imaging (MRI), and positron emission tomography (PET)-CT
The CT scan should cover the entire abdominal and pelvic region and the layer of thickness should be ≤5 mm. The maximum tumor diameter should be measured with axial line. The overall CT value (Hu) should be obtained in the layer with maximum diameter of tumor in venous enhancement phase, by curve edge tracing method. If possible, the average CT value of the lesions should be reported (61).
PET-CT scanning is the most sensitive method in diagnosis of GIST and evaluation response of molecular targeted drug therapy, but the relative high cost limits its widespread use. PET-CT can be used to determine the response to targeted therapy at earlier phase than CT, but is not recommended for routine follow-up.
Magnetic resonance diffusion weighted imaging (MR-DWI) may be another imaging technique to provide functional quantitative indicators other than PET-CT (62,63). But the exact clinical significance of MR-DWI needs to be further studied.
Principles of surveillance
Surveillance for patients after complete resection
The most common sites of metastasis after operation are the peritoneum and the liver, so abdominal and pelvic enhanced CT or MRI scan is recommended as a routine follow-up. But PET-CT scan is sometimes required when CT scans are inconclusive. Intermediate and high-risk patients should undergo CT or MRI examinations every 3 months for 3 years, then every 6 months until the 5th year, and once per year after then. Low-risk patients should undergo CT or MRI examination every 6 months for 5 years. Since metastasis to lungs and bone is rare, the chest X-ray examination is suggested to be carried out at least once a year. Emission computed tomography (ECT) bone scan is recommended for the patients with associated symptoms.
Surveillance for recurrent/metastatic/unresectable/neoadjuvant patients
Baseline enhanced CT or MRI is necessary before the treatment. After the start of the treatment, CT or MRI should be carried out at least every 3 months during the follow-up. When decision making is involved, increased frequency of follow-up is acceptable. Closely monitoring in the initial stage of treatment, the first 3 months, is very important, and PET-CT scan should be considered to confirm the response to treatment, if necessary. Therapeutic drug monitoring, for example plasma imatinib concentration, is helpful to guide clinical management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. WHO Classfication of Tumours of Soft Tissue and Bone. 4th edition. Lyon: IARC Press, 2013,164-7.
- Wada R, Arai H, Kure S, et al. " Wild type” GIST: Clinicopathological features and clinical practice. Pathol Int 2016;66:431–7. [PubMed] DOI:10.1111/pin.12431
- Hou YY, Lu SH, Zhou Y, et al. Predictive values of clinical and pathological parameters for malignancy of gastrointestinal stromal tumors. Histol Histopathol 2009;24:737–47. [PubMed] DOI:10.14670/HH-24.737
- Agaram NP, Baren A, Arkun K, et al. Comparative ultrastructural analysis and KIT/PDGFRA genotype in 125 gastrointestinal stromal tumors . Ultrestruct Pathol 2006;30:443–52. [PubMed] DOI:10.1080/01913120600854186
- Wang M, Xu J, Zhao W, et al. Prognostic value of mutational characteristics in gastrointestinal stromal tumors: a single-center experience in 275 cases. Med Oncol 2014;31:819. [PubMed] DOI:10.1007/s12032-013-0819-x
- Huss S, Pasternack H, Ihle MA, et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events . Hum Pathol 2017;62:206–14. [PubMed] DOI:10.1016/j.humpath.2017.01.005
- Wada N, Kurokawa Y, Takahashi T, et al. Detecting secondary C-KIT mutations in the peripheral blood of patients with imatinib-resistant gastrointestinal stromal tumor . Oncology 2016;90:112–7. [PubMed] DOI:10.1159/000442948
- Gao J, Li J, Li Y, et al. Intratumoral KIT mutational heterogeneity and recurrent KIT/PDGFRA mutations in KIT/PDGFRA wild-type gastrointestinal stromal tumors. Oncotarget 2016;7:30241–9. [PubMed] DOI:10.18632/oncotarget.7148
- Jasek K, Buzalkova V, Minarik G, et al. Detection of mutations in the BRAF gene in patients with KIT and PDGFRA wild-type gastrointestinal stromal tumors . Virchows Arch 2017;470:29–36. [PubMed] DOI:10.1007/s00428-016-2044-4
- Miettinen M, Fetsch JF, Sobin LH, et al. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol 2006;30:90–6. [PubMed]
- Pantaleo MA, Nannini M, Corless CL, et al. Quadruple wild-type (WT) GIST: defining the subset of GIST that lacks abnormalities of KIT, PDGFRA, SDH, or RAS signaling pathways. Cancer Med 2015;4:101–3. [PubMed] DOI:10.1002/cam4.325
- Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations . Proc Natl Acad Sci U S A 2011;108:314–8. [PubMed] DOI:10.1073/pnas.1009199108
- Italiano A, Chen CL, Sung YS, et al. SDHA loss of function mutations in a subset of young adult wild-type gastrointestinal stromal tumors. BMC Cancer 2012;12:408. [PubMed] DOI:10.1186/1471-2407-12-408
- Oudijk L, Gaal J, Korpershoek E, et al. SDHA mutations in adult and pediatric wild-type gastrointestinal stromal tumors . Mod Pathol 2013;26:456–63. [PubMed] DOI:10.1038/modpathol.2012.186
- Pantaleo MA, Astolfi A, Urbini M, et al. Analysis of all subunits, SDHA, SDHB, SDHC, SDHD, of the succinate dehydrogenase complexin KIT/PDGFRA wild-type GIST. Eur J Hum Genet 2014;22:32–9. [PubMed] DOI:10.1038/ejhg.2013.80
- Doyle LA, Nelson D, Heinrich MC, et al. Loss of succinate dehydrogenase subunit B (SDHB) expression is limited to a distinctive subset of gastric wild-type gastrointestinal stromal tumours: a comprehensive genotype-phenotype correlation study. Histopathology 2012;61:801–9. [PubMed] DOI:10.1111/j.1365-2559.2012.04300.x
- Daniels M, Lurkin I, Pauli R, et al. Spectrum of KIT/PDGFRA/BRAF mutations and Phosphatidylinositol-3-Kinase pathway gene alterations in gastrointestinal stromal tumors (GIST). Cancer Lett 2011;312:43–54. [PubMed] DOI:10.1016/j.canlet.2011.07.029
- Agaimy A, Terracciano LM, Dirnhofer S, et al. V600E BRAF mutations are alternative early molecular events in a subset of KIT/PDGFRA wild-type gastrointestinal stromal tumours . J Clin Pathol 2009;62:613–6. [PubMed] DOI:10.1136/jcp.2009.064550
- Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer 2008;47:853–9. [PubMed] DOI:10.1002/gcc.20589
- Anderson J, Sihto H, Meis-Kindblom JM, et al. NF1-associated gastrointestinal stromal tumors have unique clinical, phenotypic and genotypic characteristics. Am J Surg Pathol 2005;29:1170–6. [PubMed]
- Maertens O, Prenen H, Debiec-Rychter M, et al. Molecular pathogenesis of multiple gastrointestinal stromal tumors in NF1 patients. Hum Mol Genet 2006;15:1015–23. [PubMed] DOI:10.1093/hmg/ddl016
- Miranda C, Nucifora M, Molinari F, et al. KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors. Clin Cancer Res 2012;18:1769–76. [PubMed] DOI:10.1158/1078-0432.CCR-11-2230
- Nannini M, Astolfi A, Urbini M, et al. Integrated genomic study of quadruple-WT GIST (KIT/PDGFRA/SDH/RAS pathway wild-type GIST). BMC Cancer 2014;14:685. [PubMed] DOI:10.1186/1471-2407-14-685
- Lasota J, Felisiak-Golabek A, Wasag B, et al. Frequency and clinicopathologic profile of PIK3CA mutant GISTs: molecular genetic study of 529 cases . Mod Pathol 2016;29:275–82. [PubMed] DOI:10.1038/modpathol.2015.160
- Brenca M, Rossi S, Polano M, et al. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST . J Pathol 2016;238:543–9. [PubMed] DOI:10.1002/path.4677
- Gao Z, Wang C, Xue Q, et al. The cut-off value of tumor size and appropriate timing of follow-up for management of minimal EUS-suspected gastric gastrointestinal stromal tumors. BMC Gastroenterol 2017;17:8. [PubMed] DOI:10.1186/s12876-016-0567-4
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411–9. [PubMed] DOI:10.1016/j.humpath.2008.06.025
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83. [PubMed]
- von Mehren M, Randall RL, Benjamin RS, et al. Soft Tissue Sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:758–86. [PubMed] DOI:10.6004/jnccn.2016.0078
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265–74. [PubMed] DOI:10.1016/S1470-2045(11)70299-6
- Gold JS, Gönen M, Gutiérrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 2009;10:1045–52. [PubMed] DOI:10.1016/S1470-2045(09)70242-6
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1–41;quiz S42-4. [PubMed]
- Du CY, Zhou Y, Song C, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a prospective randomised trial in China. Eur J Cancer 2014;50:1772–8. [PubMed] DOI:10.1016/j.ejca.2014.03.280
- Ford SJ, Gronchi A. Indications for surgery in advanced/metastatic GIST. Eur J Cancer 2016;63:154–67. [PubMed] DOI:10.1016/j.ejca.2016.05.019
- Huang CM, Chen QF, Lin JX, et al. Can laparoscopic surgery be applied in gastric gastrointestinal stromal tumors located in unfavorable sites?: A study based on the NCCN guidelines. Medicine (Baltimore) 2017;96:e6535. [PubMed] DOI:10.1097/MD.0000000000006535
- Feng X, Li R, Zhang P, et al. Current status of surgical treatment of gastric gastrointestinal tumors: a national multi-center retrospective study. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2016;19:1258–64. [PubMed]
- Blesius A, Cassier PA, Bertucci F, et al. Neoadjuvant imatinib in patients with locally advanced non metastatic GIST in the prospective BFR14 trial. BMC Cancer 2011;11:72. [PubMed] DOI:10.1186/1471-2407-11-72
- Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 2009;99:42–7. [PubMed]
- Wang C, Zheng B, Chen Y, et al. Imatinib as preoperative therapy in Chinese patients with recurrent or metastatic GISTs. Chin J Cancer Res 2013;25:63–70. [PubMed] DOI:10.3978/j.issn.1000-9604.2012.12.01
- Choi H. Response evaluation of gastrointestinal stromal tumors. Oncologist 2008;13 Suppl 2:4–7. [PubMed] DOI:10.1634/theoncologist.13-S2-4
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [PubMed] DOI:10.1016/j.ejca.2008.10.026
- Sjölund K, Andersson A, Nilsson E, et al. Downsizing treatment with tyrosine kinase inhibitors in patients with advanced gastrointestinal stromal tumors improved resectability. World J Surg 2010;34:2090–7. [PubMed] DOI:10.1007/s00268-010-0639-5
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097–104. [PubMed] DOI:10.1016/S0140-6736(09)60500-6
- Zhan WH, Wang PZ, Shao YF, et al. Efficacy and safety of adjuvant post-surgical therapy with imatinib in gastrointestinal stromal tumor patients with high risk of recurrence: interim analysis from a multicenter prospective clinical trial. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2006;9:383–7. [PubMed]
- Li J, Gong JF, Wu AW, et al. Post-operative imatinib in patients with intermediate or high risk gastrointestinal stromal tumor. Eur J Surg Oncol 2011;37:319–24. [PubMed] DOI:10.1016/j.ejso.2011.01.005
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265–72. [PubMed] DOI:10.1001/jama.2012.347
- Lin JX, Chen QF, Zheng CH, et al. Is 3-years duration of adjuvant imatinib mesylate treatment sufficient for patients with high-risk gastrointestinal stromal tumor? A study based on long-term follow-up. J Cancer Res Clin Oncol 2017;143:727–34. [PubMed] DOI:10.1007/s00432-016-2334-x
- Wu X, Li J, Shen L, et al. Imatinib adjuvant therapy in intermediate risk gastrointestinal stromal tumor — a multi-center retrospective study. The 11th International Gastric Cancer Congress, São Paulo, Brazil, 2015.
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472–80. [PubMed] DOI:10.1056/NEJMoa020461
- Zalcberg JR, Verweij J, Casali PG, et al. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer 2005;41:1751–7. [PubMed] DOI:10.1016/j.ejca.2005.04.034
- Li J, Gong JF, Li J, et al. Efficacy of imatinib dose escalation in Chinese gastrointestinal stromal tumor patients. World J Gastroenterol 2012;18:698–703. [PubMed] DOI:10.3748/wjg.v18.i7.698
- Cao G, Li J, Shen L, et al. Transcatheter arterial chemoembolization for gastrointestinal stromal tumors with liver metastases. World J Gastroenterol 2012;18:6134–40. [PubMed] DOI:10.3748/wjg.v18.i42.6134
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329–38. [PubMed] DOI:10.1016/S0140-6736(06)69446-4
- Li J, Gao J, Hong J, et al. Efficacy and safety of sunitinib in Chinese patients with imatinib-resistant or -intolerant gastrointestinal stromal tumors. Future Oncol 2012;8:617–24. [PubMed] DOI:10.2217/fon.12.29
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295–302. [PubMed] DOI:10.1016/S0140-6736(12)61857-1
- Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 2008;26:5352–9. [PubMed] DOI:10.1200/JCO.2007.15.7461
- Gao J, Tian Y, Li J, et al. Secondary mutations of c-KIT contribute to acquired resistance to imatinib and decrease efficacy of sunitinib in Chinese patients with gastrointestinal stromal tumors. Med Oncol 2013;30:522. [PubMed] DOI:10.1007/s12032-013-0522-y
- Weisberg E, Wright RD, Jiang J, et al. Effects of PKC412, nilotinib, and imatinib against GIST-associated PDGFRA mutants with differential imatinib sensitivity. Gastroenterology 2006;131:1734–42. [PubMed] DOI:10.1053/j.gastro.2006.09.017
- Xu H, Ma L, Xu W, et al. A Chinese multi-center study on the significance of monitoring imatinib plasma concentration in patients with gastrointestinal stromal tumor before and after administration. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2016;19:1271–6. [PubMed]
- Yin Y, Xiang J, Tang S, et al. A lower dosage of imatinib in patients with gastrointestinal stromal tumors with toxicity of the treatment. Medicine (Baltimore) 2016;95:e5488. [PubMed] DOI:10.1097/MD.0000000000005488
- Tang L, Zhang XP, Sun YS, et al. Gastrointestinal stromal tumors treated with imatinib mesylate: apparent diffusion coefficient in the evaluation of therapy response in patients. Radiology 2011;258:729–38. [PubMed] DOI:10.1148/radiol.10100402
- Tang L, Sui Y, Zhong Z, et al. Non-Gaussian diffusion imaging with a fractional order calculus model to predict response of gastrointestinal stromal tumor to second-line sunitinib therapy. Magn Reson Med 2017. [Epub ahead of print]
- Pan F, Den J, Zhang C, et al. The therapeutic response of gastrointestinal stromal tumors to imatinib treatment assessed by intravoxel incoherent motion diffusion-weighted magnetic resonance imaging with histopathological correlation. PLoS One 2016;11:e0167720. [PubMed] DOI:10.1371/journal.pone.0167720