Evaluating efficacy of screening for upper gastrointestinal cancer in China: a study protocol for a randomized controlled trial
Introduction
Upper gastrointestinal cancer including esophageal cancer and gastric cancer remains the second leading cause of cancer deaths in China (1) despite steady declines in death rates over the last several decades. These cancers place a heavy burden on the Chinese population – based on the most recent estimates (2013), 704,000 new cases were diagnosed and 507,700 people died of these cancers in China (2). These cancers account for nearly one-half (47%) of the global number of deaths from upper gastrointestinal cancer (3).
Prognosis of upper gastrointestinal cancer depends mainly on disease stage at diagnosis. In China, 5-year survival rate of esophageal cancer was less than 10% when diagnosed at an advanced stage but was as high as 86% if detected at an earlier stage (4). This has formed the rationale for early detection programs which may have potential to improve outcomes. It can help to detect and remove pre-cancer lesions, and detect early stage tumors when treatment is more effective, and thus offers a great opportunity to reduce incidence and mortality.
As a result, a series of nationwide screening programs have been established in several East Asian countries where the incidence rates are relatively high. In Japan, radiographic screening was developed for gastric cancer early in the 1960s and has been conducted as a national screening program since 1983 (5,6). South Korea has introduced both radiographic screening and endoscopic screening for upper gastrointestinal cancer to the national screening programs since 2000 (7). Over the last decade, evidence regarding the effectiveness of upper gastrointestinal cancer screening has been increasingly accumulated. However, most of them are obtained from studies conducted in Japan and Korea, and the study design is limited to observational studies since both countries have already performed national screening.
In China, several screening programs for upper gastrointestinal cancer have been carried out in high-risk areas since the 1970s (8-11), and the “National Key Public Health Projects” was initiated in 2005 (12), which included upper gastrointestinal cancer screening in areas with high incidence rate. Although some promising evidences of mortality reduction by screening emerging from some studies, most of them were based on cohort and case-control studies in high-risk areas, and thus, the results remained inconclusive. For example, Lee et al. reported that the incidence of gastric cancer has been reduced by 25% through “screen and treat” program in an area in the Taiwan Strait (13), and other studies showed that endoscopic screening and intervention significantly reduced the mortality of esophageal cancer in a high-risk area in China (14,15). However, inconsistent result was reported by Riecken et al. They found that there was no impact of repeated endoscopic screens on gastric cancer mortality in a high-risk Chinese population (16).
Despite some evidence on the effectiveness of upper gastrointestinal cancer screening (17), none of previous studies has evaluated its efficacy based on a prospective randomized controlled trial with a large sample size. Whether the screening can achieve a significant reduction in upper gastrointestinal cancer mortality remains unclear. Moreover, there is insufficient information regarding the health economics of the screening.
In this study, we intend to determine whether screening with endoscopic examination, as compared with unscreened controls, would reduce mortality from upper gastrointestinal cancer in China among both high- and non-high-risk persons using a prospective randomized control trial.
Aims
This is a prospective, randomized controlled study based on community population, aiming at evaluating the efficacy of upper gastrointestinal cancer screening in Chinese population. The major objectives of our study are: 1) to establish a multi-center large upper gastrointestinal cancer screening cohort in both high-risk and non-high-risk areas for long time follow-ups in China; 2) to evaluate the efficacy and feasibility of upper gastrointestinal cancer screening for in Chinese population; and 3) to build up a large database and biobank for the cohort subjects for further search.
Methods
Study areas
The study fields were selected based on following consideration: 1) representing three economical-geographical regions (Eastern, Central and Western) in China; 2) the selected centers had established cancer registry and death surveillance systems; 3) the target population were relatively stable; and 4) areas are stratified as high risk or non-high risk of upper gastrointestinal cancer based on the mortality rates of these cancers during the first National Death Survey in 1973–1975 (18).
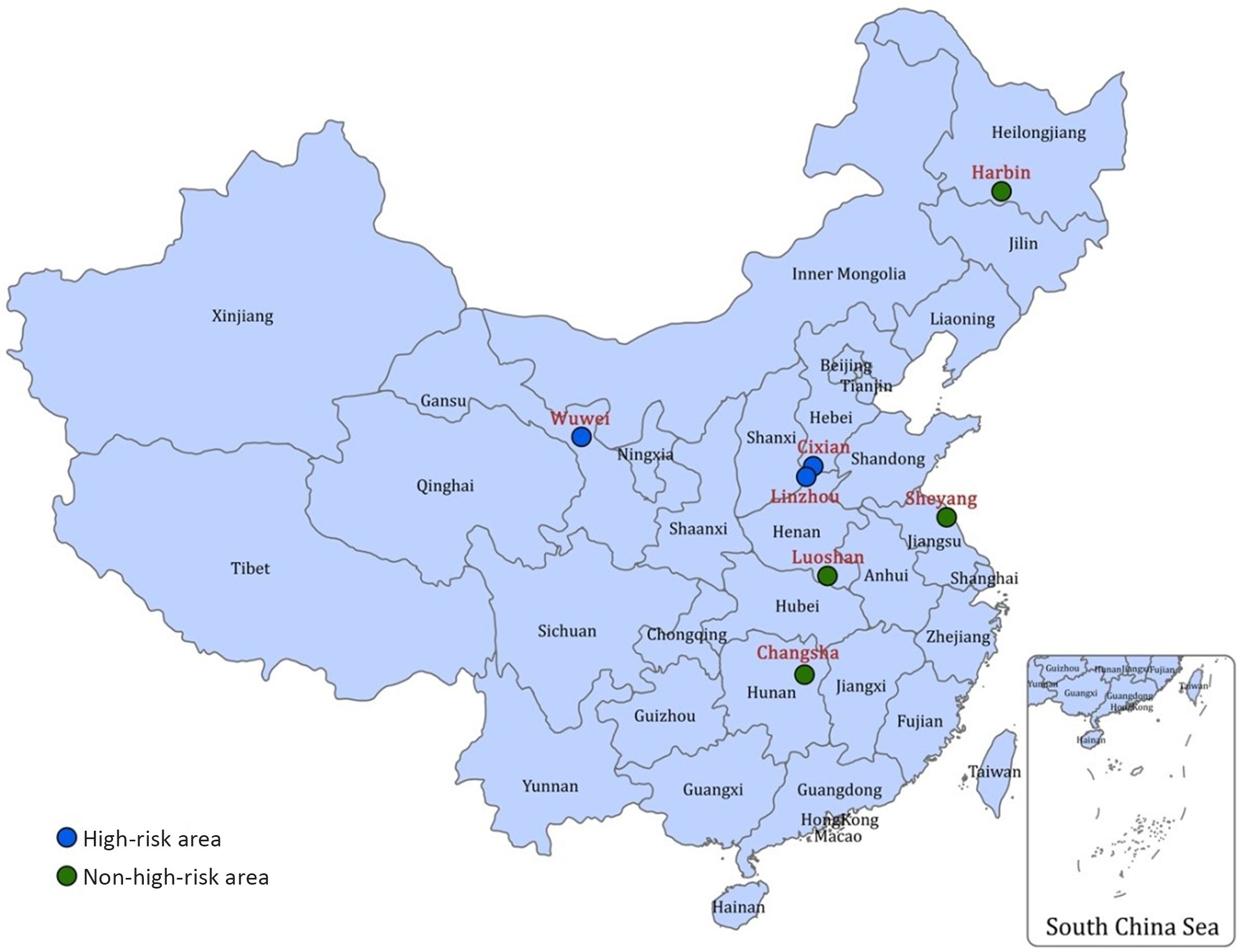
A total of seven cities/counties were selected as the screening centers and locations of them are shown in Figure 1. The areas with high risk of upper gastrointestinal cancer are Linzhou County of Henan Province, Cixian County of Hebei Province, and Wuwei County of Gansu Province, while the non-high-risk areas are Sheyang County of Jiangsu Province, Luoshan County of Henan Province, Harbin City of Heilongjiang Province, and Changsha City of Hunan Province.
Sample size calculation
We estimated that the screening would be effective if it provided a 35% relative decrease in the mortality rate of cancer screening participation in areas with high risk over 3 years. The mortality rate between 40 and 69 years old in these areas was 170/100,000 based on the data from annual report on status of cancer in China (19). The sample size of individual randomization was calculated using parameters and formula as follows (20):
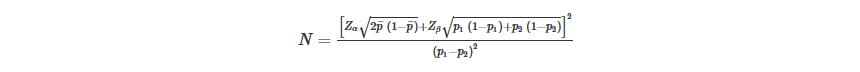
|
p1 and p2 are the expected mortality of upper gastrointestinal cancer in screening and control group, ${\bar p}$ is the mean of p1 and p2, we set significance level α=0.05 and Zα=1.96, statistic power (1-β)=0.8 and Zβ=0.84. Taking into an assumption of design effect of 1.38 based on previously reported (21,22) and account 5% of the loss of follow-up rate, a total of 60,000 subjects are needed to accomplish the hypotheses.
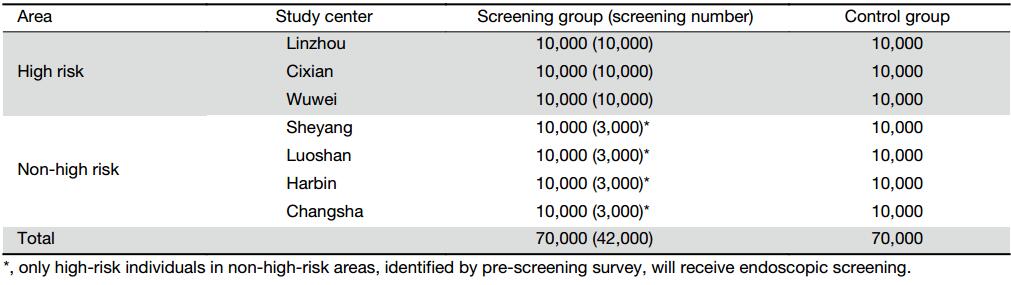
The calculation for sample size in non-high-risk areas was similar, except that the mortality rate between 40 and 69 years old was 60/100,000 (17) and the expected decrease rate is 30% after 10 years of follow-up. A total of 80,000 subjects are needed. The distribution of sample size is presented in Table 1.
Full table
Randomization and enrollment
We identified participants for study inclusion using a stratified cluster sampling design. Before enrollment, a list of the villages/communities in these seven selected areas was provided by each center and a simple random sampling procedure was performed. Villages/communities regarded as clusters were randomly assigned by a computer-generated list to either intervention group (screening by endoscopic examination) or control group (with normal community care - only seeking screening when symptoms occur) in a 1:1 ratio stratified by each center.
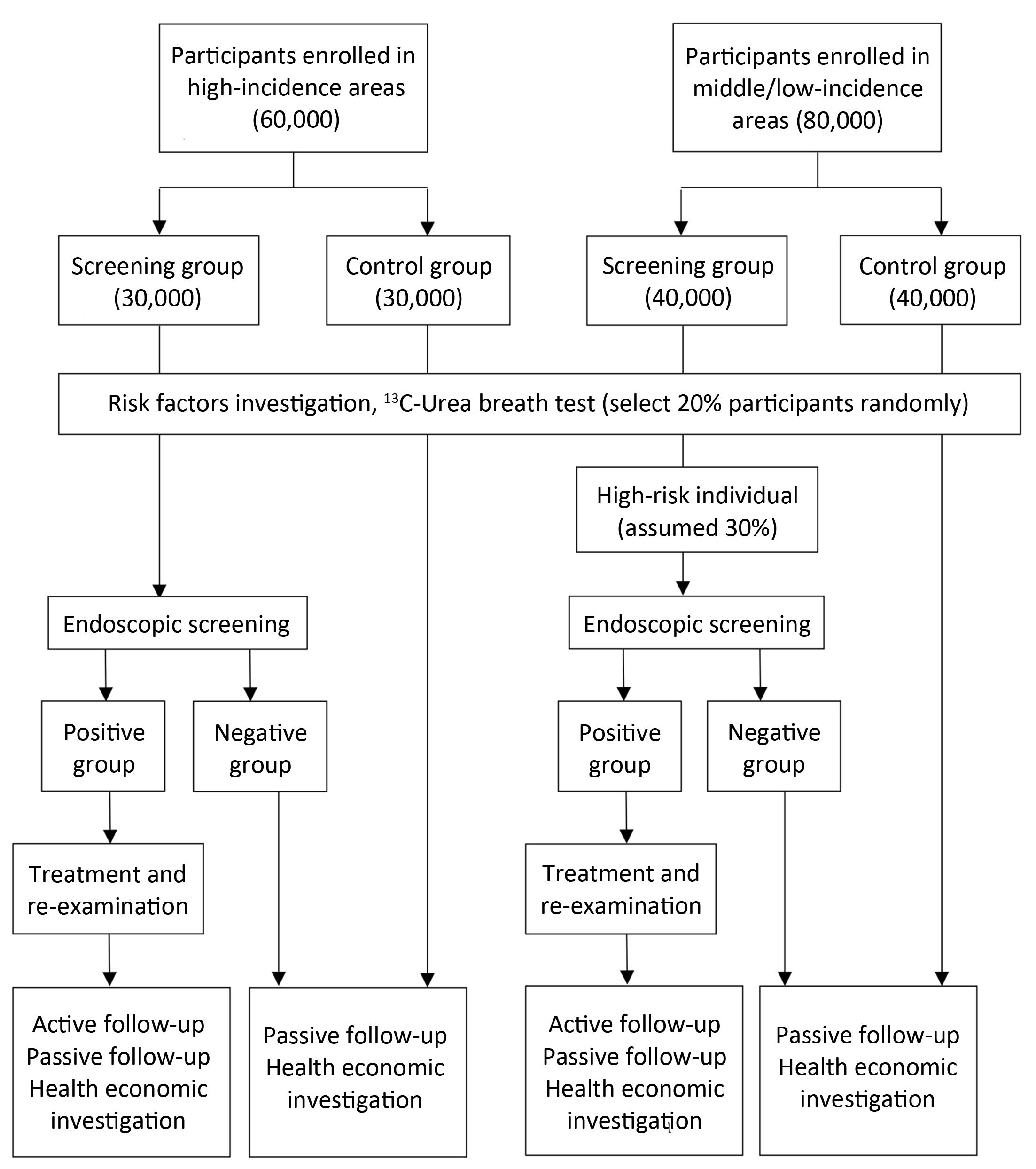
Eligible participants in randomly selected villages/communities, local residents aged 40 to 69 years with no history of cancer or endoscopic examination in the latest 3 years who are mentally and physically competent, will be asked to sign written informed consent for enrollment. Participants’ enrollment began on May 1st, 2015 and a total of 140,000 subjects are expected to be enrolled up to December 31th, 2017 (Figure 2).
The exclusion criteria are as follows: 1) subjects who are not willing to take endoscopic examination; or 2) individuals who are unwilling to sign the consent form.
Screening procedure
Screening subjects
In areas with high risk of upper gastrointestinal cancer, all subjects in screening group will be screened by endoscopy. In non-high-risk areas, subjects in screening group will be surveyed and those with high risk of upper gastrointestinal cancer (i.e., family history of cancer, unhealthy diet or lifestyle, etc.) will be identified and then screened by endoscopy. The assumed proportion of high-risk population is 30%, and thus an average of 3,000 subjects from screening group in each center will take endoscopy screening (Table 1, Figure 2). The remaining 70% will be followed up as controls.
Endoscopic examination and therapy
All endoscopic examinations and therapies are conducted by local doctors after training by and under the supervision of experienced doctors from the National Cancer Center in China. Screened participants are provided a local anesthetic and the entire esophagus and stomach are visually examined including careful examination of the cardia because it is a frequent site of gastric adenocarcinoma. Suspicious lesions are targeted for biopsy, and the number of biopsies taken depended on the size of the lesion. Participants are defined as “positive patients” if they are diagnosed as esophageal severe dysplasia, carcinoma in situ, esophageal cancer, gastric severe dysplasia (high-grade intraepithelial neoplasia), gastric cancer and other cancer patients by screening or other clinical settings.
Corresponding treatment will be provided according to the result of diagnosis. If early lesions are histologically confirmed, participants will be recalled to the clinic, and intervention methods appropriate to the lesions’ severity would be used. For esophageal severe dysplasia/carcinoma in situ or intramucosal carcinomas, cardia and gastric high-grade intraepithelial neoplasia and mucosal carcinoma, endoscopic mucosal resection and/or endoscopic submucosal dissection treatments will be used as local therapies. For esophageal cancers, gastric cardia or gastric cancer, therapies include esophagectomy, radical operation, radiotherapy, and other conventional treatments.
Sample collection
Before endoscopy, up to 10 mL blood will be collected from each participant in the fasting state for routine test for infectious diseases including hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infections, and syphilis. The remaining blood will be dispensed into two pipes and stored at –20℃. After screening, the blood will be transported on dry ice to the National Cancer Center, and be placed in –80℃ freezer for long-term preservation, contributing to the establishment of biobank. Biopsy specimens are fixed in 10% buffered formalin, embedded in paraffin, and transported to the National Cancer Center for long-term preservation for biobank establishment.
Follow up and re-examination
Active follow-up
An active follow-up procedure will be conducted to all “positive patients” every year during the project. Active follow-up refers to the process of identifying patients whose follow-up information is out of date and contacting someone who can provide more recent information. In our study, positive patients or their relatives will receive an interview by telephone, home visit or other contact methods for their condition and outcome each year (Figure 2).
Passive follow-up
A passive follow-up procedure will be carried out in all participants once a year. Passive follow-up refers to the process of updating follow-up information using the linkage of data. We will collect data from local clinical settings, cancer registry system, death surveillance system, as well as medical insurance and claim databases as supplement (Figure 2).
Re-examination
Re-examinations are required based on diagnosis of conditions other than cancer. A re-examination is required in three years for esophageal mild dysplasia, and an annual re-examination is required for esophagus moderate dysplasia, cardia or gastric low-grade intraepithelial neoplasia, severe atrophic gastritis or severe intestinal metaplasia.
For all new cases diagnosed with esophageal cancer, gastric cancer or esophageal/gastric severe dysplasia during the project, detailed information about treatment such as anatomical site, histological type, clinical staging, therapeutic schedule and medical expenses will also be acquired from hospitals where they are treated.
Data collection
Risk factor investigation
A uniform questionnaire will be administered by trained interviewers for all participants to investigate the risk factors of upper gastrointestinal cancer. Information on demography, occupational risk factors, living habits, and dietary, medical and family history will be collected. Twenty percent of participants will be selected randomly in each center to perform the 13C-urea breath test to detect a current infection with Helicobacter pylori.
Health economic information
EuroQol five dimensions questionnaire (EQ-5D) is a standardized instrument for measuring generic health status and EQ-5D-3L uses a three-level scale to describe the health state (11). All positive patients identified by screening will be surveyed through a face-to-face or telephone interview to investigate their medical behavior and related costs, as well as health-related quality of life assessed with the EQ-5D-3L. Three hundred negative participants in each center will also be selected randomly to accomplish the EQ-5D-3L investigation and a questionnaire about the non-medical cost associated with screening. The direct costs on materials, equipment, personnel, medicine and other resources will be collected from all centers to estimate the operating cost of this screening project.
All the data for risk factors and health economic information are entered into database on the spot by the trained interviewer using iPad and a small proportion of the records will be randomly checked for data accuracy.
Outcome assessment
The primary outcome is the mortality caused by upper gastrointestinal cancer. The secondary outcomes include detection rate, incidence rate, survival rate, and clinical stage distribution. Additional data on quality of life and cost-effectiveness (as mentioned in the previous paragraph) will also be collected to answer important questions regarding screening effects.
Data management
We treated all data as protected health information and stored it securely in an encrypted and password-protected database at the National Cancer Center. We securely stored paper charts in a locked room. We developed data management procedures using web-based technology to ensure accurate, efficient and real-time data collection and analysis.
Statistical analyses
The primary analysis is a comparison of upper gastrointestinal cancer mortality between the screening group and the controls. Mortality rate will be calculated as the ratio of the number of death due to upper gastrointestinal cancer to the person-year at risk for each group. Person-years will be estimated from the time of randomization to the date of upper gastrointestinal cancer death or censoring at the end of the study, which will be decided at a later time. The secondary outcomes including incidence rate and detection rates will be estimated in a similar way. SAS software (version 9.2; SAS Institute, Cary, NC, USA) will be used for these statistical analyses. χ2 tests and t-tests are used to compare categorical and continuous variables between the two groups, respectively. The Cox proportional hazards regression model is chosen to analyze the difference of incidence and mortality between screening group and control group.
We will create a decision tree-Markov model to evaluate the cost-effectiveness of endoscopic screening for upper gastrointestinal cancer in China, using the software of TreeAge Pro 2016 (TreeAge Software, Inc., MA, USA) (23). The perspective for the evaluation was societal, and the model was run with a cycle length of 1 year for 36 cycles (i.e. from 40 to 75 years old) in order to cover the entire upper gastrointestinal cancer experiences of the vast majority of cohort members. Base-case values and ranges of parameters used in the model will be estimated from a variety of sources: 1) the results of our prospective evaluation study of screening for upper gastrointestinal cancer; 2) surveillance data of Chinese National Cancer Center; 3) published literatures; 4) government documents; and 5) our survey. Costs and utility scores of upper gastrointestinal cancer-related diseases will be estimated based on our survey. Given the uncertainty about some parameters, deterministic and probabilistic sensitivity analyses will be used to assess the robustness of the model results.
The study is approved by the independent ethics committee of National Good Clinical Practice Center for Anticancer Drugs, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (2015SQ00223), and the study used the Protocol Registration System in Chinese Clinical Trial Registry (identifier: ChiCTR-EOR-16008577).
Discussion
Our study aims at evaluating the efficacy and feasibility of upper gastrointestinal cancer screening in both high-risk and non-high-risk areas in China. To our knowledge, this is the first randomized controlled study on upper gastrointestinal cancer screening based on a community population in China. The study design ensures the most robust method to determine the balance of benefit vs. harm of screening upper gastrointestinal cancer using endoscopic examination. Since previous studies mainly focused on high-risk areas and the study designs were usually observational, the results of our study will contribute unique and high quality information to the evaluation of upper gastrointestinal cancer screening, and make reference for the development and implement of screening strategy.
This trial designed to evaluate upper gastrointestinal cancer screening may have great potential to reduce the disease burden in selected centers and other areas in China. Within the constraints of the design assumptions, the sample size is sufficient to ensure scientifically valid assessment of the impact of the screening on cause-specific mortality. In addition, the trial will investigate secondary endpoints and disease natural history questions, and will provide data that can be used to address relationships among costs, risks, and benefits.
Upper gastrointestinal cancer has remained a serious burden worldwide, particularly in East Asian countries (24). However, prevention and screening programs particularly at the national level have not yet been established in most countries except for Japan and Korea. Many low-income and middle-income countries are experiencing comparable challenges with a high incidence and mortality rate of upper gastrointestinal cancer and rapidly rising healthcare costs in the setting of limited healthcare resources. We expect that the knowledge generated from this study will be useful internationally. Our experience may help guide other undeveloped countries in their desire to develop the screening strategy themselves. The screening tests being examined in this trial have been in use for nationwide screening in developed countries, but until now none has been rigorously evaluated in a proper scientific trial. Thus this trial will also provide evidence of the efficacy and feasibility of screening for developed countries.
This trial calls for recruitment to be completed by the end of 2017 but follow-up will be continued to estimate the long-term effect of screening. Unlike other cohorts that were mainly established in high-risk areas, we also evaluate the screening procedure in non-high-risk areas. Compared with high-risk areas, non-high-risk areas have a higher proportion in both regions and population and are more representative of the general situation in China. Screening strategy evaluated in those areas with positive findings may be promoted nationally and applied to the majority of Chinese people. On the other hand, negative findings will provide scientific evidence for abandoning a test and shifting resources elsewhere.
The major strength of the present study is the prospective randomized design, which was chosen in consideration of the uncertainty in interpretation of observational studies of cancer screening reducing selection bias. Secondly, both active and passive follow-ups are adopted to collect the outcome information. In addition, a biobank containing blood and tissue samples are established, which lays the foundation for further exploration on the genetic risk factors of upper gastrointestinal cancer.
The potential risk of this study includes the control of loss to follow-up and quality control of multi-centre project. We will employ full-time doctors to contact and visit the participants from their sites to improve the follow-up rate. Besides, a group of experts from multiple fields including epidemiology, statistics, health economics, endoscopy, pathology, laboratory, surgery, chemo radiotherapy, disease registration and disease surveillance will be set up to provide technical guidance and quality control.
In conclusion, this is a multi-center prospective study, seeking to evaluate the efficacy and feasibility of upper gastrointestinal cancer screening in Chinese population. This project will provide answers to questions about the mortality reduction, survival rate improvement and utility of upper gastrointestinal cancer screening procedure in both high-risk and middle/low-risk areas. The findings will be an important reference for the government to develop and implement national screening programs in the future.
Acknowledgements
The authors would like to thank the team of National Central Cancer Registry and Oncological Epidemiology Department of Cancer Hospital, Chinese Academy of Medical Sciences, and thank all staff in seven study sites working on this project for their efforts on the project. This study is supported by the Special Fund for Health Research in the Public Interest (No. 201502001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [PubMed] DOI:10.3322/caac.21338
- Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China, 2013. Cancer Lett 2017;401:63–71. [PubMed] DOI:10.1016/j.canlet.2017.04.024
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon: IARC, 2013. Available online: http://globocan.iarc.fr
- Wang GQ, Jiao GG, Chang FB, et al. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann Thorac Surg 2004;77:1740–4. [PubMed] DOI:10.1016/j.athoracsur.2003.10.098
- Matsumoto S, Yamasaki K, Tsuji K, et al. Results of mass endoscopic examination for gastric cancer in Kamigoto Hospital, Nagasaki Prefecture. World J Gastroenterol 2007;13:4316–20. [PubMed]
- Hamashima C. Current issues and future perspectives of gastric cancer screening. World J Gastroenterol 2014;20:13767–74. [PubMed] DOI:10.3748/wjg.v20.i38.13767
- Kim Y, Jun JK, Choi KS, et al. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev 2011;12:725–30. [PubMed]
- Shu YJ. Cytopathology of the esophagus. An overview of esophageal cytopathology in China. Acta Cytol 1983;27:7–16. [PubMed]
- Pan QJ, Roth MJ, Guo HQ, et al. Cytologic detection of esophageal squamous cell carcinoma and its precursor lesions using balloon samplers and liquid-based cytology in asymptomatic adults in Llinxian, China. Acta Cytol 2008;52:14–23. [PubMed]
- Qin DX, Wang GQ, Yuan FL, et al. Screening for upper digestive tract cancer with an occult blood bead detector. Investigation of a normal north China population. Cancer 1988;62:1030–4. [PubMed]
- Dawsey SM, Wang GQ, Weinstein WM, et al. Squamous dysplasia and early esophageal cancer in the Linxian region of China: distinctive endoscopic lesions. Gastroenterology 1993;105:1333–40. [PubMed]
- Dai M, Bai Y, Pu H, et al. Application of cohort study in cancer prevention and control. Zhonghua Liu Xing Bing Xue Za Zhi (in Chinese) 2016;37:303–5. [PubMed]
- Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention . Gut 2013;62:676–82. [PubMed] DOI:10.1136/gutjnl-2012-302240
- He Y, Wu Y, Song G, et al. Incidence and mortality rate of esophageal cancer has decreased during past 40 years in Hebei Province, China. Chin J Cancer Res 2015;27:562–71. [PubMed] DOI:10.3978/j.issn.1000-9604.2015.10.06
- Wei WQ, Chen ZF, He YT, et al. Long-term follow-up of a community assignment, one-time endoscopic screening study of esophageal cancer in China. J Clin Oncol 2015;33:1951–7. [PubMed] DOI:10.1200/JCO.2014.58.0423
- Riecken B, Pfeiffer R, Ma JL, et al. No impact of repeated endoscopic screens on gastric cancer mortality in a prospectively followed Chinese population at high risk. Prev Med 2002;34:22–8. [PubMed] DOI:10.1006/pmed.2001.0925
- Wong Kee Song LM, Wilson BC. Endoscopic detection of early upper GI cancers. Best Pract Res Clin Gastroenterol 2005;19:833–56. [PubMed] DOI:10.1016/j.bpg.2005.04.006
- The National Cancer Control Office of the Ministry of Health, China cancer death survey. Beijing: People’s Medical Publishing House, 1980.
- China Statistical Yearbook, 2010. Beijing: China Statistics Press, 2010.
- Schlesselman JJ. Sample size requirements in cohort and case-control studies of disease. Am J Epidemiol 1974;99:381–4. [PubMed]
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009;360:1385–94. [PubMed] DOI:10.1056/NEJMoa0808516
- Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol 1999;28:319–26. [PubMed]
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993;13:322–38. [PubMed] DOI:10.1177/0272989X9301300409
- Hamashima C. Benefits and harms of endoscopic screening for gastric cancer. World J Gastroenterol 2016;22:6385–92. [PubMed] DOI:10.3748/wjg.v22.i28.6385