Extent of lymphadenectomy has no impact on postoperative complications after gastric cancer surgery in Sweden
Introduction
Despite its decreasing incidence, gastric cancer worldwide maintains its lethal impact representing the third most common cause of death among malignant diseases (1). Radical surgical resection of the tumor and surrounding lymph node stations is the mainstay of curative treatment. The extent of lymphadenectomy is subdivided into category D0, D1, D1+ and D2 according to the third version of the Japanese Gastric Cancer Association (JGCA) treatment guidelines (2). For routine surgical practice, the extent of lymphadenectomy has been debated between Eastern and Western therapeutic traditions (3). The outcomes of three European randomized trials had a significant impact on current surgical tradition in the West (4-6). None of these trials could show an overall survival benefit of D2 lymphadenectomy. The lack of superiority might to a large extent be explained by high postoperative mortality following D2 dissection in the first two trials, and also by major problems with procedure compliance (less extensive lymphadenectomy required in the D2 arm) as well as contamination (more extensive lymphadenectomy required in the D1 arm) in all three trials. In the Italian trial (6), a significant proportion (33%) of enrolled patients had early gastric cancer, where the impact of D2 on survival is questionable. On the other hand, a Taiwanese randomized controlled trial (RCT) comparing D1 vs. an extended lymphadenectomy, equivalent to current D2 classification, was able to demonstrate a survival benefit for extended lymphadenectomy (7). This was also supported by a European subgroup and post hoc analysis with follow-up beyond 5 years, suggesting a gender-dependent survival benefit for D2 lymphadenectomy as well as in cases with regional lymph node metastatic disease (8).
A recent meta-analysis (9) concluded that in western patients, evidence now supports use of D2 rather than D1 lymphadenectomy. The risk of more extensive lymphadenectomy is allegedly a higher frequency and severity of postoperative complications (10,11), even suggesting that operative mortality and morbidity may outweigh the potential oncological benefit of D2 lymphadenectomy. However, these European RCTs (4,5) were conducted almost two decades ago and since then perioperative management and surgical technique including the introduction of pancreas- and spleen-preserving D2 techniques, have improved substantially (12). The latest European RCT (13), accordingly demonstrated lower postoperative mortality and morbidity after D2 lymphadenectomy compared to the older trials (10,11), which might better reflect modern surgical standards.
Well-designed RCTs have strong internal validity and are considered as the golden standard method in investigational medicine. Nonetheless, RCTs have some weaknesses of which a potential lack of external validity, i.e. shortcomings in generalizability to routine clinical practice is the most important. To address the risk of postoperative mortality and morbidity in routine clinical practice following different extent of lymphadenectomy (D0–D2) we conducted a population-based cohort study including the entire population of Sweden.
Materials and methods
Study design
The population-based study cohort was recruited from prospectively collected data in the National Register of Esophageal and Gastric Cancer (NREV) covering all gastric cancer resections performed in Sweden between January 1, 2006 and December 31, 2013. Exposure data were also collected from other nationwide health care registers. Patients were divided into three groups regarding extent of lymphadenectomy in association with gastrectomy (see definition below). The cohort was followed up until emigration, death or end of study period (December 31, 2013), whichever came first.
Study population
All patients that underwent gastric resection due to adenocarcinoma or undifferentiated cancer in the stomach or gastro-esophageal junctional cancer Siewert III [International Statistical Classification of Disease and Related Health Problems (ICD) 10th version, C16.1-9 and C16.0C] during the study period were included. Comprehensive classification of the extent of lymphadenectomy required exclusion of cases involving: local excisions, pylorus-preserving and proximal gastrectomy, previous gastric surgery cases, unclear surgical procedures, or patients with palliative resections.
Compliance with ethical standards
This article does not contain any studies with human or animal subjects performed by any of the authors, and thus formal informed consent is not required. This study has been approved by the Regional Ethics Committee (EPN Stockholm Dnr: 2013/596-31/3) and conforms to the Declaration of Helsinki.
Lymphadenectomy
Extent of lymphadenectomy was determined according to the JGCA Treatment Guidelines version 3 (2), as either D0, D1, D1+ or D2. Classification was based on the resected nodes as reported by the surgeon. Exceptions were made for total gastrectomy where the procedure was classified as D2 even if lymph node station 10 was not dissected. For distal gastrectomy, the procedure was accepted as D1 even if lymph node stations 1 and 7 were not dissected. We combined D1+ and D2 gastrectomy, since the D1+ group was relatively small in this cohort and the lymphadenectomy is more similar to the D2 procedure than to D1.
Data sources
NREV
NREV is a national quality register operational since 2006. It covers all gastric and esophageal cancer cases in Sweden and is used for national quality assurance for the diagnosis and treatment of esophageal and gastric cancer. All Swedish institutions detecting or treating gastric cancer report to the register. The reports are monitored by Sweden’s six regional oncological centers and collected into the NREV. Detailed information regarding diagnostic work-up, surgical treatment and postoperative follow-up is available. After surgery, the surgeon, typically the consultant surgeon, registers if the performed resection was done with curative, borderline curative/palliative, or palliative intent. Registration of complications following surgery is done at follow-up appointment, and covers complications occurring within 30 d after surgery (Table 1). Pathological tumor stage was classified according to the Union for International Cancer Control (UICC) TNM classification system, version 7 (14). Hospital volume was defined as the mean annual number of gastric cancer resections at each respective hospital during the study period and arbitrarily categorized into low, intermediate, moderately high, or high volume (0–<5, 5–<10, 10–<15 and ≥15 cases per year, respectively). In Sweden, the annual volume of gastric cancer resections for most hospitals is generally very low, and cut-off limits for the reported hospital volumes were therefore arbitrarily chosen and not according to expert consensus recommendations (15). The register has recently been evaluated, finding completeness of more than 95% and high validity of individual data (16).
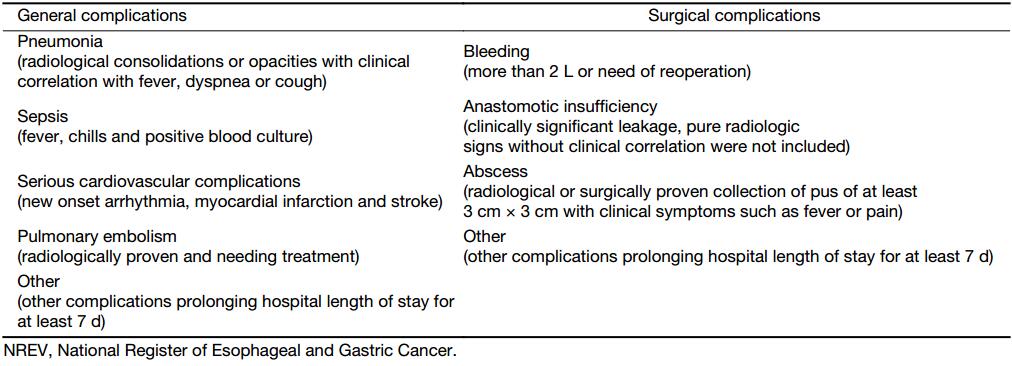
Full table
National Register of Education, Emigration Register and Death Register
Information regarding educational status was collected from Sweden’s National Register of Education. The level of education of the patients was categorized as ≤9 years, 10–12 years, or >12 years of education. Information on emigration was collected from the Emigration Register. Time of death was gathered from the Death Register.
National Patient Register
The National Patient Register, which was initiated in 1964, is administrated by the National Board of Health and Welfare. It has had complete coverage of inpatient data in Sweden since 1987, and of specialized outpatient data since 2001 (17). This register contains the main diagnosis and up to 18 secondary diagnoses from each hospital admission event. The National Patient Register was used to obtain Charlson comorbidity index.
Statistical analysis
Continuous variables were analyzed with analysis of variance (ANOVA), and non-parametric analysis was performed with the Kruskall-Wallis test. The Chi-square test and Fisher’s exact test were used for categorical variables and multivariable logistic regression for multivariable analysis. Tests were two-sided and statistical significance level set at 0.05. Associations between extent of lymphadenectomy and postoperative complications and mortality were tested in a multivariable model, calculating odds ratio (OR) with a 95% confidence interval (95% CI). The models were constructed by a stepwise simple testing of all relevant potential confounding factors, and thereafter including the variables in the final model that were statistically significantly associated or defined as standard confounding variables for the association. Final multivariable model included patient- and tumor-related factors: age (categorized into <60, 60–69, 70–79, and ≥80 years), body mass index (BMI) (categorized into quartiles), gender, American Society of Anesthesiologists (ASA) score (1–4), Charlson comorbidity index (categorized into 0–2 and ≥3), pathological tumor stage (categorized as stage 0/1, 2, 3, and 4), and treatment-related factors: surgical procedure, multivisceral resection, hospital volume and calendar year. All analyses were done using IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA).
Results
From 2006 to 2013, a total of 4,128 cases of adenocarcinoma of the stomach including cancer of the gastric cardia Siewert type III were registered in NREV. The tumor was resected in 1,494 (36%) patients, of which 1,140 (76%) were considered curative or borderline curative/palliative procedures by the surgeon. Thirty-nine cases were excluded, comprising local excisions (n=14), previous gastric resection (n=7), proximal or pylorus-preserving central gastrectomy (n=19), and unknown surgical procedure (n=1) (some cases fit into more than one group). Thus, 1,101 cases remained for final analyses (Figure 1). Basic characteristics are shown in Table 2.


Full table
Three hundred and forty-nine (31.7%) surgical procedures were classified as D0, 494 (44.9%) as D1 and 258 (23.4%) as D1+/D2. A statistically significant difference in the number of lymph nodes retrieved during gastric cancer surgery was found between different categories of lymphadenectomy (P<0.001) (Table 3). A trend was recognized to indicate that those who received a D1+/D2 lymphadenectomy were younger (P<0.001), had more advanced disease (P=0.032), had received neoadjuvant chemotherapy (P<0.001), had surgery in more recent years (P<0.001), or had surgery in a University Hospital (P<0.001) (Table 2). In total 48 patients had stage IV disease (Table 2), and they are classified as curative intent since the procedure was initially performed as a curative procedure and perioperatively isolated omental/peritoneal lesion was resected with pathological finding of metastases. There were a total of 146 patients with unknown tumor stage. All these patients have M0 disease but either unknown T or N stage.

Full table
After surgery, 31 (2.8%) patients died within 30 d and 65 (5.9%) within 90 d (Table 4). The highest postoperative mortality was observed after D0, and the lowest after D1 lymphadenectomy. A D1+/D2 lymphadenectomy was associated with a higher frequency of any kind of complication (P=0.017), pulmonary embolism (P=0.003), and the need for postoperative anastomotic stenting (P=0.017), as compared to D0 lymphadenectomy. Simple logistic regression identified that extent of lymphadenectomy, age, gender, tumor stage, ASA score, hospital volume, hospital type, calendar year, surgical procedure, bleeding, operative time and multivisceral resection were significant risk factors for overall postoperative complication. Bleeding, operative time and hospital type were not included in the final multivariable model due to high number of missing information (bleeding and operative time) and variables not significantly affecting the adjusted point estimates when tested in the model (hospital type). Due to the relatively high number of missing data in tumor stage, a sensitivity analysis was performed and it did not affect the main results significantly (data not shown). After analysis in the multivariable model we found that more extensive lymphadenectomy no longer remained as a significant risk factor for postoperative morbidity (Table 5). Regarding postoperative 90-d mortality, multivariable logistic regression identified a lower mortality risk when comparing D1 with D0, but no difference between D1+/D2 and D0, or between D1 and D1+/D2 (Table 5).

Full table
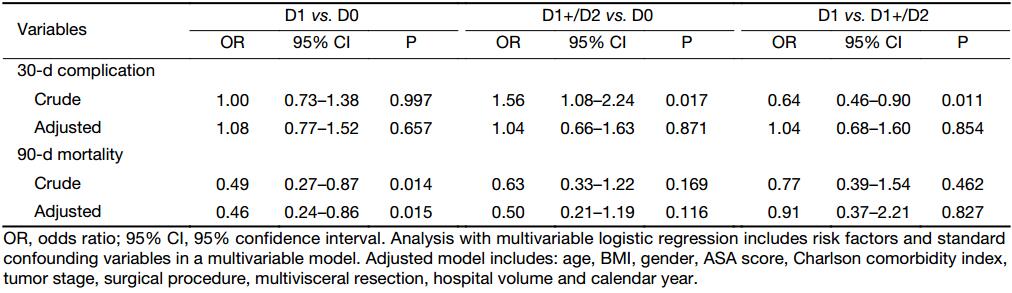
Full table
Discussion
This nationwide population-based, observational study found large differences in the extent of lymphadenectomy during curative intended gastric cancer surgery in routine clinical practice in Sweden from 2006 to 2013. A substantial number of these patients (32%) underwent a resection without any meaningful lymphadenectomy (D0). The majority of D1+/D2 dissections were done in university hospitals and during the latter part of the study period. After adjustment for confounding variables there remained no association between the extent of lymphadenectomy and the risk for postoperative complications or mortality.
During the last decade, a worldwide consensus has been reached that D2 lymphadenectomy is recommended as the curative procedure for a non-early gastric cancer. In the pivotal RCT addressing the value of D2 lymphadenectomy (4), a subsequent analysis demonstrated a clear advantage for D2 confined to certain subgroups of patients. Follow-up analysis revealed increased gastric cancer-related death for the D1 group compared to D2, a difference which did not become apparent until 15 years of follow-up (8). Our observations contrast to the results presented in the two of the European RCTs showing that postoperative complications were more common after D2 dissection compared the more limited D1 (43%–46% vs. 25%–28%, respectively) (10,11), while, in the latest RCT from Taiwan, China postoperative complication rates were reported as low as 17.9% in the D2 and 12.0% in the D1 group (13). The differences in results may be explained by differences in study design, surgical technique and availability of some potential confounding factors. There is a gap in the literature concerning population-based results after gastric cancer surgery. we herein report on nationwide data from Sweden, where a substantial proportion of lymphadenectomies were still performed less extensively than D2 during the study period; a finding that is most likely to be found in many western countries where gastric cancer surgery has not yet been centralized. A high surgical volume of extensive surgical procedures has been shown to render less postoperative comorbidity and mortality (18,19). High volume hospitals in Sweden resected 204 gastric cancers of which 134 (66%) were D1+/D2 while the corresponding numbers for low-volume hospitals were 370 resections and 34 (9%) D1+/D2. The low proportion of D1+/D2 (21%) in distal gastrectomy noted in this study was mainly due to an avoidance of resecting station 1 and 7. A corresponding high frequency of D0 was also reported in the American Intergroup study covering the period 1991–1998, where as many as 54% of patients had a D0 dissection (20). This study’s results support that more extensive lymphadenectomy does not increase postoperative morbidity or mortality in real life and that there is ample room for quality improvements in the surgical care of patients with gastric cancer (21,22).
The main advantage of this study is the nationwide, population-based study design offering a unique and large sample size of prospectively collected unselected data. This minimizes the risk of limited external validity that can be seen in randomized trials restricting the study population by inclusion and exclusion criteria. The completeness of the register can adequately and reliably present the actual circumstances and results of routine health care in the entire nation. The registered data within the NREV have recently reported a completeness of over 95% and an accuracy of more than 90% (16). The specific lymph node stations in the surgical specimen were stated by the reporting surgeon, which should add quality assurance. Based on these reports a retrospective and uniform categorization of the extent of lymphadenectomy grade was performed according to the third version of JGCA Treatment Guidelines (2). We observed a good correlation between the total number of lymph nodes retrieved and the designated lymphadenectomy category. This observation supports the notion that our classification truly represents the different lymphadenectomy categories. The number of lymph nodes in the specimen according to the pathology report is also in accordance with previous randomized trials on D2 gastrectomy (4-6).
Despite the strength of the size and completeness of the register, there are some inherent limitations. Some baseline characteristics of the enrolled patients, such as smoking, alcohol use, diet or change in weight prior to surgery, were not available. Treatment information such as neoadjuvant oncological therapy was only registered as the intention to give neoadjuvant treatment, but detailed information was lacking on what type and extent of chemotherapy individual patients actually received. Most patients in Sweden, during the studied time period, received regimens containing epirubicin, cisplatin and fluorouracil (ECF), according to the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) protocol (23), as well as subsequent modifications of the regime with oxaliplatin and oral capecitabine replacing cisplatin and fluorouracil. A small number of patients received preoperative chemotherapy combined with postoperative chemotherapy or chemoradiotherapy as part of the Chemoradiotherapy after Induction chemotherapy in Cancer of the Stomach (CRITICS) trial (24).
Definition of postoperative complications (Table 1) was according to a national consensus, since at the time of the launch of the NREV in 2006 there were no such international guidelines. The Clavien-Dindo classification (25) was added to the NREV in 2012. The current classification of postoperative complications should harmonize with higher or equal to Clavien grade III. The risk of underreporting is minimal as complications are routinely reported during the one month follow up appointment. In our multivariable analysis of postoperative complications, minimal invasive surgery or enhanced recovery after surgery (ERAS) was not assessed, as these management strategies had not yet been introduced in Sweden for gastric cancer. In the multivariable analysis of 90-d mortality, the total number of events was only 65 and the event per variable is 6.5. Thus the results must be interpreted with some caution. The variables were selected in the model as they are either biologically important confounders or found to be significant risk factors in simple logistic regression for overall complication rate. It was noteworthy that both 30- and 90-d mortality rates were the highest in the D0 group, potentially reflecting residual confounding, in spite of adjusting for hospital volume, preoperative risk and comorbidity.
Conclusions
This prospective, nationwide register-based study demonstrates that the majority of gastrectomy in Swedish daily clinical practice from 2006 to 2013 contained a limited lymphadenectomy (D0 and D1). An extended lymphadenectomy, D1+ or D2 was, however, not associated with an increased risk of postoperative morbidity or mortality.
Acknowledgements
This study was funded by unrestricted research grants from the County Council of Västerbotten (VLL-481721), and the Stockholm County Council (ALF Project 20140126).
Footnote
Conflicts of Interest: Jon A Tsai declares employment at Sanofi Genzyme as a medical advisor. All other authors have no conflicts of interests to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [PubMed] DOI:10.1002/ijc.29210
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113–23. [PubMed] DOI:10.1007/s10120-011-0042-4
- Markar SR, Karthikesalingam A, Jackson D, et al. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: comparison between West and East. Ann Surg Oncol 2013;20:2328–38. [PubMed] DOI:10.1245/s10434-012-2862-9
- Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908–14. [PubMed] DOI:10.1056/NEJM199903253401202
- Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522–30. [PubMed] DOI:10.1038/sj.bjc.6690243
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23–31. [PubMed] DOI:10.1002/bjs.9345
- Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 2006;7:309–15. [PubMed] DOI:10.1016/S1470-2045(06)70623-4
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439–49. [PubMed] DOI:10.1016/S1470-2045(10)70070-X
- Seevaratnam R, Bocicariu A, Cardoso R, et al. A meta-analysis of D1 versus D2 lymph node dissection. Gastric Cancer 2012;15(Suppl 1):S60–9. [PubMed] DOI:10.1007/s10120-011-0110-9
- Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995;345:745–8. [PubMed]
- Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996;347:995–9. [PubMed]
- Maruyama K, Sasako M, Kinoshita T, et al. Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 1995;19:532–6. [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg 2010;97:643–9. [PubMed] DOI:10.1002/bjs.6936
- Sobin LH, Gospodarowicz MK, Wittekind C, et al. TNM Classification of Malignant Tumours (7th edition). Hoboken: Wiley-Blackwell, 2010.
- Dixon M, Mahar A, Paszat L, et al. What provider volumes and characteristics are appropriate for gastric cancer resection? Results of an international RAND/UCLA expert panel. Surgery 2013;154:1100–9. [PubMed] DOI:10.1016/j.surg.2013.05.021
- Linder G, Lindblad M, Djerf P, et al. Validation of data quality in the Swedish National Register for Oesophageal and Gastric Cancer. Br J Surg 2016;103:1326–35. [PubMed] DOI:10.1002/bjs.10234
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [PubMed] DOI:10.1186/1471-2458-11-450
- Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care 2011;49:1076–81. [PubMed] DOI:10.1097/MLR.0b013e3182329b97
- Sahni NR, Dalton M, Cutler DM, et al. Surgeon specialization and operative mortality in United States: retrospective analysis. BMJ 2016;354:i3571. [PubMed] DOI:10.1136/bmj.i3571
- Hundahl SA, Macdonald JS, Benedetti J, et al. Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment. Ann Surg Oncol 2002;9:278–86. [PubMed]
- Coburn N, Seevaratnam R, Paszat L, et al. Optimal management of gastric cancer: results from an international RAND/UCLA expert panel. Ann Surg 2014;259:102–8. [PubMed] DOI:10.1097/SLA.0b013e318288dd2b
- Tegels JJ, De Maat MF, Hulsewé KW, et al. Improving the outcomes in gastric cancer surgery. World J Gastroenterol 2014;20:13692–704. [PubMed] DOI:10.3748/wjg.v20.i38.13692
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [PubMed] DOI:10.1056/NEJMoa055531
- Dikken JL, van Sandick JW, Maurits Swellengrebel HA, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer 2011;11:329. [PubMed] DOI:10.1186/1471-2407-11-329
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–96. [PubMed] DOI:10.1097/SLA.0b013e3181b13ca2
