Postoperative complications and weight loss following jejunostomy tube feeding after total gastrectomy for advanced adenocarcinomas
Introduction
Gastric cancer is the third leading cause of cancer death, accounting for almost 9% of cancer-related deaths worldwide (1). Nowadays, gastrectomy with perioperative chemotherapy is standard of care for patients with resectable gastric cancer in the West (2).
Weight loss is an inevitable but important problem in patients with gastric cancer. After total gastrectomy, inadequate caloric intake and poor absorption of essential nutrients further contribute to a poor nutritional status (3). It has been suggested that after total gastrectomy a poor nutritional status impedes patients to start and complete adjuvant therapy and therewith decrease the survival chances of these patients (4). In order to maintain a proper nutritional status, patients can receive nutritional support after total gastrectomy (5,6).
A frequently performed method of nutritional support after total gastrectomy is by starting jejunostomy tube feeding (JTF). JTF maintains mucosal integrity and enteric flora, and improves immune competence (7), and it has been demonstrated to be superior compared to keeping patients on a nil-by-mouth diet in the first seven to ten days postoperatively (8). However, there are contradictory views on the use of JTF. Some studies found an increase in postoperative complications after jejunostomy placement (9-11), whereas others did not find this association (12). As consent regarding this topic is warranted, the aim of this study is to examine the effect of JTF on the nutritional state and the incidence of postoperative complications in patients undergoing total gastrectomy for cancer with curative intent. Secondly, this study aims to identify risk factors associated with postoperative weight loss.
Materials and methods
Patients
Consecutive patients who underwent total gastrectomy for cancer with curative intent and jejunostomy placement were included from a prospective database of the University Medical Center Utrecht, a tertiary referral center. The study included patients who underwent surgery between December 2003 and August 2014. During this period, jejunostomy placement was considered standard of care after total gastrectomy, whereas after August 2014, the Enhanced Recovery After Surgery (ERAS) protocol was introduced and JTF was only performed on indication. The local ethical review board approved this study and waived the need for informed consent (protocol nr. 13-061/C).
Treatment
Patients received primary gastrectomy or gastrectomy with perioperative chemotherapy according to the MAGIC-trial (2). Total gastrectomy plus omentectomy was performed by means of an open or minimally invasive procedure as described before (13,14). A D1+ lymphadenectomy was performed according to the Japanese Gastric Cancer Treatment Guidelines (15). A Roux-Y reconstruction was established with a circular stapler for the esophagojejunostomy, and manually or with a linear stapler for the jejunojejunostomy. A jejunal pouch was created according to the surgeons’ preference (16). A jejunostomy was routinely placed in the first jejunal loop distal to the ligament of Treitz. A small caliber tube was introduced into the efferent limb of the jejunal loop and attached to the jejunum with a purse string suture. The jejunal loop was fixed to the anterior abdominal wall with a single purse string suture. Resection specimens were reviewed by a dedicated gastro-intestinal pathologist according to the International Union against Cancer (UICC) Tumor Node Metastases (TNM) staging system (17). After surgery, enteral feeding over the jejunostomy commenced directly with protein rich tube feeding (Nutrison Protein Plus® Nutricia), starting at a dosage of 25 mL/h. The dosage was increased with 25 mL per 6 h until calorie needs were met according to the modified Harris-Benedict formula (18). Patients were kept on nil-by-mouth for 3 d before the introduction of oral feeding. Feeding over the jejunostomy was ceased if oral intake was adequate, or if needed do for medical reasons. Patients were discharged home with full or partial JTF if oral intake was not sufficient or contraindicated due to complications such as anastomotic leakage.
Outcomes
Data on patient and tumor characteristics, and postoperative complications were extracted from the prospective database. All postoperative complications were graded according to the Clavien-Dindo (CD) scoring system (19). Jejunostomy-related complications were divided in leakage, obstruction, dislocation, infection, and torsion. Infection of the jejunostomy insertion site was registered if antibiotic treatment was given. Data on the length of JTF, postoperative body weight and body mass index (BMI) were retrospectively extracted from the patients’ electronic files up to 12 months after surgery (stop of JTF, 6 weeks, 3 months, 6 months, and 12 months) as the largest weight changes are expected in this period (20).
Statistical analysis
Data were analyzed using IBM SPSS Statistics (Version 21.0; IBM corp., New York, USA). Continuous variables were presented as medians [interquartile range (IQR)] or x±s, whereas categorical variables were presented as frequencies and percentages. After evaluation of the data distribution, changes in weight were analyzed based on a self-controlled method [investigating an outcome (weight) during and after a certain exposure (JTF)] using the paired Wilcoxon signed rank test (medians) or the repeated measures analysis of variance (ANOVA) (x±s). Total weight loss (in percentages) at 12 months of patients with a preoperative high BMI (≥25 kg/m2) was compared with patients with a low BMI (<25 kg/m2) using the students’ t-test. A comparable analysis was performed on patients who were discharged with or without JTF. Last, to identify factors associated with weight loss 12 months after gastrectomy, a multivariable linear regression analysis was performed including relevant perioperative variables. P<0.05 (two-sided) was considered statistically significant.
Results
Patients
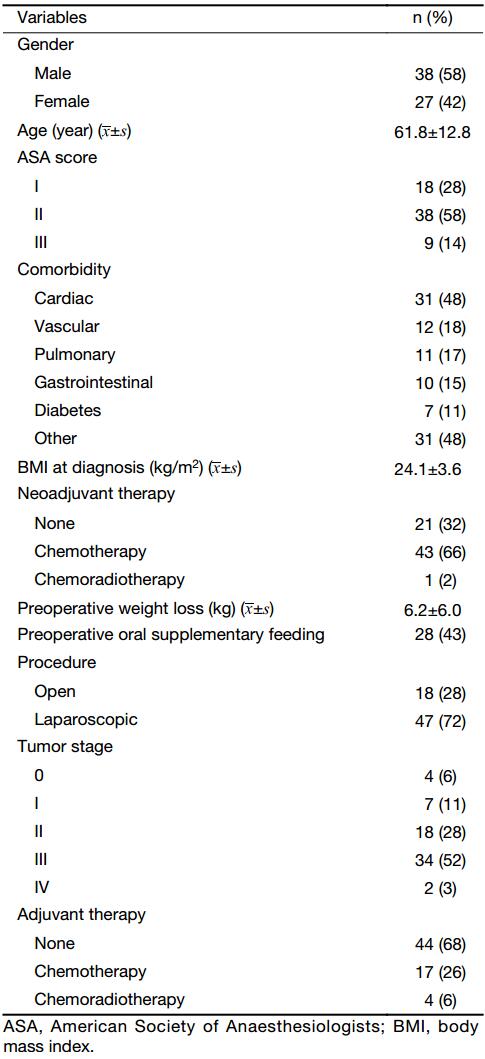
Of the 113 patients who underwent a gastrectomy during the study period, 68 patients underwent total gastrectomy with curative intent. After exclusion of 3 patients who did not receive a jejunostomy (due to preference of the surgeon), 65 patients remained for the analysis. The baseline characteristics of these patients are presented in Table 1. Most of the patients were male (58%), underwent neoadjuvant chemotherapy (66%), had an American Society of Anaesthesiologists (ASA)-score of II (58%), underwent a minimally invasive procedure (72%), and had an advanced tumor (83% >stage I). The mean BMI at diagnosis was 24.1±3.6 kg/m2, the average weight loss before surgery was 6.2±6.0 kg, and 43% of patients received supplementary oral feeding preoperatively. A total of 21 (32%) patients started adjuvant treatment, and 15 (23%) completed adjuvant therapy, which was 34% of patients who started neoadjuvant chemotherapy.
Full table
Perioperative complications
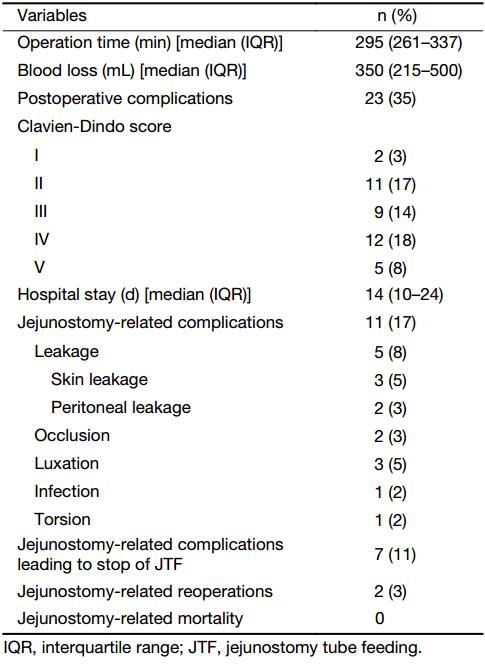
Table 2 demonstrates the perioperative outcomes of all patients. Jejunostomy-related complications occurred in 11 (17%) patients, including skin leakage (n=3) and peritoneal leakage (n=2) from the insertion site, luxation of the jejunostomy (n=3), occlusion of the jejunostomy (n=2), infection of the jejunostomy insertion site (n=1), and torsion of the jejunostomy (n=1). As a result of jejunostomy-related complications, 2 (3%) reoperations were needed (1 due to persistent leakage and 1 due to torsion of the jejunostomy), and 7 (11%) patients unintentionally stopped JTF.
Full table
Perioperative nutritional status
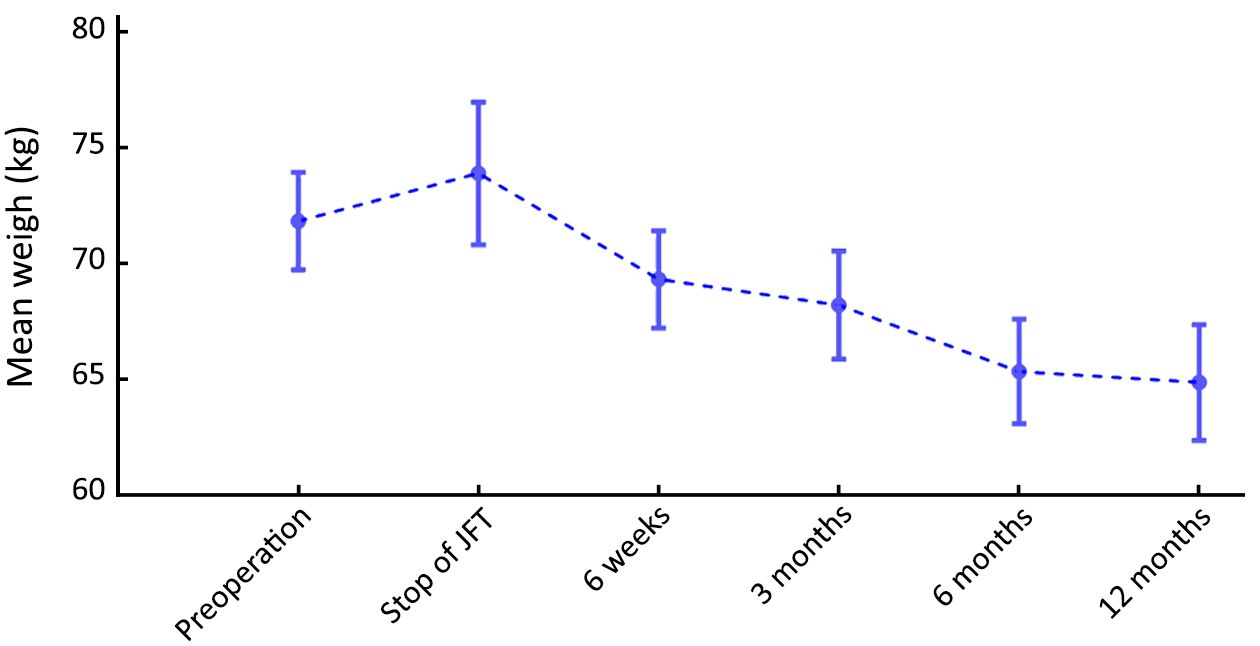
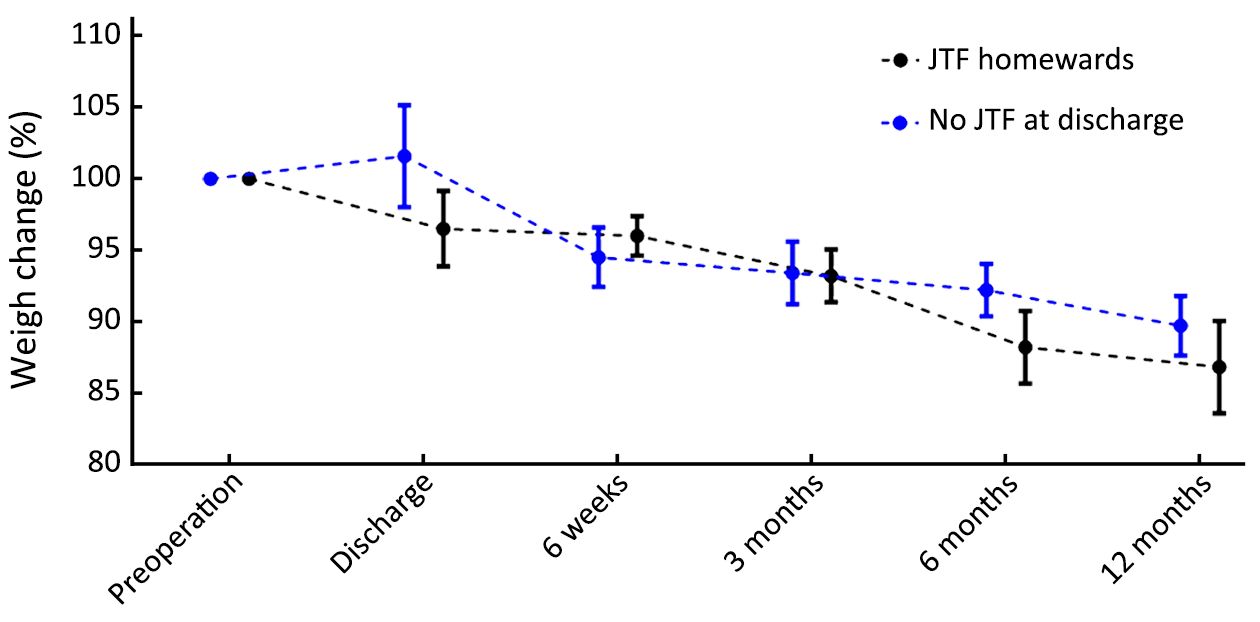
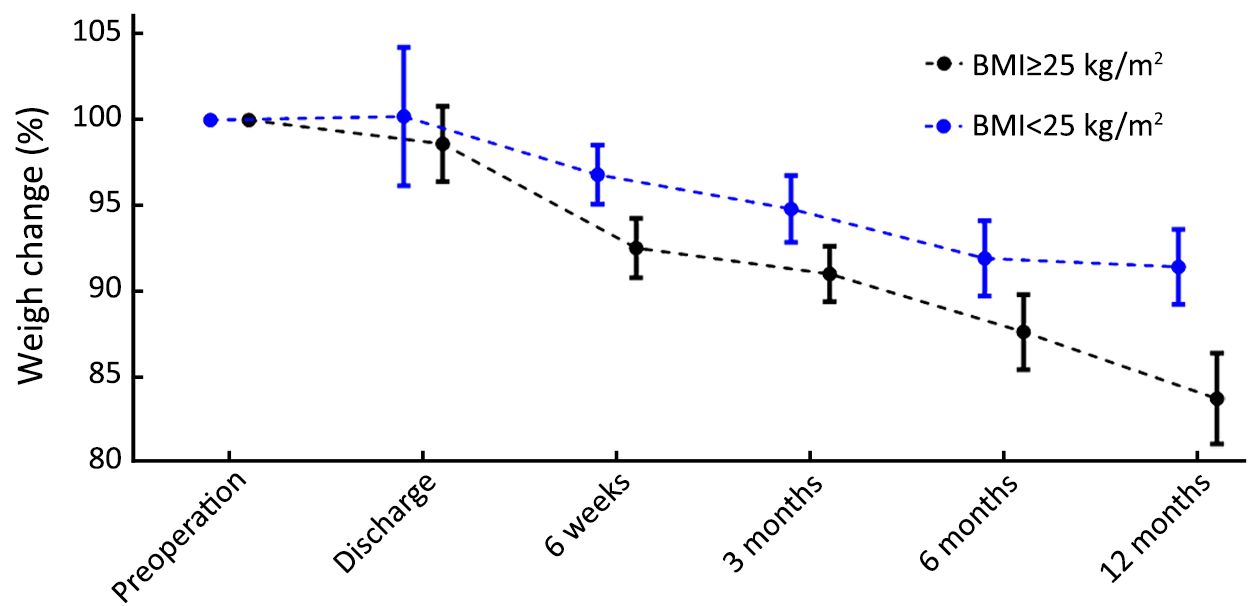
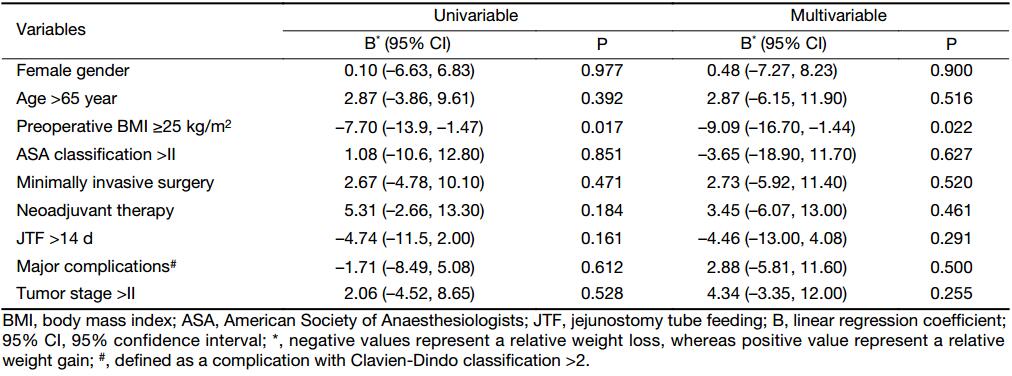
The median duration of JTF was 18 d (IQR, 10–55 d). A total of 32 (49%) patients received JTF for more than 14 d, whereas 7 (11%) patients received JTF for more than 90 d, all of whom had insufficient oral intake. The mean preoperative weight of patients was 71.8 kg (100%), and remained stable during JTF (73.9 kg, 103%, P=0.331) (Figure 1). After JTF was stopped, the mean weight of patients decreased to 64.9 kg (90%) at 12 months after surgery (P<0.001). And 31 (48%) patients were discharged without JTF, of whom 5 restarted JTF due to insufficient oral intake. Although there was a trend that patients who were discharged without JTF had less weight loss at discharge, this difference was not statistically significant (102% vs. 96%, P=0.200) (Figure 2). During follow-up, the weight changes of patients who were discharged with or without JTF did not significantly change. Patients with a higher preoperative BMI (≥25 kg/m2) were at a higher risk of postoperative weight loss (Figure 3): the weight loss at 12 months was 8.6%±8.9% in patients with a low BMI (<25 kg/m2) compared to 16.3%±8.3% in patients with a high BMI (P=0.016). In multivariable linear regression analysis, only a high preoperative BMI was identified as a factor associated with an increased postoperative weight loss [–9.09%; 95% confidence interval (95% CI): –16.70%, –1.44%; P=0.022] (Table 3).
Full table
Discussion
This single-center study demonstrates that jejunostomy-related complications occur in 17% of patients, leading to reoperations in 3% of patients. JTF does not prevent weight loss, but postpones weight loss. After stopping JTF, patients lose an average of 9 kg up to 12 months after surgery. A high preoperative BMI (≥25 kg/m2) was associated with more postoperative weight loss.
Malnutrition following total gastrectomy is a serious, and often inevitable complication, leading to a higher risk of postoperative complications and patient morbidity (21). Jejunostomies are therefore often placed for nutritional support and malnutrition prevention. One potential benefit from an improved nutritional state would be to enable more patients to complete adjuvant therapy and therewith increasing the survival chances of these patients (4). In this study, JTF prevented weight loss in the early postoperative phase but not in the longer term, and only 34% of all patients who started perioperative chemotherapy completed adjuvant therapy. This is in line with a study performed by Patel et al. who compared 66 patients who underwent jejunostomy placement to 66 patients without jejunostomy placement after gastrectomy (9). This study did not find a difference in postoperative nutritional status measured by serum albumin, nor did they find a difference in the amount of patients receiving adjuvant chemotherapy. The results from the study by Patel et al. and the present study imply that JTF may not be beneficial regarding nutritional status or the chance of completing adjuvant therapy.
Besides the limited benefit of jejunostomies, the present study demonstrated that jejunostomies were accompanied by additional risks. Jejunostomy placement resulted in complications in 17% of patients, leading to reoperations in 3% of patients. Previously, three studies from the USA also demonstrated a high risk for complications due to jejunostomy placement (9-11). On the other hand, another large comparative study from the USA could not verify these results, demonstrating similar morbidity and mortality rates in patients with jejunostomy placement compared to patients without (12). Rationally, it seems likely that jejunostomy-related complications, such as leakage, obstruction and torsion, would not occur in patients without jejunostomy. Moreover, jejunostomy forms a potential site for wound infections. Altogether, it can be assumed that jejunostomies lead to an additional risk for complications in patients who undergo gastrectomy.
Alternatively, to routine jejunostomy placement and nil-by-mouth, patients may start early oral feeding after gastrectomy according to the Enhanced Recovery After Surgery (ERAS) protocol (22). The ERAS protocol involves multiple factors such as early feeding, fast mobilization and adequate pain control. Early oral feeding as proposed by ERAS protocol is not associated with an increased risk of postoperative complications (23), and enhances recovery by stimulating the gastro-intestinal tract in a natural way (7). Moreover, the ERAS protocol may even reduce complications, hospitalization, weight loss, and health care costs (24). Based on these positive reports, University Medical Center Utrecht implemented the ERAS protocol in August 2014 and we have positive experiences ever since. The ERAS protocol was therefore also included in the currently ongoing LOGICA trial, a randomized controlled trial evaluating open vs. laparoscopic gastrectomy in the Netherlands (14). Nevertheless, it remains important to realize that in patients receiving early oral feeding, total calorie intake may be low in the first period after surgery. Although it is unclear what the consequences are of a low intake in the early postoperative period, patients should be offered high-energy and high-protein oral sip feeds if their intake does not meet a desired level (25).
In some patients, however, postoperative supplementary enteral feeding will be necessary beforehand. The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommend supplementary enteral feeding if patients are at risk of malnutrition (6). These include patients with a contraindication to oral feeding, with severe trauma, with obvious malnutrition at the time of surgery (>10% weight loss in the last 6 months), or patients whose oral intake will be inadequate (<60%) for more than 10 d postoperatively (5). Alternatively, to jejunostomy placement, enteral tube feeding can be applied through a nasojejunal tube. Comparable amounts of nutrients can be applied via this route, and serious complications have not been reported (26). However, the main drawback, apart from patients’ discomfort, is frequent dislocation, occurring in 20%–35% of all patients (26-28). Thus in case of an indication for long supplementary feeding, jejunostomy-placement may be preferred. In University Medical Center Utrecht, we now give JTF after gastrectomy if patients have lost >10% of their weight in the last 6 months, which is in accordance with literature and the ESPEN criteria (6,29).
In the case of jejunostomy placement, we advocate careful management of the jejunostomy to prevent complications. For instance, torsion of the jejunostomy might be prevented by careful placement, which occurred in 1 patient is this study. Although the torsion was successfully managed by reoperation, it may be a fatal complication as a result of intestinal ischemia, sepsis and multi-organ failure (30). To prevent this potentially lethal complication of a jejunostomy, we advise careful placement of the jejunostomy by attaching it to the abdominal wall with a purse-string suture to prevent rotation of the jejunostomy.
According to results from a previous study, it was expected that patients with a higher preoperative BMI were at a higher risk for postoperative weight loss (20). Indeed, this study found that patients with a high preoperative BMI (≥25 kg/m2) were more prone to postoperative weight loss than patients with a low preoperative BMI (<25 kg/m2). The previous study demonstrated that a preoperative BMI >30 kg/m2 was a risk factor for postoperative weight loss after gastrectomy (20). However, patients in the present study had a BMI >30 kg/m2 only occasionally, thus a lower cut-off value had to be used to remain statistical power. As weight loss has been demonstrated to be associated with poor oncological outcomes in other studies (31), obese patients should be carefully monitored and receive supplementary feeding if needed. Interestingly, there was no difference in postoperative weight between patients who were discharged with or without JTF. However, patients who were discharged with JTF seemed to have a lower weight at the time of discharge, which probably implies that the reason for discharge with JTF was a worse condition of these patients.
There are some limitations of this study, which should be addressed. First, due to the retrospective evaluation of data on weight loss after gastrectomy, postoperative weight data were missing in some patients, which could have influenced the results. Moreover, of patients who died in the first year after surgery no data were available as well after their death, possibly leading to selection bias. Last, as we used a self-controlled method, no control group was used in this study to compare patients without JTF, which is because patients without jejunostomy placement were all operated after August 2014. This would introduce the risk for historical bias as more differences in perioperative regime existed in this period except jejunostomy placement. An advantage of the self-controlled method is that it controls confounding factors that do not vary with time but between patients, whereas a disadvantage is that it is more difficult to control the time-varying factors. Nevertheless, this study is important as it focused solely on patients who underwent total gastrectomy, whereas other studies do not separate total and subtotal gastrectomy. This distinction is important, as the extensiveness of the gastrectomy influences postoperative weight loss (20). Moreover, there are only few studies focusing on postoperative weight loss. As there is still no consensus on the use of jejunostomy, this study contributes to current literature on postoperative nutrition after total gastrectomy.
Conclusions
JTF can prevent weight loss in the early postoperative phase. However, this is at the prize of possible complications. As weight loss in the long term is not prevented, routine JTF should be re-evaluated and balanced against the selected use in preoperatively malnourished patients. Special attention should be paid to patients with a high preoperative BMI, who are at risk of more postoperative weight loss.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [PubMed] DOI:10.1002/ijc.29210
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [PubMed] DOI:10.1056/NEJMoa055531
- Bae JM, Park JW, Yang HK, et al. Nutritional status of gastric cancer patients after total gastrectomy. World J Surg 1998;22:254–60. [PubMed]
- Aoyama T, Kawabe T, Fujikawa H, et al. Loss of lean body mass as an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol 2015;22:2560–6. [PubMed] DOI:10.1245/s10434-014-4296-z
- Weimann A, Braga M, Harsanyi L, et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr 2006;25:224–44. [PubMed] DOI:10.1016/j.clnu.2006.01.015
- Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11–48. [PubMed] DOI:10.1016/j.clnu.2016.07.015
- Mercadante S. Parenteral versus enteral nutrition in cancer patients: indications and practice. Support Care Cancer 1998;6:85–93. [PubMed] DOI:10.1007/s005200050140
- Barlow R, Price P, Reid TD, et al. Prospective multicentre randomised controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clin Nutr 2011;30:560–6. [PubMed] DOI:10.1016/j.clnu.2011.02.006
- Patel SH, Kooby DA, Staley CA 3rd, et al. An assessment of feeding jejunostomy tube placement at the time of resection for gastric adenocarcinoma. J Surg Oncol 2013;107:728–34. [PubMed] DOI:10.1002/jso.23324
- Dann GC, Squires MH 3rd, Postlewait LM, et al. An assessment of feeding jejunostomy tube placement at the time of resection for gastric adenocarcinoma: A seven-institution analysis of 837 patients from the U.S. gastric cancer collaborative. J Surg Oncol 2015;112:195–202. [PubMed] DOI:10.1002/jso.23983
- Choi AH, O’Leary MP, Merchant SJ, et al. Complications of feeding jejunostomy tubes in patients with gastroesophageal cancer. J Gastrointest Surg 2017;21:259–65. [PubMed] DOI:10.1007/s11605-016-3297-6
- Sun Z, Shenoi MM, Nussbaum DP, et al. Feeding jejunostomy tube placement during resection of gastric cancers. J Surg Res 2016;200:189–94. [PubMed] DOI:10.1016/j.jss.2015.07.014
- Haverkamp L, Ruurda JP, Offerhaus GJ, et al. Laparoscopic gastrectomy in Western European patients with advanced gastric cancer. Eur J Surg Oncol 2016;42:110–5. [PubMed] DOI:10.1016/j.ejso.2015.09.018
- Haverkamp L, Brenkman HJ, Seesing MF, et al. Laparoscopic versus open gastrectomy for gastric cancer, a multicenter prospectively randomized controlled trial (LOGICA-trial). BMC Cancer 2015;15:556. [PubMed] DOI:10.1186/s12885-015-1551-z
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113–23. [PubMed] DOI:10.1007/s10120-011-0042-4
- Brenkman HJ, Correa-Cote J, Ruurda JP, et al. A step-wise approach to total laparoscopic gastrectomy with jejunal pouch reconstruction: how and why we do it. J Gastrointest Surg 2016;20:1908–915. [PubMed] DOI:10.1007/s11605-016-3235-7
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th Edition. Hoboken: Wiley, 2009.
- Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr 1984;40:168–82. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6,336 patients and results of a survey. Ann Surg 2004;240:205–13. [PubMed]
- Davis JL, Selby LV, Chou JF, et al. Patterns and predictors of weight loss after gastrectomy for cancer. Ann Surg Oncol 2016;23:1639–45. [PubMed] DOI:10.1245/s10434-015-5065-3
- Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr 2003;22:235–9. [PubMed]
- Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations . Br J Surg 2014;101:1209–29. [PubMed] DOI:10.1002/bjs.9582
- Lassen K, Dejong CH, Ljungqvist O, et al. Nutritional support and oral intake after gastric resection in five northern European countries. Dig Surg 2005;22:346–52. [PubMed] DOI:10.1159/000089770
- Tanaka R, Lee SW, Kawai M, et al. Protocol for enhanced recovery after surgery improves short-term outcomes for patients with gastric cancer: a randomized clinical trial. Gastric Cancer 2017. [Epub ahead of print]
- Mariette C, De Botton ML, Piessen G. Surgery in esophageal and gastric cancer patients: what is the role for nutrition support in your daily practice? Ann Surg Oncol 2012;19:2128–34. [PubMed] DOI:10.1245/s10434-012-2225-6
- Han-Geurts IJ, Hop WC, Verhoef C, et al. Randomized clinical trial comparing feeding jejunostomy with nasoduodenal tube placement in patients undergoing oesophagectomy. Br J Surg 2007;94:31–5. [PubMed] DOI:10.1002/bjs.5283
- Gabor S, Renner H, Matzi V, et al. Early enteral feeding compared with parenteral nutrition after oesophageal or oesophagogastric resection and reconstruction. Br J Nutr 2005;93:509–13. [PubMed]
- Page RD, Oo AY, Russell GN, et al. Intravenous hydration versus naso-jejunal enteral feeding after esophagectomy: a randomised study. Eur J Cardiothorac Surg 2002;22:666–72. [PubMed]
- Bozzetti F, Gianotti L, Braga M, et al. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr 2007;26:698–709. [PubMed] DOI:10.1016/j.clnu.2007.06.009
- Han-Geurts IJ, Verhoef C, Tilanus HW. Relaparotomy following complications of feeding jejunostomy in esophageal surgery. Dig Surg 2004;21:192–96. [PubMed] DOI:10.1159/000079345
- Aoyama T, Yoshikawa T, Shirai J, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol 2013;20:2000–6. [PubMed] DOI:10.1245/s10434-012-2776-6
