Clinical and pathological portraits of axillary presentation breast cancer and effects of preoperative systemic therapy
Introduction
Axillary metastases are the first indication of occult breast cancer (OBC) in less than 1% (1) of breast cancer patients. The first case of axillary presentation in breast cancer was detected by Halsted in 1907 (2), and many studies have since indicated that the prognosis of patients presenting with OBC is generally better than that reported for stage II (TxN1-2M0) breast cancer (3). However, to date no studies have described the biological characteristics of axillary metastasis in detail, and few studies have discussed preoperative systemic therapy (PST) options for patients. Due to the low incidence of OBC, previous studies were performed retrospectively, and optimal treatment approaches have yet to be established. Here, we present our experience about OBC in Breast Disease Center of Peking University First Hospital.
Case presentation
From January 2008 to December 2015, 2,705 cases were diagnosed as primary breast cancer in Breast Disease Center of Peking University First Hospital. Eleven (0.4%) cases presented as axillary metastasis, with no foci detected in the ipsilateral breast on bilateral ultrasound and mammography. All cases were diagnosed based on histopathological reports of axillary lymph node adenocarcinoma-compatible mammary carcinoma. All patients were female with the median follow-up period was 26 (range, 6–96) months; and the median age was 56 (range, 29–75) years. Among these cases, one patient had a family history of breast cancer. At the time of diagnosis, one case presented with metastasis in the mediastinal and retroperitoneal lymph nodes and in the liver. The study was approved by the Ethics Committee of the Peking University First Hospital.
Magnetic resonance imaging (MRI)
Of the 11 patients, 9 agreed to MRI, and we found abnormalities in the ipsilateral breast in 4 of these patients. In one of the 5 cases with negative findings, surgery was not accepted because of the stage IV diagnosis; this case was excluded from our analysis without pathology evidence. Among the 4 patients with abnormal MRI findings, no invasive or in situ tumors were found by surgery in two, whereas one had invasive ductal cancer and one fibrosis after PST. The sensitivity of MRI was 100% (2/2+0), and the false positive rate was 33.3% (2/2+4).
Histologic-pathological characters of axillary lymph nodes
In this study, lymphatic pathology was obtained via B-scanultrasound (GE S60)-guided core needle biopsy (16G, Bard Urological) in all cases prior to treatment. Histological grade was evaluated according to the Elston-Ellis modification of the Scarff-Bloom-Richardson grading system (4).
For all patients, immunohistochemistry (IHC) staining for estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor 2 (HER2) and Ki67 were performed on consecutive tissue sections from the nodal disease. ER and PR status was classified as negative (lack of any ER and PR immunoreactivity or <1% tumor cell immunoreactivity, with positive inner control) or positive (≥1% tumor cell immunoreactivity) (5). Among those classified as positive, we defined samples as uncertain endocrine responsiveness (ER and/or PR <10% of cells positive) and endocrine responsiveness (both ER and PR ≥10% of cells positive) (6). The value of the Ki67 labeling index was divided into low (<14%) and high (≥14%) (7). Only intense and complete membrane staining in >10% of the tumor cells was considered as HER2 overexpression (3+). Fluorescence in situ hybridization (FISH) assays were performed in cases with IHC equivocal (2+) to identify cases with gene amplification as HER2 to chromosome 17 centromere ratio ≥2 (8).
We defined the clinicopathological surrogate subtypes of breast cancer according to the 2013 St. Gallen consensus (9) through IHC evaluation of ER, PR, HER2 and Ki67.
All 11 cases were subjected to lymphatic pathology before treatment and were found to be invasive adenocarcinoma considered to have derived from the mammary gland, as based on combined IHC and clinical features. Histologic analysis of the axillary node showed that 9 of the 11 (81.8%) cases were G3; 2 (18.2%) cases were G2, and none were G1. Of the 11 patients, 6 were ER negative, 1 was 2% weakly positive, and 4 were ≥10% positive; among the last four, three patients were PR negative. Of the 11 patients, 8 were PR negative, 2 were 2% positive, and only one was ≥10% positive. HER2 overexpression was observed in 6 of the 11 patients. Most of the patients (81.8%, 9/11) had a high Ki67 index, over 30%, and 72.7% (8/11) were ≥70%. In contrast, only one patient had a Ki67 index lower than 14%. Defined as surrogate subtypes, no Luminal A-like subtype was found; 3 were triple negative, 3 were HER2 positive, and 5 were Luminal B-like (3 HER2 positive, 2 HER2 negative).
PST regimens and response evaluation
Patients who were HER2 positive were administered TCH (docetaxel 75 mg/m2, carboplatin AUC=5–6, trastuzumab 8 mg/kg in the first week and followed by 6 mg/kg every 3 weeks). The remaining patients were given TA (docetaxel 75 mg/m2 or paclitaxel 175 mg/m2, combined with epirubicin 75 mg/m2 every 3 weeks).
The clinical response was evaluated by ultrasound according to RECIST 1.1 (10). In addition, we evaluated the pathological response of the primary site according to the Miller-Payne grading system (11). The pathological response of the axillary lymph node, as proposed by Sataloff in 1995 (12), is presented in Table 1.
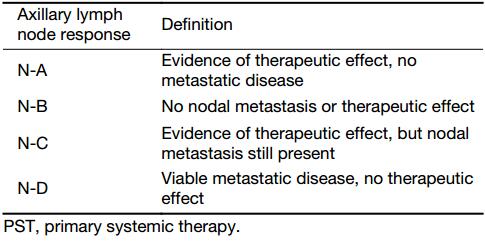
Full table
Other treatments
Regardless of whether PST was performed, modified radical mastectomy (MRM) with radiotherapy or axillary lymph node dissection (ALND) with radiotherapy (the whole breast included) was recommended. Other standard therapies, such as hormone therapy and an anti-HER2 regimen (TCH, as described above), were also commonly recommended.
Upon initial diagnosis, one case was at stage IV, with mediastinal lymph node, retroperitoneal lymph node and liver metastases. The patient underwent MRI, and no abnormality was found in the breast. This patient received a TCH regimen (6 cycles) and achieved a clinically complete response (CR). She underwent radiotherapy, instead of mastectomy and ALND, and insisted on treatment with trastuzumab every 3 weeks.
Of the other 10 cases, 5 patients accepted 6 cycles of PST (Table 2), and surgery (MRM) was then performed. Pathology after PST and surgery showed a 5 N-C response, except for one case in which we found a 2 cm fibrosis in the breast consistent with the abnormality on MRI. Four of the patients received radiotherapy, and only one refused. One of the 5 patients was HER2 positive, for whom one year of trastuzumab therapy was completed.
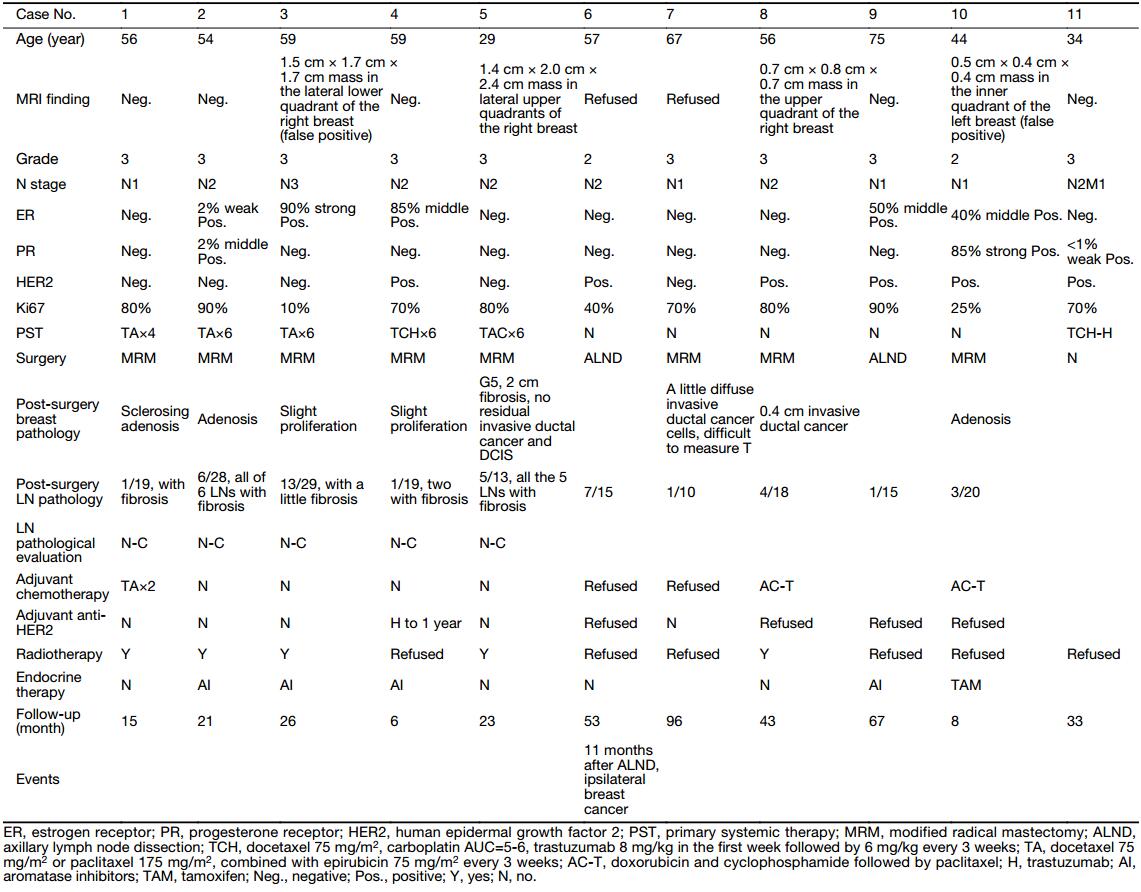
Full table
The other 5 cases underwent surgery directly. Two cases refused mastectomy, and only ALND was performed. Moreover, these 2 cases were HER2 positive but refused adjuvant chemotherapy, anti-HER2 therapy and radiotherapy. MRM was performed on the 3 other patients, 2 of whom were found to have invasive ductal cancer in the breast. One of the 3 MRM patients refused adjuvant chemotherapy and radiotherapy. The other two MRM patients were HER2 positive, and they accepted doxorubicin and cyclophosphamide followed by paclitaxel (AC-T) as adjuvant chemotherapy. In addition, both patients refused trastuzumab therapy: one accepted adiotherapy, whereas the other refused. All ER- and/or PR-positive patients accepted hormone therapy.
Follow-up
The cases were followed up until July 2016, with a median follow-up period of 26 (range, 6–96) months. One patient on whom only ALND was performed showed ipsilateral breast cancer 11 months later. The case diagnosed as de novo stage IV, achieved a clinically CR after 6 cycles TCH. As of the last follow-up, CR was maintained, and the patient was undergoing continuous trastuzumab therapy for over 2 years. The others survived without recurrence or metastasis.
Discussion
Patients of OBC comprise a rare subset. After decades of retrospective studies, some experts’ opinions are trending toward the recommendation of MRM. However, controversy remains, as there are few reports describing biological markers, molecular typing and PST for OBC.
Although MRI is a good method for identifying abnormalities more sensitively than mammography or breast ultrasound, the technique has a high false positive rate [29% in one study (13)]. The same was shown in our study. In our study, 8 patients underwent MRM (with or without PST), and we found signs of primary breast foci in 3 of them. Only ALND without radiotherapy was performed in 2 cases, and ipsilateral breast cancer was found 11 months later in one of them. Hence, MRM or breast radiotherapy should be performed, regardless of the breast abnormality found on MRI. According to a recent meta-analysis (14), the effects of ALND with breast radiotherapy were equal to those of MRM.
PST is rarely reported in OBC. A single-institutional review (15) by MD Anderson reported data for 25 patients who met criteria for OBC and underwent PST. Moreover, some patients were subjected to excisional biopsy when first diagnosed, and the data for these cases did not differ from the data for cases with no positive lymph nodes after ALND. Hence, the high pathologic complete response (pCR) rate in the study remains to be explained. In our study, all patients underwent 4–6 cycles of PST and exhibited clinical and pathological responses, yet no pCR was achieved in cases with lymph nodes metastasis. PST is a good approach for evaluating therapeutic responses and may promote a deeper understanding of the biological behavior of OBC. Hence, further investigation is needed.
Previous studies have reported controversial conclusions about OBC prognosis, most indicating that OBC has a better prognosis compared to other breast cancers with lymph node metastasis (1). However, due to its rarity, few studies have investigated the biological characteristics of OBC. In our analysis, most cases showed biomarkers indicated worse prognosis as high histological grade, ER negative, HER2 overexpression, and a very high Ki67 index, ≥70%. Actually, only one case had local breast recurrence during follow-up. Indeed, even the patient diagnosed at stage IV with hepatic metastasis had a good response to therapy, with 33-month survival to date. Hence, more data are necessary to promote our understanding of the biological characteristics of OBC.
Conclusions
OBC is a rare form of breast cancer. Its biological characteristics have not been well described to date. PST might be considered as an effective therapy for patients with OBC.
Acknowledgements
This work is supported by grants of the Precision Medicine Special Project of National Key Research and Development Program of China (2016YFC0901302).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pentheroudakis G, Lazaridis G, Pavlidis N. Axillary nodal metastases from carcinoma of unknown primary (CUPAx): a systematic review of published evidence. Breast Cancer Res Treat 2010;119:1–11. [PubMed] DOI:10.1007/s10549-009-0554-3
- Halsted WS. The results of radical operations for the cure of carcinoma of the breast. Ann Surg 1907;46:1–19. [PubMed]
- Rosen PP, Kimmel M. Occult breast carcinoma presenting with axillary lymph node metastases: a follow-up study of 48 patients. Hum Pathol 1990;21:518–23. [PubMed]
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 2002;41:154–61. [PubMed]
- Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–95. [PubMed] DOI:10.1200/JCO.2009.25.6529
- Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 2005;16:1569–83. [PubMed] DOI:10.1093/annonc/mdi326
- Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–50. [PubMed] DOI:10.1093/jnci/djp082
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [PubMed] DOI:10.1200/JCO.2013.50.9984
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206–23. [PubMed] DOI:10.1093/annonc/mdt303
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [PubMed] DOI:10.1016/j.ejca.2008.10.026
- Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 2003;12:320–7. [PubMed]
- Sataloff DM, Mason BA, Prestipino AJ, et al. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg 1995;180:297–306. [PubMed]
- Olson JA Jr, Morris EA, Van Zee KJ, et al. Magnetic resonance imaging facilitates breast conservation for occult breast cancer. Ann Surg Oncol 2000;7:411–5. [PubMed]
- Macedo FI, Eid JJ, Flynn J, et al. Optimal surgical management for occult breast carcinoma: A Meta-analysis. Ann Surg Oncol 2016;23:1838–44. [PubMed] DOI:10.1245/s10434-016-5104-8
- Rueth NM, Black DM, Limmer AR, et al. Breast conservation in the setting of contemporary multimodality treatment provides excellent outcomes for patients with occult primary breast cancer. Ann Surg Oncol 2015;22:90–5. [PubMed] DOI:10.1245/s10434-014-3991-0
