Low hypoxia inducible factor-1α (HIF-1α) expression in testicular germ cell tumors — a major reason for enhanced chemosensitivity?
Introduction
When Vranic et al. discovered a low hypoxia inducible factor-1α (HIF-1α) expression in testicular germ cell tumors (GCT), specifically seminomas and mixed GCT (1), they may have accidentally stumbled upon an important underlying factor for the exquisite chemosensitivity of these tumors. Indeed, the molecular basis for enhanced chemosensitivity of GCT has been an area of great interest, as it could potentially give us therapeutic leads in other resistant malignancies. Thus far, however, the increased sensitivity of GCT has been variously attributed to multiple factors, — an inability to detoxify cisplatin, a lack of export pumps, an inability to repair the DNA damage, an intact apoptotic cascade and lack of p53 mutation (2-4).
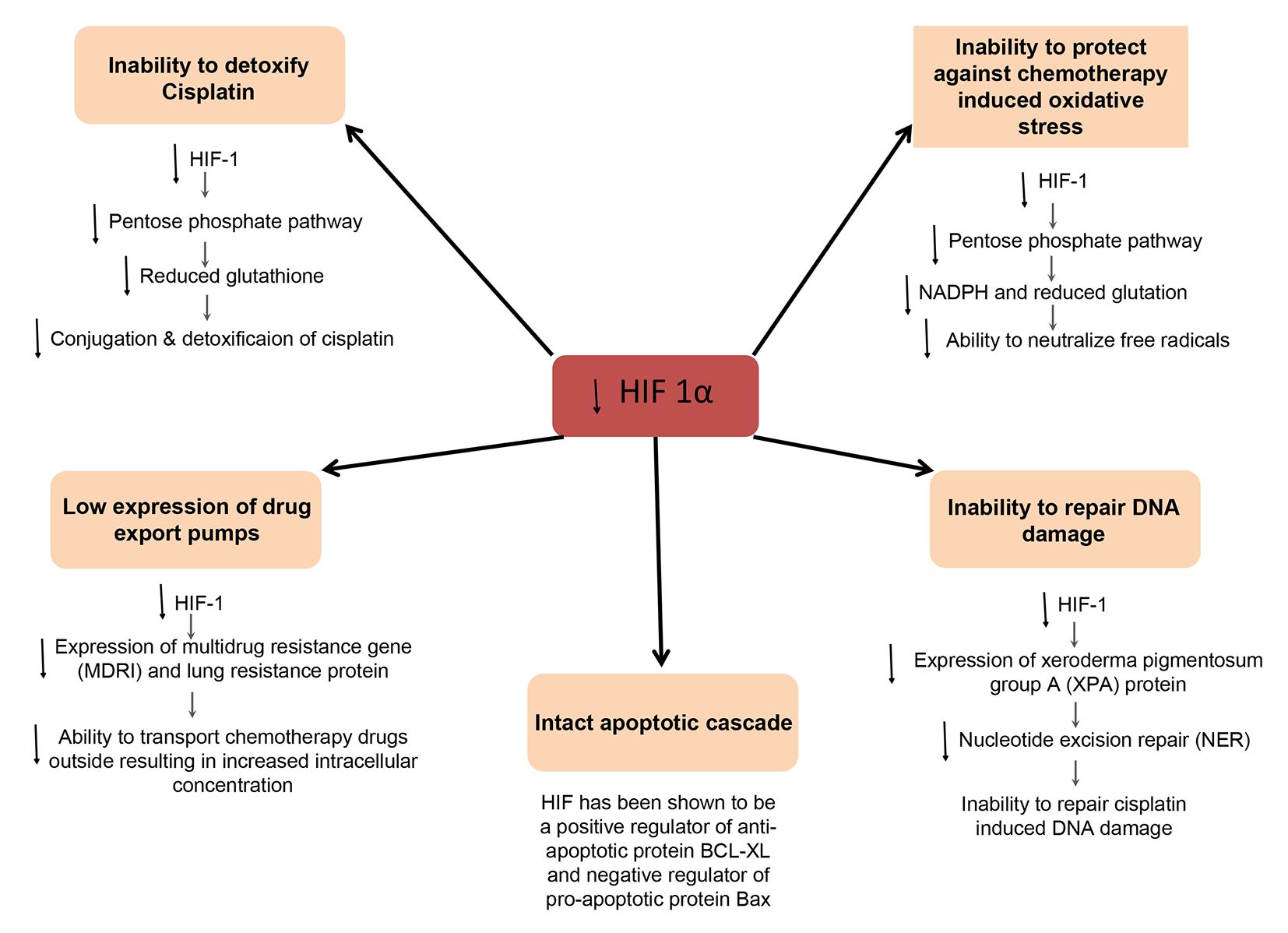
Although the immediate cause for several of these factors has been identified [for example, low intracellular reduced glutathione (5) and metallothionein (6) leading to reduced conjugation and detoxification of cisplatin; and low expression of drug transport pumps such as lung resistance protein (LRP) (7) resulting in higher intracellular retention of chemotherapeutic agents], the root cause of these factors has not been delineated (for example, the cause of low intracellular reduced glutathione or the low expression of LRP in GCT has been unknown). We believe that the demonstrated low HIF-1α expression in GCT may be the root cause for several of these noted chemotherapy sensitizing mechanisms, and have put forth postulated pathophysiologic pathways by which this may occur (Figure 1).
Inability to detoxify cisplatin
HIF-1α increases uptake of glucose and mediates the diversion of glucose 6 phosphate into the pentose phosphate pathway, which produces increased amounts of NADPH and reduced glutathione (8,9). Low HIF-1α may therefore be the reason for the low reduced glutathione found in testicular GCT, leading to reduced conjugation and detoxification of cisplatin.
Inability to protect against chemotherapy-induced oxidative stress
Reduced glutathione and NADPH generated through the pentose phosphate pathway are major defense mechanisms against the oxidative stress induced by chemotherapeutic agents. As such, tumor cells with low HIF-1α would be expected to be vulnerable to chemotherapy induced oxidative stress.
Low expression of drug transport pumps
The expression of multidrug resistance 1 (MDR1) and LRP, involved in drug transport and efflux, has been found to be synchronous with the changes in expression of HIF-1α, and increased expression of these genes has been observed upon transfection with a plasmid HIF-1α (10,11). These data suggest that HIF-1α is a key regulator of MDR1 and LRP, involved in drug transport and efflux. LRP expression has been shown to be low in GCT (7), possibly due to low HIF-1α levels.
Inability to repair DNA damage
One of the prominent reasons for the enhanced sensitivity of GCT to chemotherapy, specially cisplatin, is believed to be low levels of xeroderma pigmentosum group A (XPA) protein, a critical component of the nucleotide excision repair (NER) pathway (12). NER is the main mechanism by which DNA damage induced by cisplatin, in the form of bulky DNA adducts, is repaired (13). Binding of XPA to replication protein A (RPA) is the initiating, rate-limiting step of NER and it subsequently recruits other factors (14,15). Furthermore, increased expression of XPA has been associated with cisplatin resistance in other solid tumors (16,17). Interestingly, HIF-1 binds to hypoxia response element (HRE) in the XPA promoter, and has been shown to up-regulate XPA, leading to cisplatin resistance in lung cancer. Furthermore, inhibition of HIF-1α with siRNA has been shown to decrease the expression of XPA. Low HIF-1α in GCT thus represents a potential cause of low XPA levels, in turn leading to an innate inability to repair DNA damage induced by cisplatin.
Intact apoptotic pathway
HIF-1α is known to play a key role in regulating apoptosis resistance in tumor cells under hypoxia (18-20). GCTs have been shown to have low anti-apoptotic protein levels (BCL-2 and BCL-XL) (4) and HIF-1α has been shown to be a key positive regulator of BCL-XL (19) and a negative regulator of pro-apoptotic protein Bax (21).
HIF-1 (a heterodimeric transcription factor consisting of the constitutively expressed HIF-1β subunit and the oxygen-regulated HIF-1α subunit) is known to confer worse prognosis in multiple cancers, with prominent effects on angiogenesis, cell survival and glucose metabolism (22); and mediates chemoresistance and radioresistance. PX-478, an inhibitor of HIF-1α, has been shown to enhance radiosensitivity of various cancer cell lines and xenografts (23,24). Studies have also demonstrated reversal of chemoresistance with the use of small-interfering RNAs directed against HIF-1α or with HIF-1α destabilization (25,26). HIF-1 can up-regulate MDR gene expression and increased MDR gene expression has been shown to confer resistance to various drugs such as cisplatin, vinca alkaloids, anthracyclines, taxanes and epipodophyllotoxins (27,28). HIF-1 inhibition has been shown to down-regulate MDR1 expression (27). It is also a well-established fact that the hypoxic areas of cancers in general tend to be more radioresistant and chemoresistant, mediated by HIF-1 (29). The innate chemoresistance and radioresistance of renal cell carcinoma is thought to be caused, to a significant extent, by a constitutionally active HIF pathway due to loss of von Hippel-Lindau (VHL) function or accumulation of Krebs cycle intermediates (30-33). Pancreatic cancer, the most hypoxic of all solid tumors, remains refractory to a large extent to chemoradiotherapy with nuclear HIF-1α in 88% of human pancreatic ductal carcinoma (34). HIF-1 therefore has a prominent role in mediating resistance in many cancers, and the development of direct or indirect HIF-1 inhibitors [summarized by Semenza et al. (22) and Onnis et al. (35)] continue to hold promise in enhancing chemosensitivity and radiosensitivity in resistant malignancies. Clinical trials targeting HIF-1 in cancer are underway (ClinicalTrials.gov Identifier: NCT02564614 and NCT01120288).
Conclusions
Considering the above factors and the correlative evidence, a low level of HIF-1 may be a prominent underlying reason for the enhanced chemosensitivity of GCT. The unanswered questions that then arise are: what causes low HIF-1 in testicular GCT? Is it p53 induced degradation (36), with GCT rarely demonstrating inactivating mutation of p53, in contrast to other solid tumors (2)? If so, what explains the low p53 mutation incidence in testicular GCT? How is low HIF-1 related to isochromosome 12p, a common feature of testicular GCT?
Statement of translational relevance
Herein, we offer a molecular biology based hypothesis to support a potential significant role for the previously demonstrated low HIF-1 expression in mediating the general exquisite chemosensitivity of testicular GCT. Going forward, we plan to determine the difference in modulation of HIF-1 in platinum-resistant and sensitive GCT, and correlate it with patient outcomes.
If validated, this hypothesis could have a significant translational impact on platinum refractory GCT with the addition of HIF-1 modifying strategies to conventional chemotherapy regimens.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vranic S, Hes O, Grossmann P, et al. Low frequency of HIF-1α overexpression in germ cell tumors of the testis. Appl Immunohistochem Mol Morphol 2013;21:165–9. [PubMed] DOI:10.1097/PAI.0b013e31825e00b7
- Mayer F, Honecker F, Looijenga LH, et al. Towards an understanding of the biological basis of response to cisplatin-based chemotherapy in germ-cell tumors. Ann Oncol 2003;14:825–32. [PubMed]
- Voutsadakis IA. The chemosensitivity of testicular germ cell tumors. Cell Oncol (Dordr) 2014;37:79–94. [PubMed] DOI:10.1007/s13402-014-0168-6
- Mayer F, Stoop H, Scheffer GL, et al. Molecular determinants of treatment response in human germ cell tumors. Clin Cancer Res 2003;9:767–73. [PubMed]
- Masters JR, Thomas R, Hall AG, et al. Sensitivity of testis tumour cells to chemotherapeutic drugs: role of detoxifying pathways. Eur J Cancer 1996;32A:1248–53. [PubMed]
- Koropatnick J, Kloth DM, Kadhim S, et al. Metallothionein expression and resistance to cisplatin in a human germ cell tumor cell line. J Pharmacol Exp Ther 1995;275:1681–7. [PubMed]
- Izquierdo MA, Scheffer GL, Flens MJ, et al. Broad distribution of the multidrug resistance-related vault lung resistance protein in normal human tissues and tumors. Am J Pathol 1996;148:877–87. [PubMed]
- Masson N, Ratcliffe PJ. Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer Metab 2014;2:3. [PubMed] DOI:10.1186/2049-3002-2-3
- Lu H, Samanta D, Xiang L, et al. Chemotherapy triggers HIF-1-dependent glutathione synthesis and copper chelation that induces the breast cancer stem cell phenotype. Proc Natl Acad Sci U S A 2015;112:E4600–9. [PubMed] DOI:10.1073/pnas.1513433112
- Zhu H, Chen XP, Luo SF, et al. Hypoxia-inducible factor-1 alpha dependent expression and significance of the related multidrug resistance genes induced by hypoxia in human hepatocarcinoma cell. Zhonghua Wai Ke Za Zhi (in Chinese) 2005;43:277–81. [PubMed]
- Comerford KM, Wallace TJ, Karhausen J, et al. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 2002;62:3387–94. [PubMed]
- Köberle B, Masters JR, Hartley JA, et al. Defective repair of cisplatin-induced DNA damage caused by reduced XPA protein in testicular germ cell tumours. Curr Biol 1999;9:273–6. [PubMed]
- Chaney SG, Sancar A. DNA repair: enzymatic mechanisms and relevance to drug response. J Natl Cancer Inst 1996;88:1346–60. [PubMed]
- Matsuda T, Saijo M, Kuraoka I, et al. DNA repair protein XPA binds replication protein A (RPA). J Biol Chem 1995;270:4152–7. [PubMed]
- Buschta-Hedayat N, Buterin T, Hess MT, et al. Recognition of nonhybridizing base pairs during nucleotide excision repair of DNA. Proc Natl Acad Sci U S A 1999;96:6090–5. [PubMed]
- Dabholkar M, Vionnet J, Bostick-Bruton F, et al. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J Clin Invest 1994;94:703–8. [PubMed] DOI:10.1172/JCI117388
- Liu Y, Bernauer AM, Yingling CM, et al. HIF1α regulated expression of XPA contributes to cisplatin resistance in lung cancer. Carcinogenesis 2012;33:1187–92. [PubMed] DOI:10.1093/carcin/bgs142
- Sermeus A, Genin M, Maincent A, et al. Hypoxia-induced modulation of apoptosis and BCL-2 family proteins in different cancer cell types. PLoS One 2012;7:e47519. [PubMed] DOI:10.1371/journal.pone.0047519
- Chen N, Chen X, Huang R, et al. BCL-xL is a target gene regulated by hypoxia-inducible factor-1{alpha}. J Biol Chem 2009;284:10004–12. [PubMed] DOI:10.1074/jbc.M805997200
- Kilic M, Kasperczyk H, Fulda S, et al. Role of hypoxia inducible factor-1 alpha in modulation of apoptosis resistance. Oncogene 2007;26:2027–38. [PubMed] DOI:10.1038/sj.onc.1210008
- Erler JT, Cawthorne CJ, Williams KJ, et al. Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol 2004;24:2875–89. [PubMed]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721–32. [PubMed] DOI:10.1038/nrc1187
- Palayoor ST, Mitchell JB, Cerna D, et al. PX-478, an inhibitor of hypoxia-inducible factor-1alpha, enhances radiosensitivity of prostate carcinoma cells. Int J Cancer 2008;123:2430–7. [PubMed] DOI:10.1002/ijc.23807
- Schwartz DL, Powis G, Thitai-Kumar A, et al. The selective hypoxia inducible factor-1 inhibitor PX-478 provides in vivo radiosensitization through tumor stromal effects . Mol Cancer Ther 2009;8:947–58. [PubMed] DOI:10.1158/1535-7163.MCT-08-0981
- Jiao M, Nan KJ. Activation of PI3 kinase/Akt/HIF-1α pathway contributes to hypoxia-induced epithelial-mesenchymal transition and chemoresistance in hepatocellular carcinoma. Int J Oncol 2012;40:461–8. [PubMed] DOI:10.3892/ijo.2011.1197
- Fischer C, Leithner K, Wohlkoenig C, et al. Panobinostat reduces hypoxia-induced cisplatin resistance of non-small cell lung carcinoma cells via HIF-1α destabilization. Mol Cancer 2015;14:4. [PubMed] DOI:10.1186/1476-4598-14-4
- Chen J, Ding Z, Peng Y, et al. HIF-1α inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS One 2014;9:e98882. [PubMed] DOI:10.1371/journal.pone.0098882
- Ambudkar SV, Dey S, Hrycyna CA, et al. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 1999;39:361–98. [PubMed] DOI:10.1146/annurev.pharmtox.39.1.361
- Yeom CJ, Zeng L, Zhu Y, et al. Strategies to assess hypoxic/HIF-1-active cancer cells for the development of innovative radiation therapy. Cancers (Basel) 2011;3:3610–31. [PubMed] DOI:10.3390/cancers3033610
- Shenoy N, Pagliaro L. Sequential pathogenesis of metastatic VHL mutant clear cell renal cell carcinoma: putting it together with a translational perspective. Ann Oncol 2016;27:1685–95. [PubMed] DOI:10.1093/annonc/mdw241
- Shenoy N, Shrivastava M, Sukrithan V, et al. The regulation and interactions of the hypoxia inducible factor pathway in carcinogenesis and potential cancer therapeutic strategies. J Cancer Ther 2015;6:511–21. DOI:10.4236/jct.2015.66055>
- Xing T, He H. Epigenomics of clear cell renal cell carcinoma: mechanisms and potential use in molecular pathology. Chin J Cancer Res 2016;28:80–91. [PubMed] DOI:10.3978/j.issn.1000-9604.2016.02.09
- Rao Q, Xia QY, Cheng L, et al. Molecular genetics and immunohistochemistry characterization of uncommon and recently described renal cell carcinomas. Chin J Cancer Res 2016;28:29–49. [PubMed] DOI:10.3978/j.issn.1000-9604.2016.01.03
- Schwartz DL, Bankson JA, Lemos R, Jr, et al. Radiosensitization and stromal imaging response correlates for the HIF-1 inhibitor PX-478 given with or without chemotherapy in pancreatic cancer. Mol Cancer Ther 2010;9:2057–67. [PubMed] DOI:10.1158/1535-7163.MCT-09-0768
- Onnis B, Rapisarda A, Melillo G. Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med 2009;13:2780–6. [PubMed] DOI:10.1111/j.1582-4934.2009.00876.x
- Ravi R, Mookerjee B, Bhujwalla ZM, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev 2000;14:34–44. [PubMed]
