Clinicopathological features and surgical outcomes of four rare subtypes of primary liver carcinoma
Introduction
The most common pathological subtype of primary liver cancer is hepatocellular carcinoma (HCC) (1,2). A total of 466,100 new liver cancer patients in China were reported in 2015 (3), according to the World Health Organization (WHO) classification of liver neoplasm (4). Several rare cytological variants of primary liver malignancies exist; these subtypes have pathological features as well as characteristic clinical manifestations and prognostic features (5-10).
However, owing to the low incidences of these uncommon subtypes of primary hepatic malignancies, a relatively few clinical studies have been reported. It is difficult to diagnose these subtypes of primary liver malignancies preoperatively. The diagnosis needs to be confirmed in most patients by postoperative pathological examination or even using immunohistochemical (IHC) stains (8,11). Furthermore, little is known about the clinical and prognostic features of these subtypes.
Therefore, the present study aimed to analyze clinicopathological and prognostic features of four rare pathological subtypes of primary liver malignancies. The findings may help clinical surgeons to predict the prognosis and make a suitable treatment plan.
Materials and methods
Patients
The study retrospectively analyzed clinicopathological and prognostic data of 114 patients who underwent hepatectomy with pathologically confirmed diagnosis of rare pathological subtypes of primary liver malignancies in the Cancer Hospital & Institute of the Chinese Academy of Medical Sciences in Beijing, China, from October 1998 to August 2015. Also, the corresponding clinical survival data of 908 patients were collected. These patients underwent hepatectomy during the same time with pathologically confirmed diagnosis of common HCC. They were treated as two control group: early-stage HCC group and advanced-stage HCC group. The study was approved by the Ethics Committee of Cancer Hospital & Institute of the Chinese Academy of Medical Sciences (No. 16-024/1103). The need of individual consent was waived because of the retrospective nature of the study.
Preoperative examination
Preoperative serological examination included serum hepatitis B virus (HBV) and related viral markers, serum bilirubin, serum albumin, serum alpha-fetoprotein (AFP), and carcinoembryonic antigen (CEA) in all the patients. Abdominal ultrasonic and computed tomography (CT) scan were performed in all patients; 90 patients underwent magnetic resonance imaging (MRI) scan.
Surgical procedure
All patients underwent hepatectomy. Different surgical procedures were performed according to the size, location of the tumor, and liver function or cirrhosis level. Twelve patients underwent lymph node (LN) dissection because of preoperative diagnosis considering intrahepatic cholangiocarcinoma (ICC) or abnormal enlarged LNs in the hepatic hilar region intraoperatively. Seven patients underwent combined partial diaphragm or transverse colon resection and cholecystectomy because of the tumor invading the aforementioned organs.
Pathologic examination
All the surgical resection specimens were embedded in paraffin. The sections were stained with hematoxylin and eosin (HE). Two pathologists with expertise in the liver pathology reviewed and confirmed the histopathological characteristics of all the sections, and classified patients into the following groups: 1) clear cell carcinoma (CCC) group: patients with >50% “clear cells” in the liver tumor could be classified into the CCC group, and non-primary (renal clear cell carcinoma liver metastasis) HCC patients were excluded; 2) giant cell carcinoma (GCC): patients with more than 50% irregular-shaped, bizarre multinucleated, or fused “giant” cells were classified into the GCC group; 3) sarcomatoid carcinoma (SC): patients consisted of sarcomatous portions and sarcomatous elements predominantly composed of spindle-shaped, pleomorphic, and osteoplastic types of cells; 4) combined hepatocellular-cholangiocarcinoma (CHC): CHC was defined as a tumor containing unequivocal, intimately mixed elements of both HCC and ICC according to the WHO classification, and patients with separate nodules of HCC and ICC in the same liver were excluded (Figure 1). The control groups were classified into two groups based on the 7th American Joint Committee on Cancer staging system; 5) early-stage group: TNM stage I or II common HCC; and 6) advanced-stage group: TNM stage III or IV common HCC.

Follow-up and treatment of patients
The 114 patients were followed up by outpatient examination, telephone, or mail communication at 1 month, then every 3 months during the first 2 years postoperatively, and every 6 months after 2 years. The total follow-up duration was 1–168 months, and the median follow-up duration was 24 months.
Statistical analysis
Numerical data were presented as x±s. Differences were compared using two-way analysis of variance or nonparametric Mann-Whitney U test. Categorical data were tested using the Chi-square test or Fisher’s exact test. The survival analysis curves were determined using the life-table and Kaplan-Meier methods, and pairwise compared using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards regression model. P values less than 0.05 were considered significant. All the statistical assessment was performed using SPSS software for Windows (Version 13.0; SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinical characteristics
The demographic and clinicopathological characteristics of the patients with uncommon types of HCC are presented in Table 1. Male patients were predominant in each group. Gender and age were similar among groups (P>0.05). Sixty-seven (58.8%) patients were symptomatic at the time of diagnosis; 60 patients with abdominal pain and 2 with jaundice related to the tumor. Ninety-five (83.3%) patients had HBV infection and 75 (65.8%) patients had cirrhosis. The levels of serum tumor markers AFP and CEA were similar in the four groups (P>0.05). The patients in the CCC group had the smallest tumor size and the lowest incidence of tumor vascular emboli and adjacent organ invasion among the four groups (P<0.05). However, the patients in the CCC group had the highest incidence of pseudocapsule formation and R0 resection among the four groups (P<0.05). In contrast, the patients in the SC group patients had the biggest tumor size. Tumor vascular emboli and adjacent organ invasion were more frequently found in the SC and GCC groups than in other groups (P<0.05).

Full table
Surgical outcomes
All the patients underwent hepatectomy. Different liver resection procedures were chosen, including lobectomy, segmentectomy, and local resection according to the tumor size, location, preoperative imaging diagnosis, liver functional reserve, and intraoperative exploration. Also, perihilar LN dissection was performed in 12 patients on the basis of preoperative imaging or intraoperative finding of abnormal enlarged LNs and diagnosis of ICC preoperatively. Two patients underwent combined phrenectomy, one case underwent combined cholecystectomy, and one case underwent combined transverse colectomy because of tumor invasion. Only one case of postoperative mortality due to liver failure was reported 12 days after operation.
Prognostic factors and survival analysis
The 1-, 3- and 5-year overall survival (OS) of the patients with CCC was 85%, 62% and 43%, respectively. The median OS time was 40.5 months. Patients with CCC had the best prognosis among the four uncommon subgroups (P<0.05), and the outcome of the CCC group was similar to that of the early-stage HCC group (n=790, P>0.05), whose 1-, 3- and 5-year OS was 85%, 69% and 53%, respectively. The median OS time was 79.2 months (Table 2). The SC group had the worst outcome among the four study groups. The median OS time was 8.7 months, and the 1-year OS was only 22%. No case of survival was reported after 3 years. The prognosis was poorer than that of any other subgroups (P<0.05), including the outcome of the advanced-stage common HCC group (n=118; median OS: 23.3 months; the 1-, 3- and 5-year OS was 49%, 24% and 18%, respectively). The 1-, 3-, and 5-year OS of the patients with GCC was 48%, 20%, and 0%, respectively. The median OS was 22.9 months. The outcome of the GCC group in terms of OS seemed poorer than that of the CHC group (n=35; median OS: 24.9 months; the 1-, 3-, and 5-year OS was 51%, 36%, and 26%, respectively), but no statistical difference was observed between the GCC and CHC groups by survival analysis (P>0.05). In addition, no significant difference was found in the GCC and CHC groups compared with the advanced-stage HCC group (P>0.05). The survival function curves of the four study groups and two control groups are presented in Figure 2.
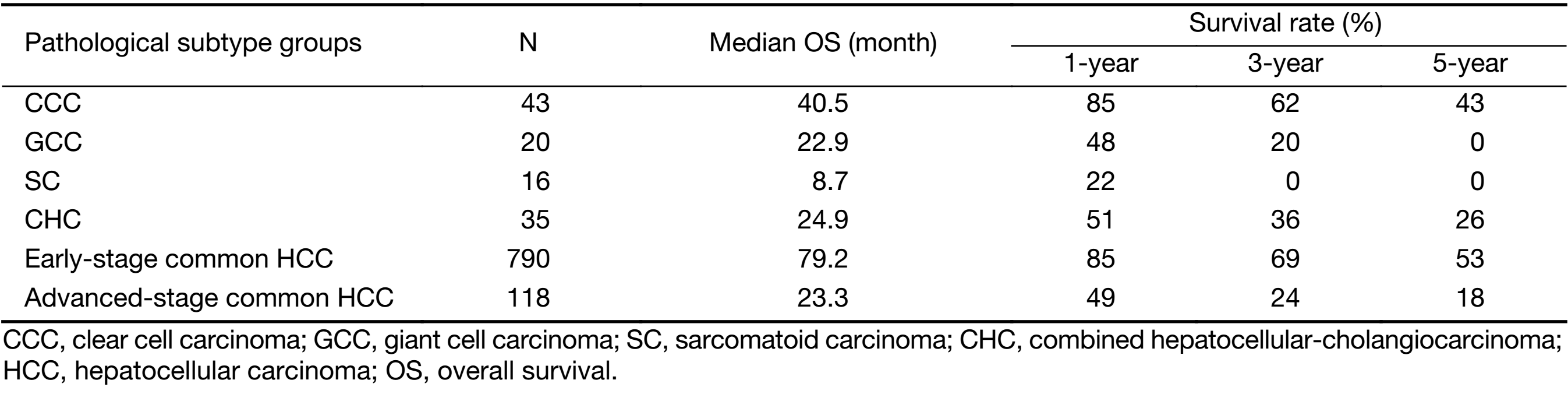
Full table

Multivariate analysis (Table 3) revealed that the incidences of tumor vascular emboli, TNM staging and nonradical resection were three risk factors of the prognosis of OS (P<0.01). However, gender, age, tumor size and AFP were excluded from the prognostic factors in the four study groups (P>0.05).
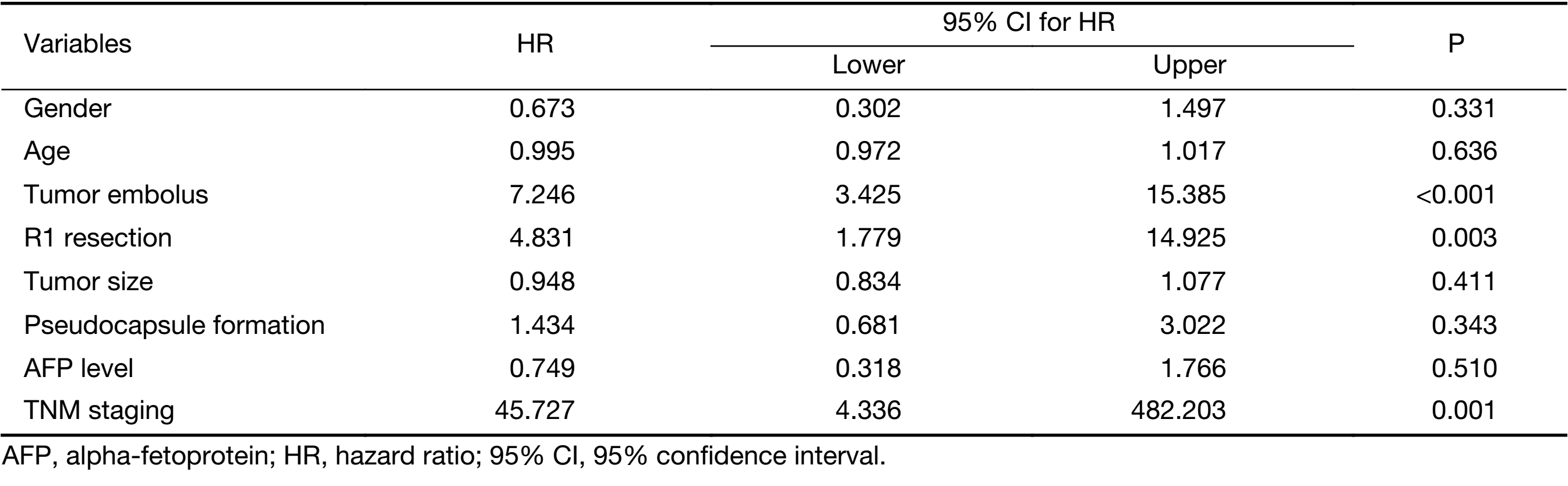
Full table
Discussion
Rare subtypes of HCC represent their distinctive clinicopathological properties with unique tumor biology (6,9). However, very few reports were documented to help and guide hepatobiliary surgeons to make reliable treatment regimens for these rare HCC subtypes. Although the present study had limitations of being a single-center study and comprising a small population, the relatively large sample from China used in this study revealed useful information to understand the tumor biology in these rare subtypes of HCC.
All these four rare pathological subtypes of HCC had their own histological features and clinical features. The features of the present study group were as follows:
CCC of liver, histologically characterized by cells with entirely or almost entirely “clear” cytoplasm that were not stained with HE under microscopy (Figure 1A), related to the cytoplasmic accumulation of glycogen and/or fat droplets that dissolved during HE staining, leaving behind a “clear” cytoplasm (12-15). In the present study group, the proportion of “clear cell” was defined as more than 50%. Clear cells were generally polygonal and of medium size, and solid or trabecular in alignment. The nuclei of clear cells were mostly centrally located or slightly eccentric with a dense or sometimes clumpy chromatin. Ultrastructural studies revealed that cytoplasmic organoids, such as rough endoplasmic reticulum, free ribosomes, and polyribosomes, appear markedly decreased (15).
Generally, CCC is classified as well or moderate degree of differentiation due to abundant cytoplasm and a low proportion of nuclei. Most of the literature reported low-grade malignancy and prognosis of CCC (16). The CCC group had the smallest tumor size, the lowest incidence of tumor emboli, the highest incidence of pseudocapsule formation, and predominance of solitary tumor (Table 1). No LN metastasis or adjacent organ invasion was presentated. These pathological and clinical manifestations suggested the low-degree malignancy of the tumor.
The median overall survival time of the CCC group was markedly better than those of the other three rare pathological subtype groups and similar to that of the early-stage HCC group (P>0.05). One case of CCC even had more than 10 years’ long-term survival after the resection of recurrent peritoneal nodules 3 years after primary lesion resection.
In addition, microscopically, CCC of liver was similar to clear cell tumors from other sites including adrenal gland, kidney, ovary, lung, and pancreas (17,18), making it difficult to differentiate it from the metastatic CCC of the liver. Murakata et al. (19) reported that the hepatocyte antibody (DAKO) IHC staining distinguished primary CCC of liver from other clear cell malignancies with a sensitivity of 90% and specificity of 100%.
GCC of liver is a rare cytological variant type, it corresponds to “pleomorphic cell variant” in WHO pathological classification. It is named so because of giant cells formed by tumor cell fusion. The pleomorphic tumor cells show a marked variation in cellular and nuclear size, shape, and staining. The bizarre multinucleated or mononuclear giant tumor cells are often seen (Figure 1B). Generally, pleomorphic tumor cells lack cohesiveness and do not show a distinct trabecular pattern. Pleomorphic cells are common in poorly differentiated tumors (20,21).
A few reports on GCC are available in the literature (22). In the present study group, most patients had marked cytological and nuclear pleomorphism and abundant mitotic activity, and were classified into Edmondson-Steiner G3 or G4 (17/19). It was difficult to confirm the diagnosis using clinical materials without pathological results. The prognosis of this GCC group was poor, the survival median time was only 13 months, and no long-term survival was reported. In addition, one case had liver tumor invading the transverse colon, which manifested highly malignant and aggressive behavior.
SC of liver is also a rare histopathological subtype. It is characterized by the proliferation of spindle cells or bizarre giant cells with the interlacing arrangement of acini. When sarcomatous features are predominant, the tumor is called SC (4) (Figure 1C). The IHC examination result suggested that SC originated from sarcomatous change or mesenchymal metaplasia of both HCC and ICC cells. CK8 might be an excellent marker for the differential diagnosis of sarcomatoid cancers from metastatic or primary sarcomas of the liver (23-26). The CT scan of lesions showed a lack of blood supply, edge enhancement, and part of patients with hilar LN metastasis (27-29). The diagnosis was easily confused with cholangiocellular carcinoma. In the present study group, seven patients were considered cholangiocellular carcinoma and four patients were considered hilar LN metastasis by preoperative CT or MRI scan. The SC CT imaging revealed that the central part of the tumor was never enhanced in the portal venous phase, which was quite different from the central part irregular enhancement in the portal venous phase of cholangiocellular carcinoma or metastatic liver lesions. Six patients underwent hilar LN dissection, 2 patients had pathologically proven LN metastasis, and 3 patients had invasion of diaphragm or gallbladder.
The median overall survival time of this group was the shortest, and the largest number of patients with LN metastasis and direct invasion into the adjacent organs were reported in all the study groups. It indicated that the SC patients had the highest malignancy and the worst prognosis among the four rare pathological subtype groups. Most published studies related to SC were case reports; the prognoses showed poor outcomes similar to those observed in the present study group (30-36).
CHC is a rare pathological type containing both hepatocellular and cholangiocarcinoma elements. Allen and Lisa (37) reported five patients of CHC and, for the first time, classified these tumors into three groups: type A with HCC and CC present at different sites within the same liver; type B with HCC and CC present at adjacent sites and mingling with each other but still recognizable as distinct tumors; and type C with HCC and CC components combined in the same tumor. According to the WHO classification criteria, CHC is a tumor containing components of HCC and cholangiocarcinoma, and belongs to type C of Allen classification, in which the two components are in the same tumor. The 34 patients in the present study group were all type C (Figure 1D). The clinical manifestations and imaging findings depended on the predominance of HCC or CHC component within the tumor. When HCC formed the majority of the tumor, the serum AFP level increased in most patients and the CT scan of the lesion revealed “wash-in and wash-out” sign, which corresponded to HCC imaging diagnostic characteristics. However, when CC formed the majority of the tumor, the serum CEA level increased and delayed enhancement in CT scan was often observed (38-42). In our study, 6 patients were diagnosed with hilar LN metastasis by preoperative imaging; 3 of these patients were pathologically confirmed postoperatively. The serum CEA level markedly increased preoperatively in 5 patients; however, none of the patients was diagnosed with CHC preoperatively.
Several studies suggested that CHC showed a poorer prognosis than HCC or ICC (38-43). Kim et al. (44) compared the prognosis of 30 CHC patients and 77 ICC patients. The results revealed that the disease-free survival of patients with CHC was shorter than that of patients with ICC, but the OS rate was similar.
The CHC patients in our study group also had a poor prognosis. No significant difference in the median OS was observed compared with the GCC and advanced-stage common HCC groups (P>0.05).
The multivariate analysis of survival risk factors revealed that the incidences of tumor vascular emboli, non-radical resection (R1) and TNM staging were three risk factors of prognosis (P<0.01).
The tumor size, tumor marker levels, HBV infection, cirrhosis, LN metastasis, adjacent invasion, and tumor pseudocapsule formation in the four uncommon types of HCC groups were not statistically significant factors. The bias due to the small population of the present study cannot be excluded. The findings need to be further evaluated by investigating more patients.
Conclusions
The CCC had low-degree malignancy and favorable prognosis. On the contrary, GCC, SC and CHC were three rare high-degree malignancy subtypes of HCC with poor prognosis. The incidence of tumor emboli, TNM staging and non-radical (R1) resection were risk factors of OS, suggesting that radical resection is an efficient way to improve the prognosis.
Acknowledgements
The study was financially supported by the State Key Project on Infection Diseases of China (No. 2018ZX10723204-005), the National Natural Science Foundation of China (No. 81672461), the National High-tech R&D (863) Program of China (No. 2015AA020408), the Capital Health Research and Development of Special (No. 2018-1-4021), the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2016-I2M-1-001), and the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2017-12M-4-002).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008;134:1752–63. [PubMed] DOI:10.1053/j.gastro.2008.02.090
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557–76. [PubMed] DOI:10.1053/j.gastro.2007.04.061
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [PubMed] DOI:10.3322/caac.21338
- Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. 4th Editon. Lyon: IARC press, 2010:196-261.
- Terasaki S, Kaneko S, Kobayashi K, et al. Histological features predicting malignant transformation of nonmalignant hepatocellular nodules: a prospective study. Gastroenterology 1998;115:1216–22. [PubMed]
- Jernigan PL, Wima K, Hanseman DJ, et al. Natural history and treatment trends in hepatocellular carcinoma subtypes: Insights from a national cancer registry. J Surg Oncol 2015;112:872–6. [PubMed] DOI:10.1002/jso.24083
- Ma X, Yang Y, Tu H, et al. Risk prediction models for hepatocellular carcinoma in different populations. Chin J Cancer Res 2016;28:150–60. [PubMed] DOI:10.21147/j.issn.1000-9604.2016.02.02
- Schlageter M, Terracciano LM, D’Angelo S, et al. Histopathology of hepatocellular carcinoma. World J Gastroenterol 2014;20:15955–64. [PubMed] DOI:10.3748/wjg.v20.i43.15955
- Li L, Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett 2016;379:191–7. [PubMed] DOI:10.1016/j.canlet.2015.07.018
- Xu XS, Chen W, Miao RC, et al. Survival analysis of hepatocellular carcinoma: A comparison between young patients and aged patients. Chin Med J (Engl) 2015;128:1793–800. [PubMed] DOI:10.4103/0366-6999.159356
- Varma V, Cohen C. Immunohistochemical and molecular markers in the diagnosis of hepatocellular carcinoma. Adv Anat Pathol 2004;11:239–49. [PubMed]
- Buchanan TF Jr. Clear-cell carcinoma of the liver. A clinicopathologic study of 13 patients. Am J Clin Pathol 1974;61:529–39. [PubMed]
- Sakhuja P, Mishra PK, Rajesh R, et al. Clear cell hepatocellular carcinoma: Back to the basics for diagnosis. J Cancer Res Ther 2015;11:656. [PubMed] DOI:10.4103/0973-1482.136041
- Yang SH, Watanabe J, Nakashima O, et al. Clinicopathologic study on clear cell hepatocellular carcinoma. Pathol Int 1996;46:503–9. [PubMed]
- Clayton EF, Furth EE, Ziober A, et al. A case of primary clear cell hepatocellular carcinoma in a non-cirrhotic liver: an immunohistochemical and ultrastructural study. Rare Tumors 2012;4:e29. [PubMed] DOI:10.4081/rt.2012.e29
- Li T, Fan J, Qin LX, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol 2011;18:1955–63. [PubMed] DOI:10.1245/s10434-010-1540-z
- Sivrioglu AK, Saglam M, Incedayi M, et al. Clear cell HCC mimicking to hepatic adenoma. BMJ Case Rep 2013;2013:pii: bcr2013008901. [PubMed] DOI:10.1136/bcr-2013-008901
- Hou TC, Wu CC, Yang CR, et al. Synchronous renal cell carcinoma and clear cell hepatocellular carcinoma mimicking metastatic disease. Pathol Res Pract 2010;206:342–5. [PubMed] DOI:10.1016/j.prp.2009.06.008
- Murakata LA, Ishak KG, Nzeako UC. Clear cell carcinoma of the liver: a comparative immunohistochemical study with renal clear cell carcinoma. Mod Pathol 2000;13:874–81. [PubMed] DOI:10.1038/modpathol.3880156
- Atra A, Al-Asiri R, Wali S, et al. Hepatocellular carcinoma, syncytial giant cell: a novel variant in children: a case report. Ann Diagn Pathol 2007;11:61–3. [PubMed] DOI:10.1016/j.anndiagpath.2005.12.005
- Dioscoridi L, Bisogni D, Freschi G. Hepatocellular carcinoma with osteoclast-like giant cells: report of the seventh case in the literature. Case Rep Surg 2015;2015:836105. [PubMed] DOI:10.1155/2015/836105
- Ikeda T, Seki S, Maki M, et al. Hepatocellular carcinoma with osteoclast-like giant cells: possibility of osteoclastogenesis by hepatocyte-derived cells. Pathol Int 2003;53:450–6. [PubMed]
- Haratake J, Horie A. An immunohistochemical study of sarcomatoid liver carcinomas. Cancer 1991;68:93–7. [PubMed]
- Dahm HH. Immunohistochemical evaluation of a sarcomatoid hepatocellular carcinoma with osteoclastlike giant cells. Diagn Pathol 2015;10:40. [PubMed] DOI:10.1186/s13000-015-0274-4
- Koda M, Maeda Y, Matsunaga Y, et al. Hepatocellular carcinoma with sarcomatous change arising after radiofrequency ablation for well-differentiated hepatocellular carcinoma. Hepatol Res 2003;27:163–7. [PubMed]
- Murata M, Miyoshi Y, Iwao K, et al. Combined hepatocellular/cholangiocellular carcinoma with sarcomatoid features: genetic analysis for histogenesis. Hepatol Res 2001;21:220–7. [PubMed]
- Koo HR, Park MS, Kim MJ, et al. Radiological and clinical features of sarcomatoid hepatocellular carcinoma in 11 cases. J Comput Assist Tomogr 2008;32:745–9. [PubMed] DOI:10.1097/RCT.0b013e3181591ccd
- Seok JY, Kim YB. Sarcomatoid hepatocellular carcinoma. Korean J Hepatol (in Korean) 2010;16:89–94. [PubMed] DOI:10.3350/kjhep.2010.16.1.89
- Giunchi F, Vasuri F, Baldin P, et al. Primary liver sarcomatous carcinoma: report of two cases and review of the literature. Pathol Res Pract 2013;209:249–54. [PubMed] DOI:10.1016/j.prp.2013.01.005
- Lao XM, Chen DY, Zhang YQ, et al. Primary carcinosarcoma of the liver: clinicopathologic features of 5 cases and a review of the literature. Am J Surg Pathol 2007;31:817–26. [PubMed] DOI:10.1097/01.pas.0000213431.07116.e0
- Kamat RN, Waghmare RS. Sarcomatoid hepatocellular carcinoma with bilateral adrenal metastases. J Assoc Physicians India 2013;61:354–6. [PubMed]
- Wang QB, Cui BK, Weng JM, et al. Clinicopathological characteristics and outcome of primary sarcomatoid carcinoma and carcinosarcoma of the liver. J Gastrointest Surg 2012;16:1715–26. [PubMed] DOI:10.1007/s11605-012-1946-y
- Nam HS, Kim HK, Ma SU, et al. A case of sarcomatoid hepatocellular carcinoma in a young female without risk factor. Korean J Gastroenterol (in Korean) 2006;47:458–62. [PubMed]
- Inoue T, Kudo M, Minami Y, et al. Case of rapidly progressed sarcomatoid hepatocellular carcinoma in a young female without risk factor. Liver Int 2007;27:1428–30. [PubMed] DOI:10.1111/j.1478-3231.2007.01584.x
- Lee JW, Kim MW, Choi NK, et al. Double primary hepatic cancer (sarcomatoid carcinoma and hepatocellular carcinoma): A case report. Mol Clin Oncol 2014;2:949–52. [PubMed] DOI:10.3892/mco.2014.402
- Han JH, Park YN, Jung WH, et al. A case with sarcomatoid hepatocellular carcinoma. Yonsei Med J 1998;39:390–4. [PubMed] DOI:10.3349/ymj.1998.39.4.390
- Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol 1949;25:647–55. [PubMed]
- Uenishi T, Hirohashi K, Shuto T, et al. Surgery for mixed hepatocellular and cholangiocellular carcinoma. Hepatogastroenterology 2000;47:832–4. [PubMed]
- Tang D, Nagano H, Nakamura M, et al. Clinical and pathological features of Allen’s type C classification of resected combined hepatocellular and cholangiocarcinoma: a comparative study with hepatocellular carcinoma and cholangiocellular carcinoma. J Gastrointest Surg 2006;10:987–98. [PubMed] DOI:10.1016/j.gassur.2006.01.018
- Singh S, Chakraborty S, Bonthu N, et al. Combined hepatocellular cholangiocarcinoma: a case report and review of literature. Dig Dis Sci 2013;58:2114–23. [PubMed] DOI:10.1007/s10620-013-2585-1
- Lee SD, Park SJ, Han SS, et al. Clinicopathological features and prognosis of combined hepatocellular carcinoma and cholangiocarcinoma after surgery. Hepatobiliary Pancreat Dis Int 2014;13:594–601. [PubMed]
- Terada T. Combined hepatocellular-cholangiocarcinoma with stem cell features, ductal plate malformation subtype: a case report and proposal of a new subtype. Int J Clin Exp Pathol 2013;6:737–48. [PubMed]
- Matsumoto M, Wakiyama S, Shiba H, et al. Combined hepatocellular-cholangiocarcinoma producing parathyroid hormone-related protein: report of a case. Surg Today 2014;44:1577–83. [PubMed] DOI:10.1007/s00595-013-0714-2
- Kim SH, Park YN, Lim JH, et al. Characteristics of combined hepatocelluar-cholangiocarcinoma and comparison with intrahepatic cholangiocarcinoma. Eur J Surg Oncol 2014;40:976–81. [PubMed] DOI:10.1016/j.ejso.2014.04.016
