Oxaliplatin plus S-1 or capecitabine as neoadjuvant or adjuvant chemotherapy for locally advanced gastric cancer with D2 lymphadenectomy: 5-year follow-up results of a phase II−III randomized trial
Introduction
Gastric cancer is the second common cause of cancer-related deaths worldwide, and China has the largest number of gastric cancer patients in the world (1). The most recent data show that the estimated age-standardized incidence rate is 21.52 per 10,000 in China (2). Surgery is the primary treatment for gastric cancer (3). In Japan, D2 gastrectomy plus adjuvant chemotherapy (ACT) using S-1 is the standard treatment for locally advanced gastric cancer (4); whereas in some others, the standard is gastrectomy with postoperative capecitabine plus oxaliplatin (CapeOX) (5). However, even with ACT, the prognosis for gastric cancer patients is not satisfactory.
Neoadjuvant chemotherapy (NACT) was proposed as an alternative to improve the prognosis of gastric cancer. The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial in UK showed that perioperative epirubicin-cisplatin-5-fluorouracil treatment was superior to surgery alone, suggesting a possible survival benefit due to preoperative treatment (6). However, evidence is limited on the comparison between NACT and ACT.
Moreover, the best regimen of NACT remains to be determined. The Fédération Nationale des Centres de Lutte contre le Cancer (FNCLCC) and the Fédération Francophone de Cancérologie Digestive (FFCD) trial in France indicated platinum and fluorouracil-based perioperative chemotherapy was safe and beneficial (7). In a retrospective analysis of Gastrointestinal Cancer Center of Peking University Cancer Hospital, NACT was shown to be more beneficial than ACT with 5-fluorouracil, folinic acid and oxaliplatin (8). Therefore, more clinical trials should be conducted to confirm the efficacy of NACT as well as to select the optimal regimens.
CapeOX is a commonly used ACT regimen, and oxaliplatin plus S-1 (SOX) was considered as effective as cisplatin plus S-1 (CS) in G-SOX trial (9). A randomized trial on advanced gastric cancer showed SOX and CapeOX regimens were equally active and well tolerated (10). However, very few studies have investigated the safety and efficacy of SOX and CapeOX used as NACT. In a phase II trial, SOX was shown to be as active and tolerable as neoadjuvant regimens, with a high response rate (11).
We therefore conducted this factorial randomized phase II−III trial comparing NACT with ACT using SOX or CapeOX regimens, to better inform clinical decisions on gastric cancer treatment.
Materials and methods
Study design
We did this phase II−III, single center, open-label, randomized controlled study at the Gastrointestinal Cancer Center of Peking University Cancer Hospital in Beijing, China. This was defined as a phase II−III trial as it started as a phase II trial and was designed to transit to phase III using failure-time data collected in phase II. Eligible patients were enrolled from September 2011 to December 2012, and were followed up till December 2017. We used a two-by-two factorial design, with four treatment arms: neoadjuvant SOX (peri-SOX), neoadjuvant CapeOX (peri-CapeOX), adjuvant SOX (post-SOX), and adjuvant CapeOX (post-CapeOX). This design enabled us to evaluate the superiority of NACT compared to ACT using SOX or CapeOX as the regimen. This study was approved by the Ethics Committee of Peking University Cancer Hospital. This trail was registered on ISRCTN registry (No. ISRCTN12206108). For more details see the Appendix file.
Patient selection and treatment
The main inclusion criteria were aged 18−80 years and locally advanced gastric cancer (T2−4NanyM0). The main exclusion criteria were serious comorbidities, distant metastasis, and patient refusal. Laparoscopic exploration and rapid cytology of peritoneal lavage fluid were routinely performed to exclude distant metastasis. Clinical stage was determined by thin-slice CT, endoscopic ultrasonography, and laparoscopic exploration to ensure the accuracy of clinical stages.
Eligible patients were randomly assigned to one of the four arms at 1:1:1:1 ratio using random number table. Patients receiving NACT started chemotherapy within 3 d after the laparoscopic exploration; and after 2 cycles of chemotherapy, the clinical stage of the tumor was evaluated before the surgery was performed. Radical dissection was aimed in gastrectomy, with standard D2 lymphadenectomy. Patients receiving ACT had surgery immediately after the randomization.
After the surgery, patients in NACT arms received 6 cycles of postoperative chemotherapy, whereas 8 cycles were administered to the adjuvant arms. Patients randomized to SOX regimens received oral S-1 (80 mg/m2 twice daily on d 1−14) and intravenous oxaliplatin (130 mg/m2 on d 1) for each cycle, whereas the CapeOX patients received oral capecitabine (1,000 mg/m2 twice daily on d 1−14) and intravenous oxaliplatin (130 mg/m2 on d 1). Dose reduction and interruptions were allowed for potentially serious and life-threatening adverse events that were determined by clinicians.
Primary and secondary endpoints
The primary endpoint was overall survival (OS), which was defined as the time interval from the time of randomization to the date of all-cause death or the last follow-up. Follow-up was conducted by phone call every six months after the completion or termination of the treatment. The secondary endpoints included treatment completion rate, surgical complications, chemotherapy safety, clinical response, and pathological complete response rate.
Statistical consideration
The common procedure of sample size calculation for a 2×2 factorial trial is to perform a separate calculation based on target effect sizes for each of the interventions compared with their respective controls. However, the major purpose of current trial was to demonstrate the superiority of NACT over ACT in terms of OS using SOX or CapeOX as the regimen. Therefore, sample size consideration was based on the effect size of chemotherapy administering approach only. On the basis of our prior study, expected 4-year OS rate in the NACT/ACT arm was 51%/78% respectively, corresponding to a hazard ratio of 0.369 (comparing NACT to ACT) (8). Using a two-sided log-rank test, 56/56 participants are needed in the arm of NACT/ACT to achieve 80.0% power at a 0.050 significance level in a study lasting for 5 years, accruing patients in the first 3 years, and with a yearly dropout rate of 4.4% (approximately 20% dropout in five years). Under the scenario that there was no intention to detect the interaction between administering approach and chemotherapy regimen, the sample size of a factorial design would be equal to the size of two 2-arm parallel trials (12,13). Therefore, a total sample size of 224 (56 per arm) was needed. The sample size calculation was carried out in NCSS-PASS v8.0.15 (NCSS LLC, Kaysville, Utah, USA). However, this trial was stopped due to the initiation of the phase III multicenter RESOLVE trial (NCT01534546) in 2012 while follow-up on enrolled patients continued.
Before the primary analysis, we assessed the interaction between administering approach (i.e. neoadjuvant or adjuvant) and regimen type (i.e. SOX or CapeOX) to examine the independence of the two research hypotheses using Cox regression including a corresponding interaction term as an explanatory variable. If no statistically significant interaction was detected, we proceeded with primary analysis by combining the four arms into two combinations. That is, the neoadjuvant and adjuvant SOX arms were combined into the SOX group, same for the CapeOX arms; likewise, the neoadjuvant SOX and CapeOX arms were combined as the NACT group, and the post-arms as the ACT group. Primary analysis was performed on an intention-to-treat (ITT) basis. The full analysis set was defined as the set of patients who started NACT or ACT. Cumulative OS curves are constructed as time-to-event plots using the Kaplan-Meier method. Differences between the curves are tested for significance using log-rank tests. If baseline characteristics were found to be unbalanced among the groups, multivariate Cox regression adjusting for the unbalanced variable(s) would be used. Considering Lauren classification has been found to interact with regimens, subgroup analyses were conducted to compare survival within each category of histology type (14). Secondary endpoints were compared using Pearson’s Chi-square tests.
All statistical analyses were conducted in Stata software (Version 14.0; StataCorp LLC, TX, USA) and RStudio (Version 1.1.419; RStudio Inc., Boston, MA) with a two-sided P<0.05 as statistically significant.
Results
Patients
Between September 2011 and December 2012, 135 patients in the Gastrointestinal Cancer Center of Peking University Cancer Hospital agreed to participate in and received laparoscopic exploration (Figure 1). One hundred were eligible and randomly assigned to one of the four treatment arms. All patients assigned to NACT initiated the therapy. Four patients randomized to post-SOX did not start chemotherapy because of metastasis (n=1) and patient refusal (n=3). Five patients randomized to post-CapeOX did not start chemotherapy due to perioperative mortality (n=1), early stage gastric cancer (n=2), and patient refusal (n=2).
Baseline characteristics for all randomized patients were similar in the four treatment arms (Table 1). Among the enrolled 100 patients, the majority were male (76%) and younger than 65 years old (69%). The most frequent T, N clinical stage, and Lauren classification were T4a (58%), N0 (34%), and diffuse type (46%), respectively. Most tumors were undifferentiated (73%) and most patients did not have comorbidities (63%).

Full table
Completion rate
The completion rate of the assigned therapy was 56% (14/25), 52% (13/25), 38% (8/21) and 30% (6/20) for the arms of peri-SOX, peri-CapeOX, post-SOX and post-CapeOX, respectively, and differed by NACT and ACT [χ2(1)=5.91, P=0.015]. Among the total of 50 patients who started but did not complete the treatment, the reasons for dropout were unacceptable toxicities (n=36), patient unwillingness to continue (n=11), changed regimens (n=2), and perioperative morality (n=1).
Surgical characteristics
Table 2 shows the surgical and pathological characteristics by four arms. All patients received D2 lymphadenectomy. All patients achieved radical gastrectomy except for one in post-SOX arm. The amount of intraoperative bleeding, surgical duration, and postoperative length of stay were not significantly different across the four arms (all P>0.05). As for the surgery complications, there were two perioperative deaths, one in the peri-SOX arm and the other in the post-CapeOX arm. The complication rate was 32% (n=8), 28% (n=7), 33% (n=7) and 35% (n=7) in arms of peri-SOX, peri-CapeOX, post-SOX and post-CapeOX, respectively [χ2(3)=0.28, P=0.986].

Full table
Two deaths occurred, one in peri-SOX arm and the other in post-CapeOX arm. In peri-SOX arm, a 66-year-old male patient died after severe chest infection by Enterobacter cloacae and pleural effusion caused by anastomotic leakage. Unfortunately, the condition deteriorated even after reexploration. The death was not considered to be attributed to chemotherapy directly. In post-CapeOX arm, a 65-year-old male died of anastomotic leakage and bleeding 3 weeks after surgery. The patients did not receive any chemotherapy.
Toxicities
Table 3 shows the descriptive frequencies of adverse chemotherapy events for the four arms. The three most frequent toxicities in the SOX arms were nausea or vomiting (50%), fatigue (50%), and anorexia (50%), whereas those for the CapeOX arms were nausea or vomiting (62%), anorexia (53%), and leukopenia (51%). The top three toxicities for the neoadjuvant group were leukopenia (50%), nausea or vomiting (48%), and anorexia (50%); and those for the adjuvant group were nausea or vomiting (66%), anorexia (56%), and weight loss (56%). No chemotherapy-related mortality was observed. Furthermore, a significantly greater number of patients with ACT experienced weight loss than patients with NACT [χ2(1)=12.7, P<0.001]. The frequencies of other toxicity factors did not significantly differ by NACT vs. ACT nor SOX vs. CapeOX.
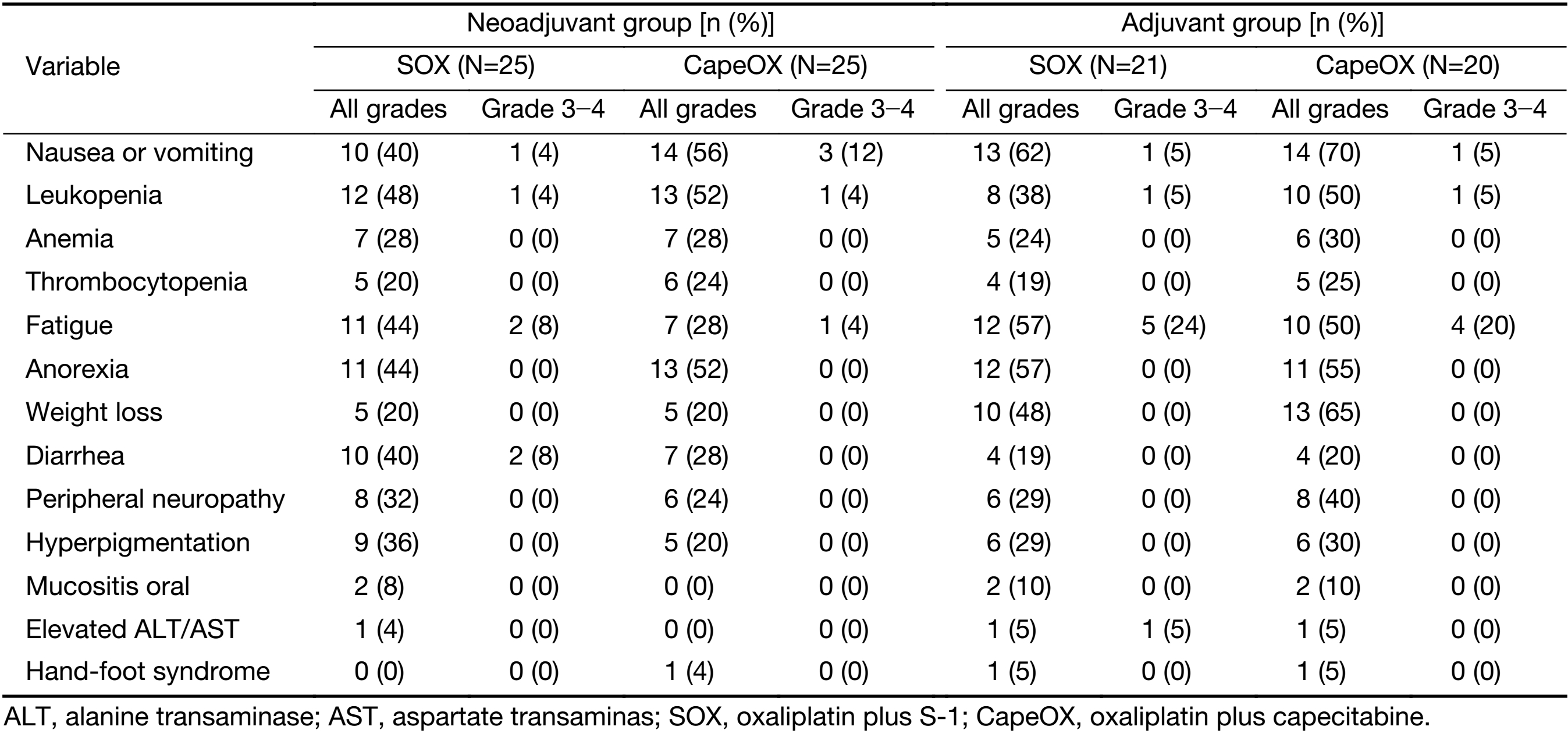
Full table
Clinical response
According to the endoscopic ultrasonography (EUS) evaluation, 15 (71.4%) and 13 (56.5%) patients had downstaged after NACT in neoadjuvant SOX and CapeOX groups, respectively (15). As for CT response, lymph nodes with a short axis of ≥10 mm are measurable and assessable as target lesions, according to the RECIST guidelines (16). Three patients had target lesions in the neoadjuvant SOX group, two of whom had partial response. Four patients had target lesions in the neoadjuvant CapeOX group, three of whom were considered partial response.
Pathological stage and response
The number of positive lymph nodes and the proportion of patients with positive lymphovascular invasion were lower in the NACT group than in the ACT group (rank-sum P=0.014; χ2(1)=9.54, P=0.002, respectively). Regarding pathology response, peri-SOX arm had three (12%) patients with complete responses and seven (28%) patients with pathology response of grade 2 that is considered as effective; the corresponding number for peri-CapeOX was one (4%) and eight (32%), respectively.
Oncologic outcome
The median follow-up period was 60.4, 63.6, 59.5, 61.0 and 59.9 months for all patients, peri-SOX, peri-CapeOX, post-SOX and post-CapeOX, respectively (Figure 2). Five-year OS was 70% and 74% in the NACT and the ACT group, 78% and 66% in the SOX and CapeOX group, respectively. A multivariate Cox regression model with three variables (NACT vs. ACT, SOX vs. CapeOX, and their interaction term) demonstrated that there was no statistically significant interaction between the two interventions (P=0.963).
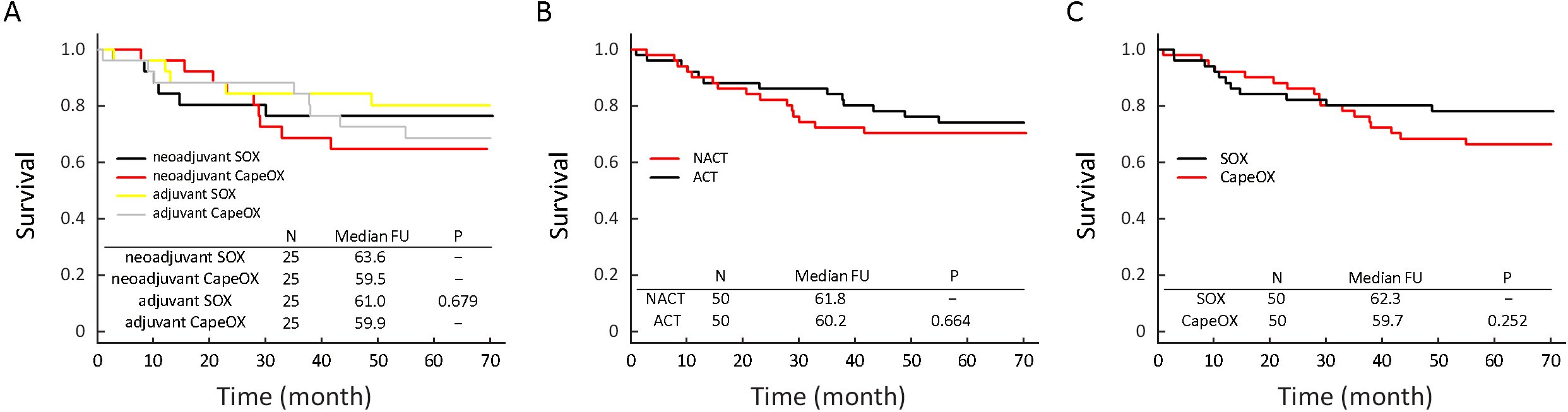
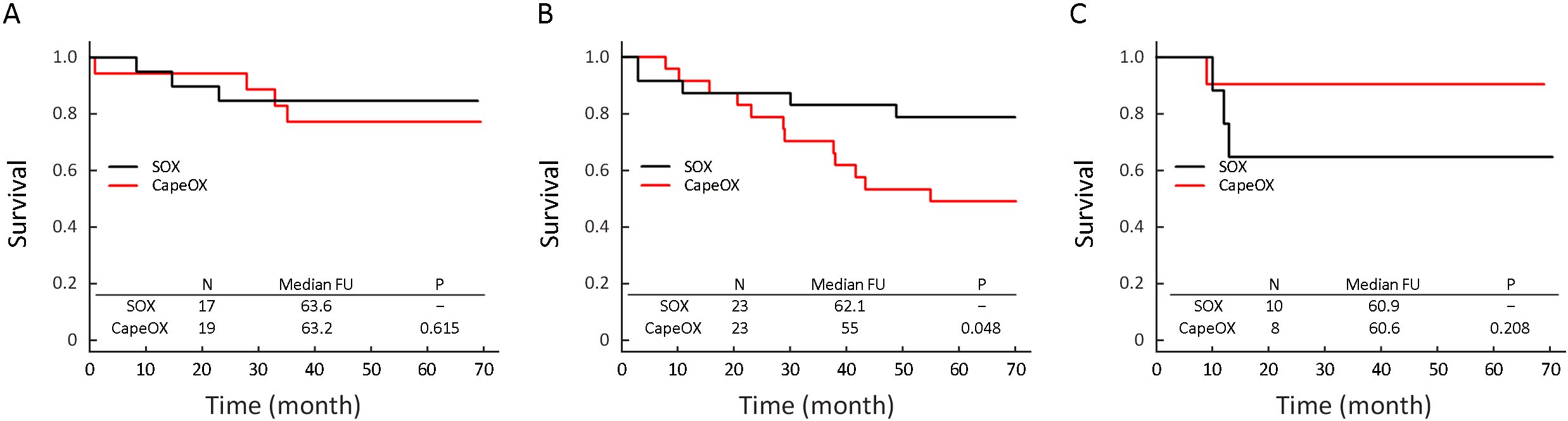
In the ITT analysis, no significant difference in OS was found between patients receiving NACT and those receiving ACT [overall: χ2(1)=0.19, P=0.664; stage II: χ2(1)=0.08, P=0.783; stage III: χ2(1)=0.15, P=0.696]. Similarly, regardless whether the chemotherapy was administered before or after the surgery, the survival in SOX and CapeOX group did not show a significant difference [χ2(1)=1.31, P=0.252]. We further used Cox regression to adjust for the Lauren type which was found to be unbalanced between the neoadjuvant and adjuvant group at baseline (Supplementary Table S1): the difference on OS between NACT and ACT was likewise not statistically significant [hazard ratio=0.76 comparing ACT to NACT, 95% confidence interval (95% CI)=0.35−1.64, P=0.483]. Subgroup analysis found significant difference of survival existed in the subgroup of diffuse type [χ2(1)=3.93, P=0.048], but not in those with intestinal type [χ2(1)=0.25, P=0.615] or mixed type [χ2(1)=1.58, P=0.208] (Figure 3).

Full table
Discussion
Previous clinical trials have confirmed the survival benefit of NACT, however, these trials were comparing NACT to surgery alone (6,7). It is still debatable if NACT is more beneficial than ACT. To our best knowledge, this is the first trial to compare NACT and ACT directly using platinum and fluorouracil-based regimens in locally advanced gastric cancer with D2 lymphadenectomy. Our trial did not find significant difference on OS between NACT and ACT or between SOX and CapeOX. However, SOX was possibly more beneficial to diffuse type patients according to subgroup analysis. Secondary analysis showed SOX and CapeOX were safe and effective as neoadjuvant regimens.
From the short-term results, we found the completion rate of treatment was significantly higher in NACT patients than in ACT patients. Previous trials have demonstrated similar results. The MAGIC trial found the completion rate of ACT was almost half of that among the NACT patients (49.5% vs. 90.7%, respectively) (6). In the Swiss group for clinical cancer research (SAKK) trial, almost all patients (33/34) started NACT, whereas one-third patients (12/35) did not even start chemotherapy in the ACT arm (17). Body weight loss after surgery was found to be an independent risk factor for continuation of S-1 ACT (18). In our study, a significantly greater number of patients with ACT experienced weight loss than patients with NACT, which could possibly explain why more patients in ACT group did not finish treatment.
However, as the follow-up proceeded, we found the better completion rate did not turn into survival benefit, which was in accordance with an SAKK trial comparing NACT to ACT using docetaxel-cisplatin-fluorouracil regimens (17). The underpower might be one reason. Another possible explanation is the questionable necessity of long-time chemotherapy. The IDEA collaboration trials have confirmed that 3 months of adjuvant therapy was as effective as 6 months in stage III colon cancer (19). A retrospective study in gastric cancer found that 6 rather than 8 (as used in this trial) cycles of ACT was the ideal duration for ACT (20). Our results suggested more trials should be taken in gastric cancer on duration of perioperative chemotherapy.
Though without significant difference on OS in primary analysis, subgroup analysis showed an improved prognosis obtained by SOX in diffuse type. This finding is consistent with several previous studies. A phase II trial in advanced gastric cancer with S-1 had found that diffuse type had better objective response rate than intestinal type (52% vs. 28%) (21). Similarly, another randomized study had shown that CS had improved survival than cisplatin plus 5-fluorouracil in advanced gastric cancer of diffuse type (14). The more significant effect of SOX on patients with diffuse type was possibly because diffuse type patients expressed more mRNA of dihydropyrimidine dehydrogenase, and gimeracil, a component of S-1, is a strong dihydropyrimidine dehydrogenase inhibitor (22). However, another recent randomized phase III trial suggested otherwise: no survival benefit of CS over cisplatin plus 5-fluorouracil in advanced diffuse type patients (23). These divergent results indicate more trials should be undertaken.
The influence on surgery is another safety concern of NACT. Our study found no significant difference on surgical complication rate between NACT and ACT groups. Similarly, in MAGIC trial, the complication rate did not increase after NACT, neither nor in the FNCLCC and FFCD trial (6,7). However, one difference is that the proportion of patients undergoing D2 gastrectomy was low in these trails. In our study, all patients received D2 lymphadenectomy, and the complication and mortality rates were still acceptable and indifferent among the arms. This suggests that SOX and CapeOX can be safe as NACT regimens, even with D2 lymphadenectomy.
An obvious limitation of our study was early stop of enrollment, which led to fewer patients than planned being enrolled and may have underpowered the study. Another one was the large proportion of dropouts. However, the dropout due to all causes was not significantly associated with NACT vs. ACT nor SOX vs. CapeOX, indicating a non-differential dropout (all P>0.05). One more potential limitation is that no correction on P-value was made even though several subgroup comparisons were conducted. This was mainly due to the concern on the power of current study as it was stopped prematurely. Adjusting the P-value to reduce the type I error for null associations would further increase the type II error, making it more difficult to observe the difference when there was one. We therefore did not make any correction on the P-value, as also advocated in the paper of Rothman “…a policy of not making adjustments for multiple comparisons is preferable because it will lead to fewer errors of interpretation when the data under evaluation are not random numbers but actual observations on nature…” (24).
Conclusions
The NACT and ACT groups did not differ in OS, nor did the SOX and CapeOX groups. However, subgroup analysis showed diffuse type patients may benefit more from SOX. SOX and CapeOX were safe and effective as neoadjuvant regimens in locally advanced gastric cancer with D2 lymphadenectomy.
Acknowledgements
This work was supported in part by the grants from Beijing Municipal Science & Technology Commission (No. D171100006517002).
Footnote
The study has been presented in part at the 87th Japanese Gastric Cancer Association (JGCA) conference in March 2015, Hiroshima, Japan, and presented at the 12th International Gastric Cancer Association (IGCC) conference in April 2017, Beijing, China.
Conflicts of Interest: The authors have no conflicts of interest to declare.
Appendix file
Eligibility
The inclusion criteria were as follows: 1) age between 18 and 80 years; 2) pathologically confirmed gastric adenocarcinoma; 3) disease at the clinical stage of resectable advanced gastric cancer (T2−4NanyM0), without peritoneal metastasis as confirmed by laparoscopy and cytological pathology; 4) an Eastern Cooperative Oncology Group performance status of 0 or 1; 5) no previous treatment history; 6) adequate organ function levels [hematological ANC ≥1.5×109/L, hemoglobin ≥9 g/dL, platelets ≥100×109/L, hepatic albumin ≥30 g/L, serum bilirubin ≤1.5 times the upper limit of normal (ULN), aspartate transaminase (AST) and alanine transaminase (ALT) ≤2.5×ULN, alkaline phosphatase (ALP) ≤2.5×ULN, total bilirubin (TBIL) ≤1.5×ULN, renal serum creatinine <1.5×ULN]; and 7) adequate lung and heart function, without electrocardiograph (ECG)-confirmed ischemic change or ventricular arrhythmias. The exclusion criteria were as follows: 1) serious comorbidities; 2) distant metastasis; 3) acute inflammation; 4) systematic steroid therapy; 5) pregnant or breast-feeding women or women considering pregnancy; 6) nervous system disorder or psychiatric disease; 7) a medical history of allergy or hypersensitivity to any drugs; or 8) patient refusal.
Treatment
Radical dissection was aimed in gastrectomy, with standard D2 lymphadenectomy, and with a combination of spleen, pancreas or transverse colon resection if necessary. Splenectomy was not mandatory. Patients started adjuvant chemotherapy (ACT) between four to six weeks after the operation. Prophylactic anti-emetic treatment, hepatic protective medications and granulocyte-colony stimulating factor were mandatory. A 25% dose reduction was mandatory in the case of grade 3 events. If the event was considered to be obviously attributed to one drug, we only reduced the dose of this drug, while the other one would be continuously used without reduction. The treatment was stopped in case of grade 4 or consistent grade 3. If S-1 or capecitabine had to be stopped, the treatment ended. If oxaliplatin had to be stopped, S-1 or capecitabine could be taken continuously.
Assessment procedure
Pathological stage was evaluated according to the 7th edition of the American Joint Committee on Cancer TNM Staging Classification for Carcinoma of the Stomach (1). Pathology response to neoadjuvant chemotherapy (NACT) was evaluated according to the 3rd English edition of the Japanese Classification of Gastric Cancer (2). The clinical response was evaluated by the Response Evaluation Criteria for Solid Tumors in computed tomography (CT) and by the down-staging assessed by endoscopic ultrasonography (EUS), as suggested by Choi (3).
- Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th edition. New York: Springer, 2010.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;4:101–12.
- Park SR, Lee JS, Kim CG, et al. Endoscopic ultrasound and computed tomography in restaging and predicting prognosis after neoadjuvant chemotherapy in patients with locally advanced gastric cancer. Cancer 2008;112:2368–76. [PubMed]
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018. [Epub ahead of print].
- Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res 2017;29:1–10. [PubMed] DOI:10.21147/j.issn.1000-9604.2017.01.01
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Gastric Cancer Version 2.2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810–20. [PubMed] DOI:10.1056/NEJMoa072252
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315–21. [PubMed] DOI:10.1016/S0140-6736(11)61873-4
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [PubMed] DOI:10.1056/NEJMoa055531
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715–21. [PubMed] DOI:10.1200/JCO.2010.33.0597
- Li ZY, Koh CE, Bu ZD, et al. Neoadjuvant chemotherapy with FOLFOX: improved outcomes in Chinese patients with locally advanced gastric cancer. J Surg Oncol (in Chinese) 2012;105:793–9. [PubMed] DOI:10.1002/jso.23009
- Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 2015;26:141–8. [PubMed] DOI:10.1093/annonc/mdu472
- Kim GM, Jeung HC, Rha SY, et al. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer 2012;48:518–26. [PubMed] DOI:10.1016/j.ejca.2011.12.017
- Honma Y, Yamada Y, Terazawa T, et al. Feasibility of neoadjuvant S-1 and oxaliplatin followed by surgery for resectable advanced gastric adenocarcinoma. Surg Today 2016;46:1076–82. [PubMed] DOI:10.1007/s00595-015-1276-2
- Pandis N, Walsh T, Polychronopoulou A, et al. Factorial designs: an overview with applications to orthodontic clinical trials. Eur J Orthod 2014;36:314–20. [PubMed] DOI:10.1093/ejo/cjt054
- Brookes ST, Whitley E, Peters TJ, et al. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5:1–56. [PubMed]
- Ajani JA, Rodriquez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1(CS) with cisplatin/5-FU (CF) as first-line therapy in patients with advanced gastric cancer (FLAGS): Secondary and subset analyses. J Clin Oncol 2009;27:4511–4511. [PubMed] DOI:10.1200/jco.2009.27.15s.4511
- Park SR, Lee JS, Kim CG, et al. Endoscopic ultrasound and computed tomography in restaging and predicting prognosis after neoadjuvant chemotherapy in patients with locally advanced gastric cancer. Cancer 2008;112:2368–76. [PubMed] DOI:10.1002/cncr.23483
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;78:228–47. DOI:10.1016/j.ejca.2008.10.026
- Fazio N, Biffi R, Maibach R, et al. Preoperative versus postoperative docetaxel-cisplatin-fluorouracil (TCF) chemotherapy in locally advanced resectable gastric carcinoma: 10-year follow-up of the SAKK 43/99 phase III trial. Ann Oncol 2016;27:668–73. [PubMed] DOI:10.1093/annonc/mdv620
- Aoyama T, Yoshikawa T, Shirai J, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol 2013;20:2000–6. [PubMed] DOI:10.1245/s10434-012-2776-6
- Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 2018;378:1177–88. [PubMed] DOI:10.1056/NEJMoa1713709
- Qu JL, Li X, Qu XJ, et al. Optimal duration of fluorouracil-based adjuvant chemotherapy for patients with resectable gastric cancer. PLoS One 2013;8:e83196. [PubMed] DOI:10.1371/journal.pone.0083196
- Koizumi W, Kurihara M, Nakano S, et al. Phase II Study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Study Group. Oncology 2000;58:191–7. [PubMed] DOI:10.1159/000012099
- Yamada Y, Yamamoto S, Ohtsu A, et al. Impact of dihydropyrimidine dehydrogenase status of biopsy specimens on efficacy of irinotecan plus cisplatin, S-1, or 5-FU as first-line treatment of advanced gastric cancer patients in JCOG9912. J Clin Oncol 2009;27:4535–4535. DOI:10.1200/jco.2009.27.15s.4535
- Ajani JA, Abramov M, Bondarenko I, et al. A phase III trial comparing oral S-1/cisplatin and intravenous 5-fluorouracil/cisplatin in patients with untreated diffuse gastric cancer. Ann Oncol 2017;28:2142–8. [PubMed] DOI:10.1093/annonc/mdx275
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. [PubMed] DOI:10.1097/00001648-199001000-00010

