A comparative study of totally laparoscopic distal gastrectomy versus laparoscopic-assisted distal gastrectomy in gastric cancer patients: Short-term operative outcomes at a high-volume center
Introduction
Laparoscopic surgery has been established as a standard treatment for early gastric cancer since long-term outcomes and better short-term surgical outcomes have been reported. Numerous studies have demonstrated that laparoscopic surgery has many advantages, such as less pain, less wound complications, cosmetic effect, and shorter hospital day over open surgery (1-3). However, most previous studies have only reported the advantages of laparoscopic-assisted distal gastrectomy (LADG) using extracorporeal anastomosis which requires at least 4−5 cm incision in the upper abdomen.
Intracorporeal anastomosis can be made during totally laparoscopic distal gastrectomy (TLDG) without the need for additional incision for extracorporeal anastomosis, thereby reducing postoperative pain, analgesic use, and wound infections and providing a better cosmetic effect (4-6). In addition, TLDG using intracorporeal anastomosis offers better visual field and working space compared to LADG using extracorporeal anastomosis reconstruction. This advantage can reduce tension of the anastomosis site and possibility of adjacent tissue damage (7,8).
Several studies have reported that TLDG has similar or better short-term surgical outcomes than LADG. However, because of the technical difficulties of intracorporeal anastomosis with linear stapler manipulation and the need for accumulated experience of laparoscopic surgery (9,10), TLDG has not been performed popularly until now, and there are only a few reports of a large number of patients with various reconstruction types. Hence, this study was conducted to compare the short-term postoperative outcomes of TLDG versus LADG in gastric cancer to analyze the feasibility and safety of intracorporeal anastomosis on a large scale using various reconstruction methods.
Materials and methods
Patients and inclusion criteria
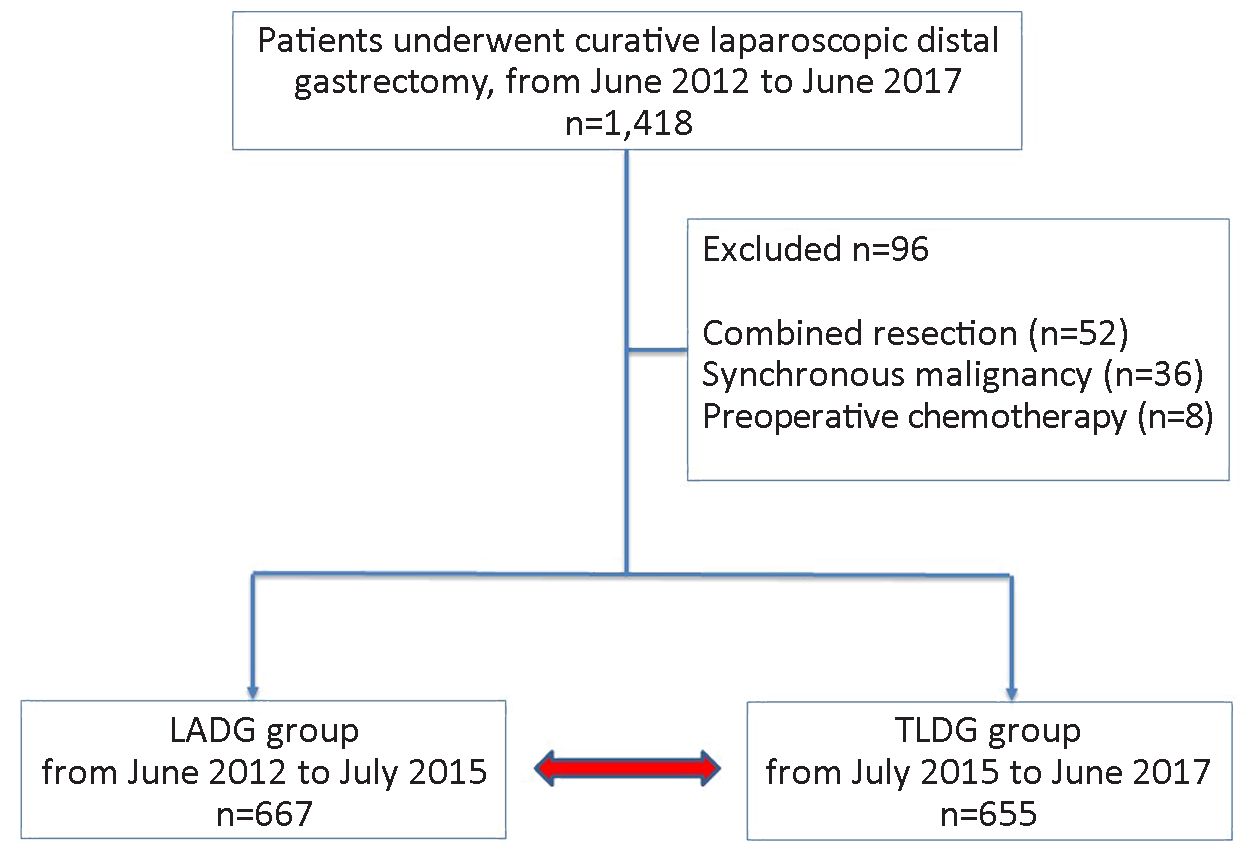
From June 2012 to June 2017, a total of 1,418 patients who were treated with laparoscopic radical distal gastrectomy at the National Cancer Center due to primary gastric adenocarcinoma were enrolled. LADG was performed in the early period (from June 2012 to July 2015), and TLDG was performed in the later period (from July 2015 to June 2017). Of these patients, combined resection, other synchronous malignancies, and those with a history of preoperative chemotherapy were excluded. The remaining 1,322 patients were enrolled in the study. LADG with a stapler anastomosis or hand-sewn suture was performed in 667 patients, and TLDG with linear stapler was performed in 655 patients (Figure 1). In LADG, epigastric incision was made longitudinally for gastrojejunostomy and transversely for gastroduodenostomy. In TLDG, Billroth I (BI) anastomosis was performed as side-to-side gastroduodenostomy using a linear stapler (delta anastomosis) (11). Billroth II (BII) anastomosis was made using a linear stapler, and closure of the entry hole of the stapler was performed using continuous suture.
Evaluation of operative outcomes
Distal gastric cancer was preoperatively diagnosed with endoscopy with biopsy, and computed tomography (CT) to assess tumor site, depth of invasion, extent of lymph node metastasis, and metastatic disease.
Clinicopathologic factors of enrolled patients were retrospectively analyzed including patients’ sex, age, preoperative body mass index (BMI), co-morbidity represented by American Society of Anesthesiologist (ASA) score, tumor size, location, stage, extent of lymph node dissection, and reconstruction type. Surgical outcomes included operating time, estimated blood loss (EBL), proximal/distal margin, postoperative pain score and first flatus passage time after operation, hospital day after operation, and postoperative complications. Pain score was measured by asking the patients the intensity of pain from 0 to 10 points on postoperative day 1 (POD 1) and POD 3. Postoperative complications were investigated according to the Clavien-Dindo classification (12). White blood cell (WBC) count, C-reactive protein (CRP) at POD 1 and 3 were analyzed to compare postoperative inflammatory response between the two groups. Subgroup analysis was performed to compare the reconstruction type between BI and BII anastomoses.
This study was approved by the Institutional Review Board of the National Cancer Center (approval No. NCC2018-0129).
Statistical analysis
All analyses were performed using SAS® for Windows® (Version 9.1.3; SAS Institute Inc., Cary, NC, USA). Categorical variables were compared using the Pearson χ2 test. Continuous variables were expressed as x±s of the mean or median (range) and compared using t-test or Wilcoxon rank sum test as appropriate. A logistic regression analysis was used to test univariate and multivariate associations between variables to investigate risk factors of postoperative complication. Statistical significance was set at two-sided, P<0.05.
Results
Patient characteristics
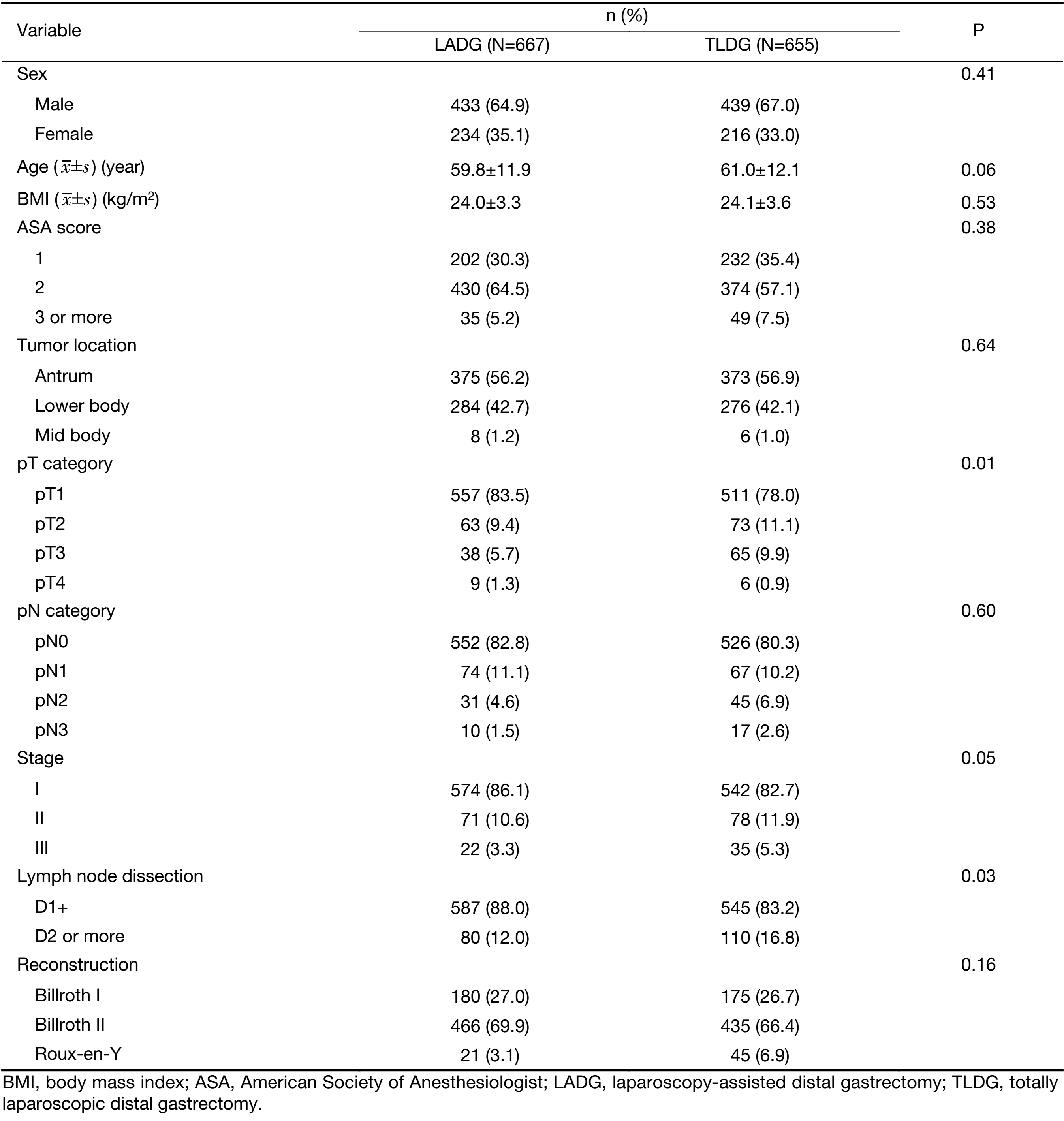
Patient characteristics were compared between TLDG and LADG groups (Table 1). pT category (P=0.01) was significantly higher in the TLDG group because the indication of laparoscopy was extended in the later period. Cancer stage was also marginally higher in the TLDG group, but without statistical significance (P=0.05). D2 or more lymph node dissection was performed more frequently in the TLDG group (P=0.03).
Full table
Intraoperative and postoperative outcome
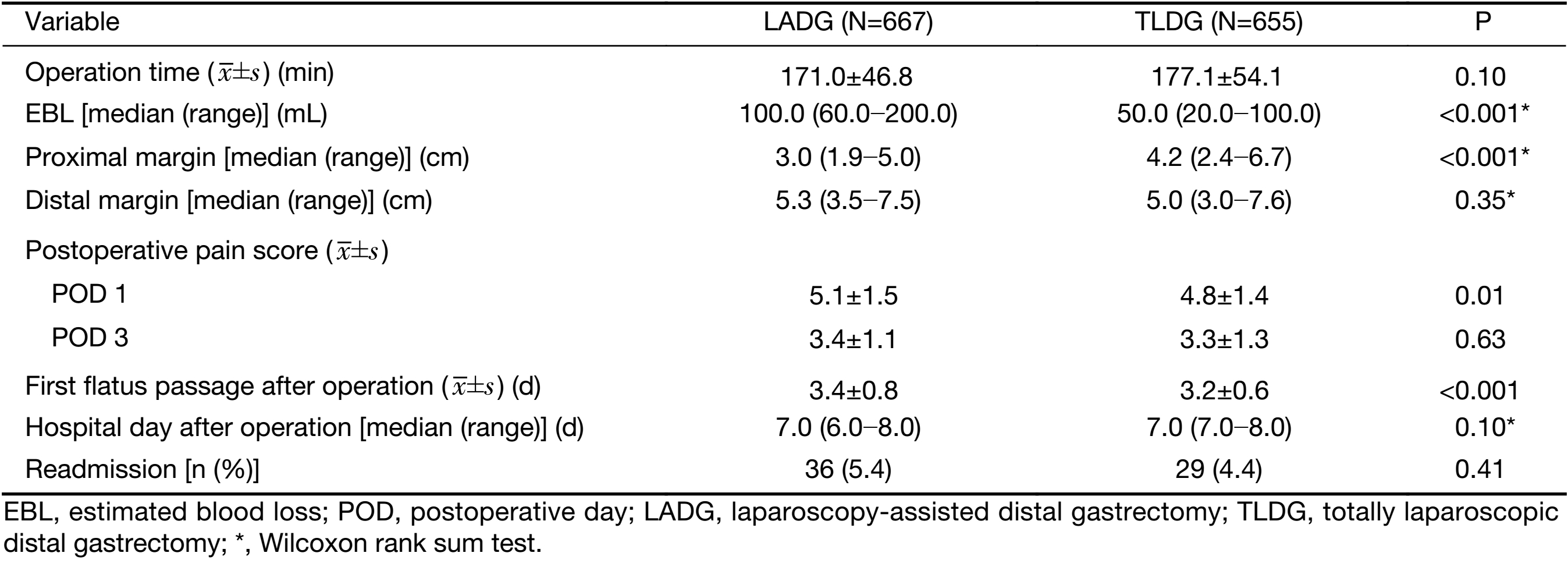
Intraoperative EBL was minimal in the TLDG group than in the LADG group (P<0.001). The proximal margin was significantly longer in the TLDG group (P<0.001). Postoperative pain score in POD 1 was significantly lower in the TLDG group (5.1±1.5 vs. 4.8±1.4, P=0.015), but postoperative pain score in the POD 3 was similar between the two groups. The first flatus passage after the operation was significantly earlier in the TLDG group than in the LADG group (3.4±0.8 d vs. 3.2±0.6 d, P<0.001) (Table 2).
Full table
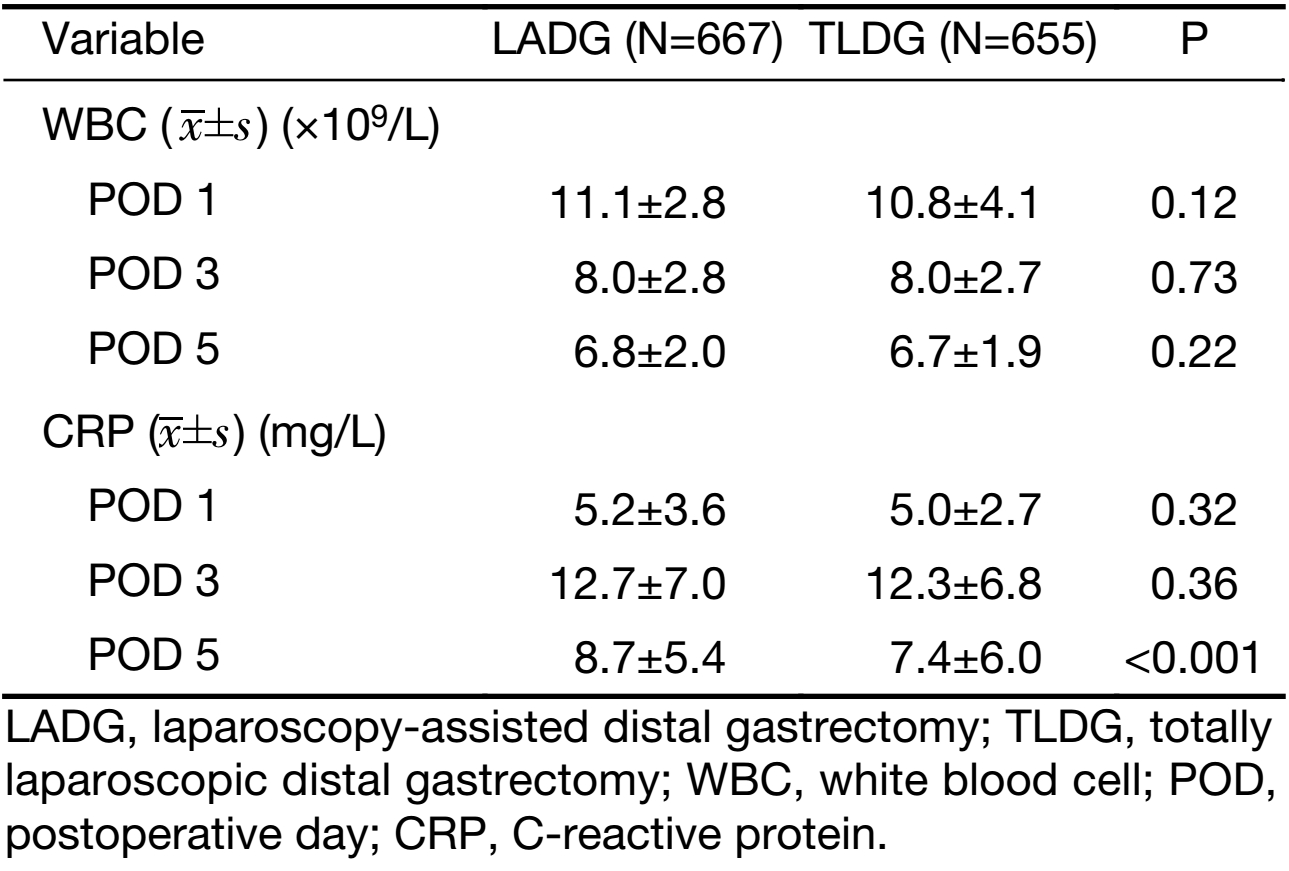
Laboratory test results are shown in Table 3. WBC and CRP were marginally lower in the TLDG group, but the differences were not statistically significant. While in POD 5, CRP was significantly lower in the TLDG group (8.7±5.4 mg/L vs. 7.4±6.0 mg/L, P<0.001).
Full table
Postoperative complication
The overall complication rates were 13.8% (92/667) in the LADG group and 13.0% (85/655) the TLDG group (Table 4). Wound complications (2.4% vs. 2.3%, P=0.96) and anastomotic leakage (2.1% vs. 2.0%, P=0.88) were similar between the two groups. There were no significant differences in severe complications between the two groups (P=0.20).
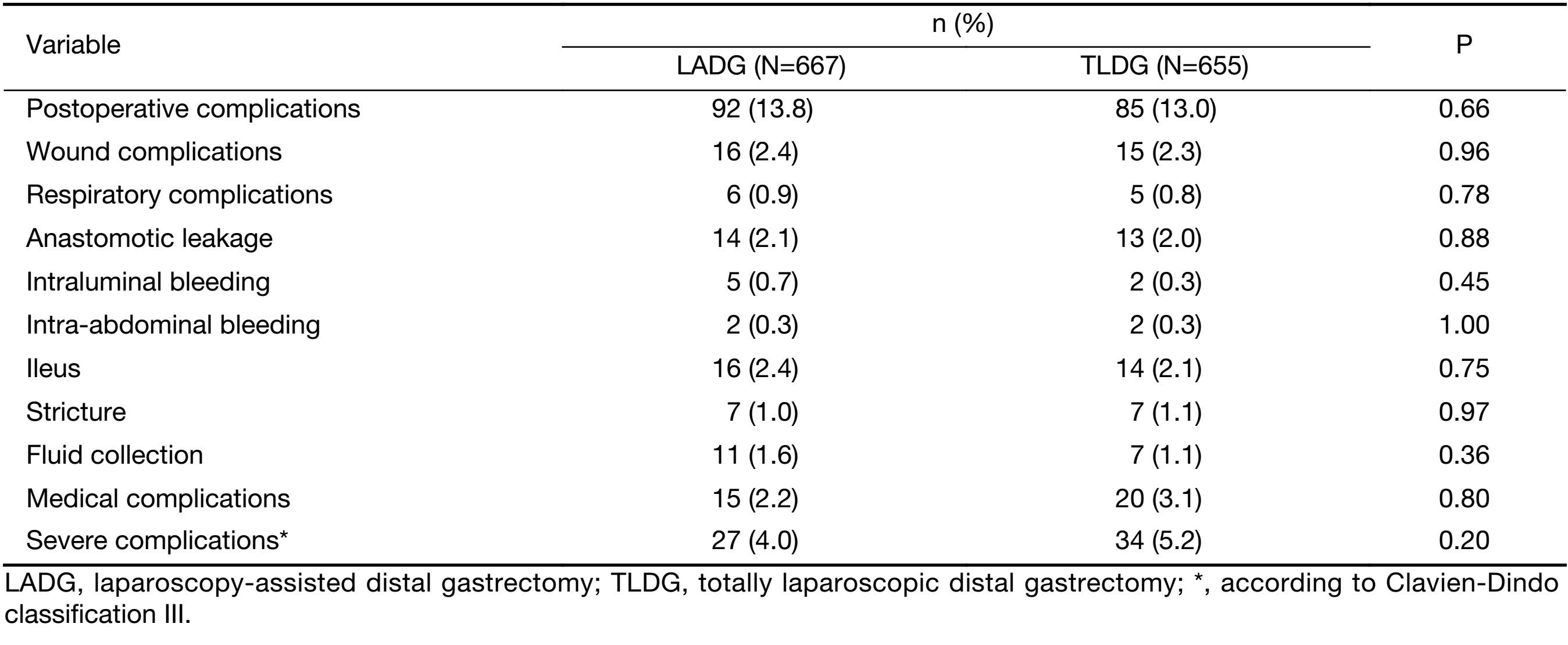
Full table
Clinicopathologic factors were compared for analysis of risk factor related to postoperative complications. In univariate analysis, ASA score (P=0.001) and operation time (P<0.001) were statistically related to postoperative complications. In multivariable analysis, ASA score [odds ratio (OR)=1.35, 2.36; P=0.04] and operation time (OR=1.64; P=0.002) were statistically significant (Table 5).
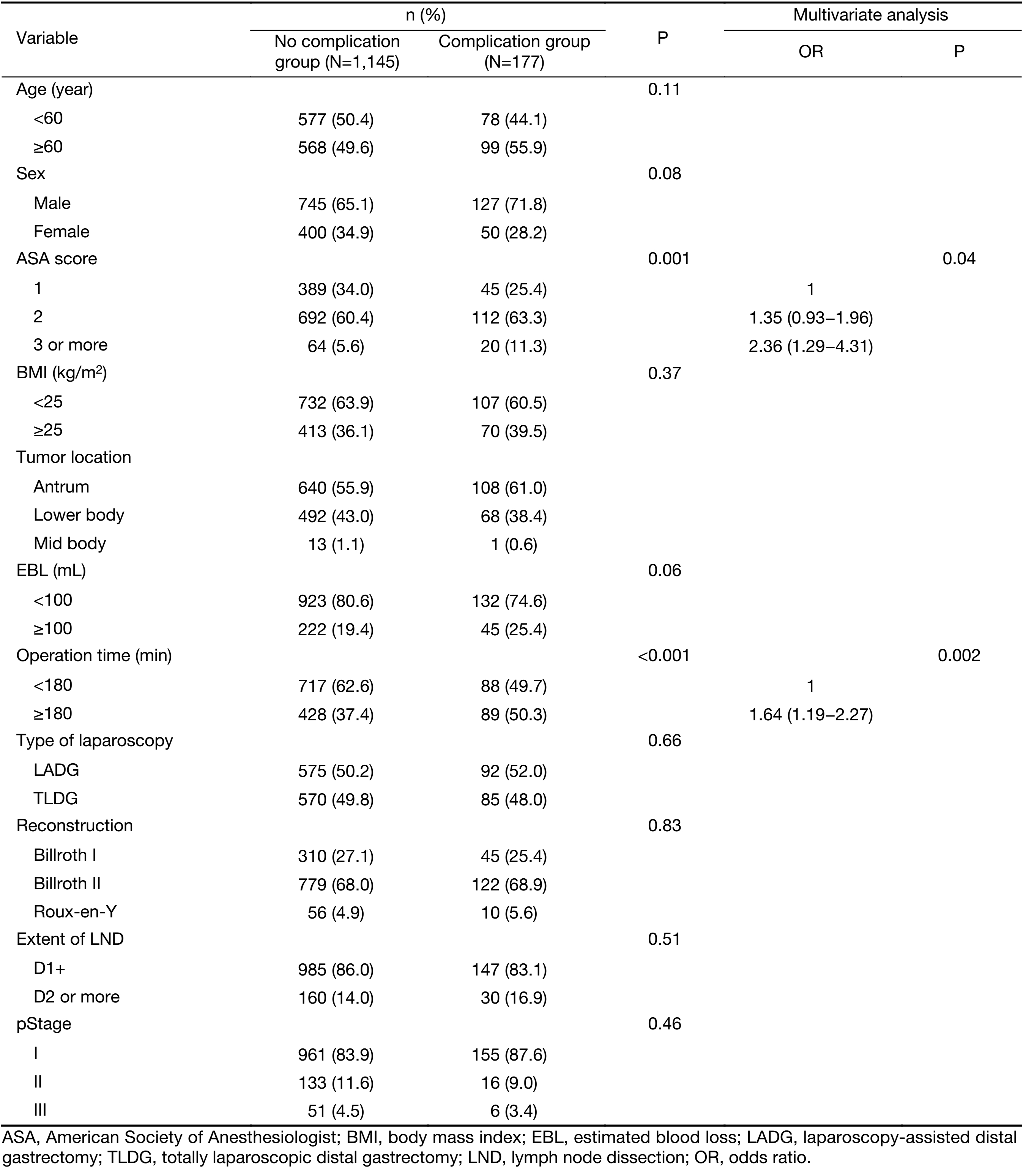
Full table
Subgroup analysis depending on reconstruction type
In the comparison of postoperative outcomes according to the reconstruction type, the operating time in TLDG was longer than that in LADG with BI anastomosis (P<0.001), while it was not significant in BII anastomosis. EBL was lower in TLDG regardless of the reconstruction type (P<0.001). Hospital stays were longer after TLDG with BI (P=0.01). Flatus passage was significantly earlier after TLDG with BII than LADG (P<0.001). Complication rates were not significantly different between the two groups in the subgroup analysis (Table 6).
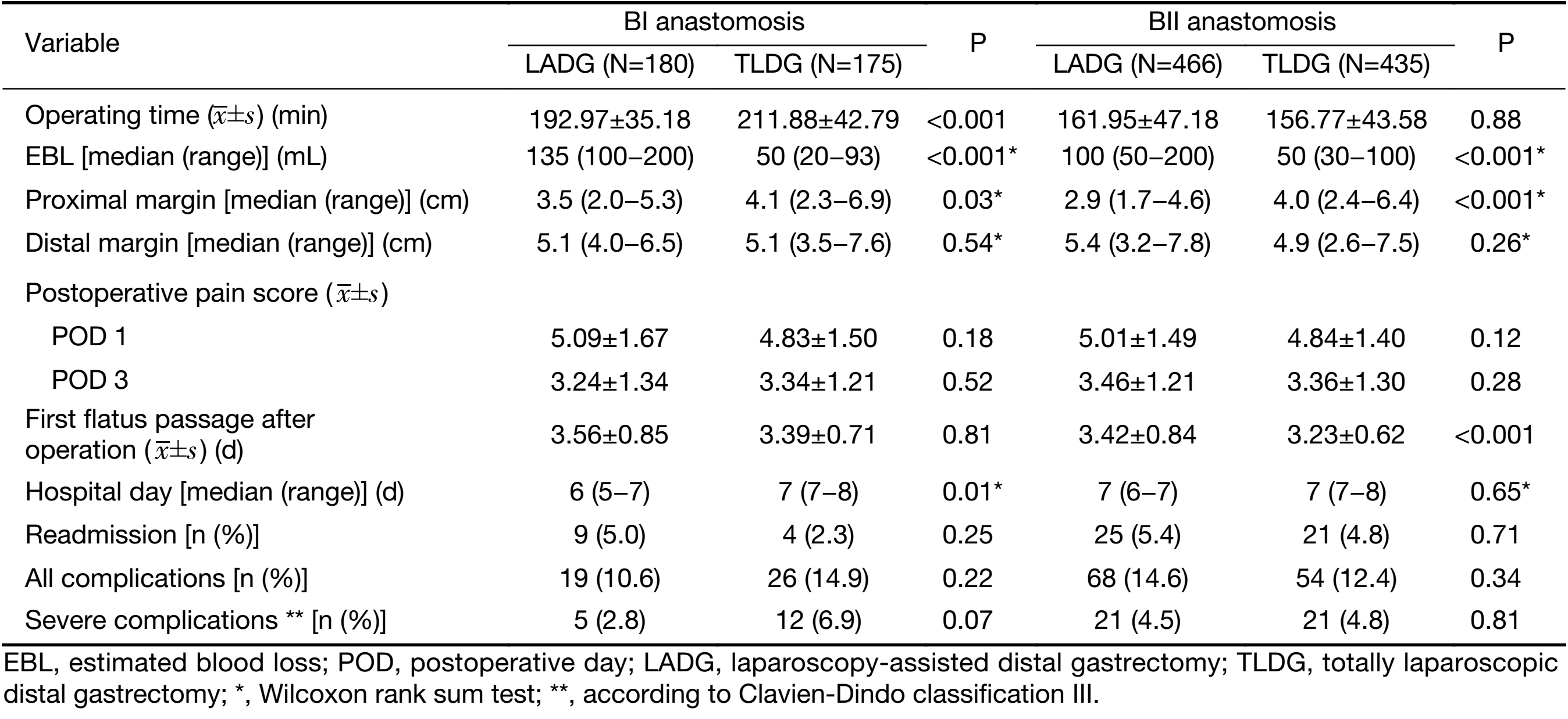
Full table
Discussion
The present study demonstrated that TLDG is safe and feasible compared with LADG in a relatively high number of patients. Moreover TLDG had several benefits, such as minimal intraoperative EBL, less postoperative pain, and enhanced bowel motility over LADG.
Previous studies have shown that TLDG has a better outcome than LADG (4-6). However, TLDG has several technical difficulties for several reasons. First, intracorporeal anastomosis requires more experience of performing laparoscopic surgery than LADG (9). Second, complications might increase with difficulty of manipulating the linear stapler in the early introductory period (13,14). Given that there were few large-scale randomized studies, the advantages of TLDG were debatable.
In the present study, EBL was significantly reduced in TLDG. This result was similar in other studies (6,13,15). Anastomosis should be performed in a relatively narrow operative field to pull out remnant stomach via mini laparotomy during the LADG. However, TLDG has an advantage of better visual field especially in obese patients (7,8). Although the present study did not compare obese patients separately, this advantage leads to reducing damage to surrounding tissues and decreasing the tension on anastomosis site not only in obese patients, but also in non-obese patients.
In TLDG, proximal resection margins should be determined in the abdominal cavity without palpation of the endoscopic clip (16). Therefore, a longer and safer length of the proximal margin was kept. In the present study, the proximal margin was significantly longer in the TLDG group than in the LADG group.
Other studies have shown that TLDG reduces postoperative pain because of the reduced wound size and shows superiority in recovery after operation compared to LADG (4,15,17,18). However, several studies have reported no significant difference in the short-term outcome between TLDG and LADG. A prospective randomized study reported no significant difference in postoperative pain between the two groups (19). Another retrospective case-control study shows no significant differences in bowel recovery, EBL, and hospital stay (20). However, the present study found that bowel recovery was faster in TLDG than in LADG based on the first flatus passage after operation. Postoperative pain score was lower only in POD 1 and not significant in POD 3, and there was no difference in the length of hospital stay. This slight difference in surgical outcomes may be due to several factors. First, the reduction of wound size is minimal between LADG and TLDG. Second, the routine use of postoperative patient-controlled analgesia can minimize the pain difference between the two groups.
Inflammatory parameters were marginally lower in TLDG. Only CRP in POD 5 was significantly lower in TLDG. Other studies have shown marginally or significantly lower inflammatory parameters in TLDG, similar to the present study (18,20,21). This result can also be attributed to the advantages of performing intracorporeal anastomosis, that is, intracorporeal anastomosis offers a better operative field and can reduce the damage to the surrounding tissue.
Postoperative complication was not different between the two groups. Previous studies have reported no difference in the postoperative complications between LADG and TLDG (4,14,15,18,19,21). Theoretically, a reduction of incision size in the TLDG may reduce wound complication, but there is no significant difference in wound complication rate between the two approaches. However, TLDG requires opening of the gastric lumen to the abdominal cavity during anastomosis, which increased abdominal infection rate. However, there was no difference in abdominal infection between the two groups. Appropriate decompression of gastric luminal contents during anastomosis may be necessary to reduce infection.
In the subgroup analysis based on the reconstruction type, TLDG has significantly longer operating time during BI gastroduodenostomy. Because linear stapler manipulation requires more precise technique in BI anastomosis than BII anastomosis (22), the anastomosis process takes a longer time. However, there is no significant difference in complications between the two groups according to reconstruction type, which suggest that TLDG is feasible regardless of the reconstruction type.
The present study has several limitations. First, as a retrospective study, this study was not performed concurrently. Surgeon’s learning curve might influence the surgical outcomes. However, since surgeons had already experienced sufficient number of cases of laparoscopic gastric cancer surgery, it might not have a significant effect on the outcome of the study. Second, as indication of laparoscopy was extended, more advanced gastric cancer patients were included, and patients with D2 dissection increased in the TLDG group. It also might affect the operative outcomes. Third, the present study could not evaluate some important factors such as quality of life and cost-effectiveness for operation and long-term survival outcome. In addition, the exact wound length for each patient was not recorded.
Conclusions
Based on the short-term postoperative outcome in this study, TLDG is safe and feasible as well as LADG. Moreover, compared with LADG, TLDG can reduce intraoperative EBL and postoperative pain and enhance bowel motility in gastric cancer surgery.
Acknowledgements
This work was supported by a grant (NCC 1710160-2) from the National Cancer Center, Republic of Korea.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kim W, Kim HH, Han SU, et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg 2016;263:28–35. [PubMed] DOI:10.1097/SLA.0000000000001346
- Viñuela EF, Gonen M, Brennan MF, et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg 2012;255:446–56. [PubMed] DOI:10.1097/SLA.0b013e31824682f4
- Shinohara T, Satoh S, Kanaya S, et al. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc 2013;27:286–94. [PubMed] DOI:10.1007/s00464-012-2442-x
- Chen K, Mou YP, Xu XW, et al. Comparison of short-term surgical outcomes between totally laparoscopic and laparoscopic-assisted distal gastrectomy for gastric cancer: a 10-y single-center experience with meta-analysis. J Surg Res 2015;194:367–74. [PubMed] DOI:10.1016/j.jss.2014.10.020
- Jun G, Ping L, Jie C, et al. Totally laparoscopic vs. laparoscopically assisted distal gastrectomy for gastric cancer: a meta-analysis. Hepatogastroenterology 2013;60:1530–4. [PubMed] DOI:10.5754/hge121240
- Lee SW, Tanigawa N, Nomura E, et al. Benefits of intracorporeal gastrointestinal anastomosis following laparoscopic distal gastrectomy. World J Surg Oncol 2012;10:267. [PubMed] DOI:10.1186/1477-7819-10-267
- Kim MG, Kawada H, Kim BS, et al. A totally laparoscopic distal gastrectomy with gastroduodenostomy (TLDG) for improvement of the early surgical outcomes in high BMI patients. Surg Endosc 2011;25:1076–82. [PubMed] DOI:10.1007/s00464-010-1319-0
- Sugimoto M, Kinoshita T, Shibasaki H, et al. Short-term outcome of total laparoscopic distal gastrectomy for overweight and obese patients with gastric cancer. Surg Endosc 2013;27:4291–6. [PubMed] DOI:10.1007/s00464-013-3045-x
- Kim HG, Park JH, Jeong SH, et al. Totally laparoscopic distal gastrectomy after learning curve completion: comparison with laparoscopy-assisted distal gastrectomy. J Gastric Cancer 2013;13:26–33. [PubMed] DOI:10.5230/jgc.2013.13.1.26
- Ahn CW, Hur H, Han SU, et al. Comparison of intracorporeal reconstruction after laparoscopic distal gastrectomy with extracorporeal reconstruction in the view of learning curve. J Gastric Cancer 2013;13:34–43. [PubMed] DOI:10.5230/jgc.2013.13.1.34
- Kanaya S, Kawamura Y, Kawada H, et al. The delta-shaped anastomosis in laparoscopic distal gastrectomy: analysis of the initial 100 consecutive procedures of intracorporeal gastroduodenostomy. Gastric Cancer 2011;14:365–71. [PubMed] DOI:10.1007/s10120-011-0054-0
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [PubMed] DOI:10.1097/01.sla.0000133083.54934.ae
- Song KY, Park CH, Kang HC, et al. Is totally laparoscopic gastrectomy less invasive than laparoscopy-assisted gastrectomy?: prospective, multicenter study. J Gastrointest Surg 2008;12:1015–21. [PubMed] DOI:10.1007/s11605-008-0484-0
- Choi BS, Oh HK, Park SH, et al. Comparison of laparoscopy-assisted and totally laparoscopic distal gastrectomy: the short-term outcome at a low-volume center. J Gastric Cancer 2013;13:44–50. [PubMed] DOI:10.5230/jgc.2013.13.1.44
- Kinoshita T, Shibasaki H, Oshiro T, et al. Comparison of laparoscopy-assisted and total laparoscopic Billroth-I gastrectomy for gastric cancer: a report of short-term outcomes. Surg Endosc 2011;25:1395–401. [PubMed] DOI:10.1007/s00464-010-1402-6
- Ryu KW, Lee JH, Choi IJ, et al. Preoperative endoscopic clipping: localizing technique of early gastric cancer. J Surg Oncol 2003;82:75–7. [PubMed] DOI:10.1002/jso.10191
- Lee J, Kim D, Kim W. Comparison of laparoscopy-assisted and totally laparoscopic Billroth-II distal gastrectomy for gastric cancer. J Korean Surg Soc 2012;82:135–42. [PubMed] DOI:10.4174/jkss.2012.82.3.135
- Kim JH, Jun KH, Chin HM. Short-term surgical outcomes of laparoscopy-assisted versus totally laparoscopic Billroth-II gastrectomy for gastric cancer: a matched-cohort study. BMC Surg 2017;17:45. [PubMed] DOI:10.1186/s12893-017-0245-7
- Woo J, Lee JH, Shim KN, et al. Does the difference of invasiveness between totally laparoscopic distal gastrectomy and laparoscopy-assisted distal gastrectomy lead to a difference in early surgical outcomes? A prospective randomized trial. Ann Surg Oncol 2015;22:1836–43. [PubMed] DOI:10.1245/s10434-014-4229-x
- Lin M, Zheng CH, Huang CM, et al. Totally laparoscopic versus laparoscopy-assisted Billroth-I anastomosis for gastric cancer: a case-control and case-matched study. Surg Endosc 2016;30:5245–54. [PubMed] DOI:10.1007/s00464-016-4872-3
- Lee SH, Kim IH, Kim IH, et al. Comparison of short-term outcomes and acute inflammatory response between laparoscopy-assisted and totally laparoscopic distal gastrectomy for early gastric cancer. Ann Surg Treat Res 2015;89:176–82. [PubMed] DOI:10.4174/astr.2015.89.4.176
- Jeong O, Jung MR, Park YK, et al. Safety and feasibility during the initial learning process of intracorporeal Billroth I (delta-shaped) anastomosis for laparoscopic distal gastrectomy. Surg Endosc 2015;29:1522–9. [PubMed] DOI:10.1007/s00464-014-3836-8
