Probe-based confocal endomicroscopy is accurate for differentiating gastric lesions in patients in a Western center
Introduction
Gastric cancer represents the third leading cause of cancer deaths worldwide. Developing countries, including Brazil, have a high incidence of gastric cancer and the majority of patients are diagnosed in advanced stage disease (1,2). Early diagnosis is desirable, since it improves the prognosis and has a relevant impact on mortality reduction.
Techniques such as probe-based confocal laser endomicroscopy (pCLE) allow acquisition of high-resolution real-time in vivo images at the cellular and microvascular levels (3,4). pCLE evaluates the microarchitecture of the mucosal epithelium allowing differentiation between normal and neoplastic mucosa. This technique has been used with high sensitivity and specificity in different scenarios involving the gastrointestinal tract, including esophageal squamous cell carcinoma, Barrett’s esophagus, pancreatic cysts, and colorectal lesions (4,5).
Zhang et al. first suggested a classification of gastric pit patterns by confocal laser endomicroscopy through the endoscope, in 2008 (6). Later, Wallace et al. proposed a Miami classification using pCLE for the differentiation of normal and pathologic conditions in the gastrointestinal tract, and, specifically for the stomach, described the findings of normal mucosa, gastritis, gastric dysplasia, and gastric adenocarcinoma (4). Further, Bok et al. classified the lesions into non-neoplastic, dysplasia, differentiated adenocarcinoma, and undifferentiated adenocarcinoma, and demonstrated high diagnostic accuracy (7). In 2016, Li et al. detailed the classification, taking into consideration the gastric pit patterns and the vessel architecture according to gastric location. These authors showed high accuracies for the diagnoses of atrophic gastritis, intestinal metaplasia, and gastric neoplasia. In addition, they described substantial interobserver agreement for the differentiation of neoplasia versus non-neoplasia (8).
The aim of this case series study was to evaluate the accuracy of pCLE for the differential diagnosis of non-neoplastic and neoplastic gastric lesions in a tertiary center of a developing country where the gastric cancer incidence is high.
Materials and methods
The Ethical Committee of the Department of Gastroenterology and University of São Paulo School of Medicine at the Cancer Institute approved the study (No. 303.460). All patients consented for participation.
Twenty gastric mucosal alterations from ten patients were evaluated during endoscopic procedure and were examined by pCLE. Routinely, the gastric mucosa was washed with 6% N-acetyl-cysteine before examination. The characteristics of suspicious lesions were carefully described and classified according to Paris Classification (9). Location and size were also reported. Photos and videos of endoscopic and pCLE images were taken.
pCLE procedure
Deep sedation was provided with propofol, fentanyl, and midazolam. Patients underwent high definition white light endoscopy, narrow band imaging (NBI), and indigo-carmine chromoendoscopy for proper characterization of the target lesion immediately followed by pCLE.
Before the procedure, the patients underwent an allergic test with intravenous injection of 0.1 mL of 10% fluorescein. If no allergic reaction was observed, 4.9 mL of 10% fluorescein diluted in 100 mL of saline solution was injected intravenously. A cap was attached to the tip of the endoscope (GIF H180; Olympus, Tokyo, Japan; 9.8 mm caliber and 2.8 mm biopsy channel) in order to stabilize the probe (pCLE). The probe was then inserted through the working channel of the endoscope into the stomach a few minutes after fluorescein injection. pCLE (2.5-mm UHD GastroFlex probe, Cellvizio; Mauna Kea Technologies, Paris, France) provided a depth of examination of 55 to 65 mm, a 240-mm field of view at a resolution of 1 mm and magnification of 1,000 at 12 frames per second.
The distribution of the fluorescein into the capillary network and connective tissue, enhancing the cellular morphology is optimal for 20 min after injection, so the pCLE procedure was kept to less than 20 min.
pCLE classification system
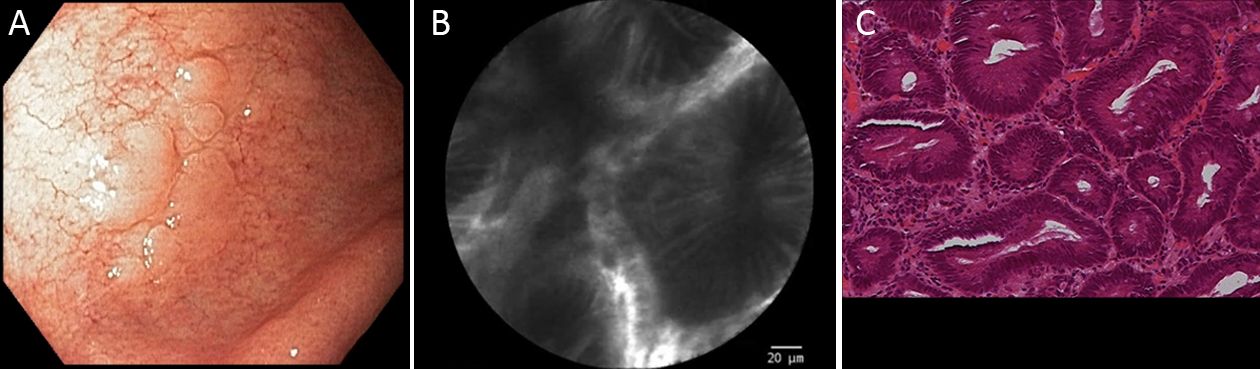
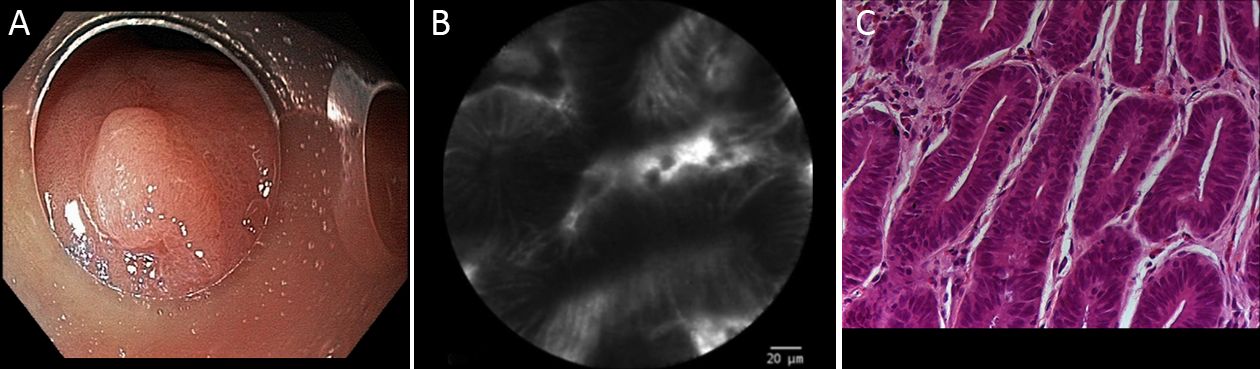
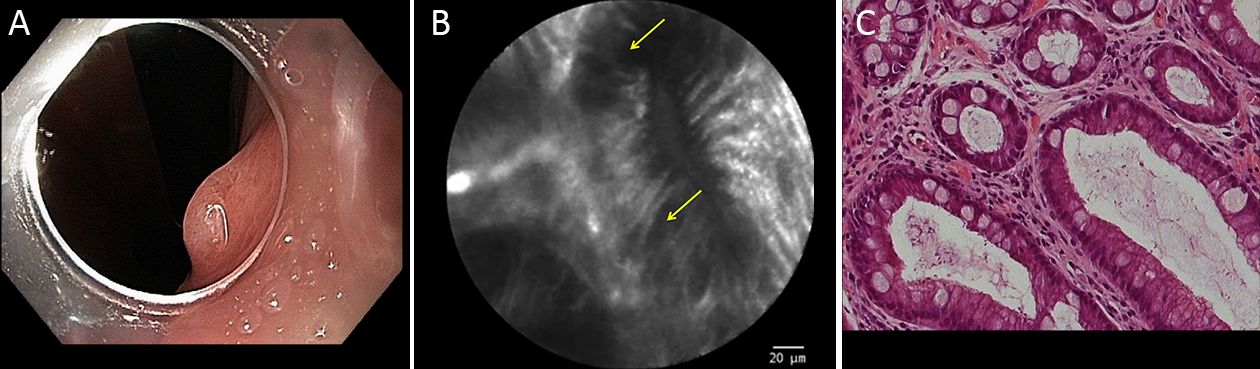
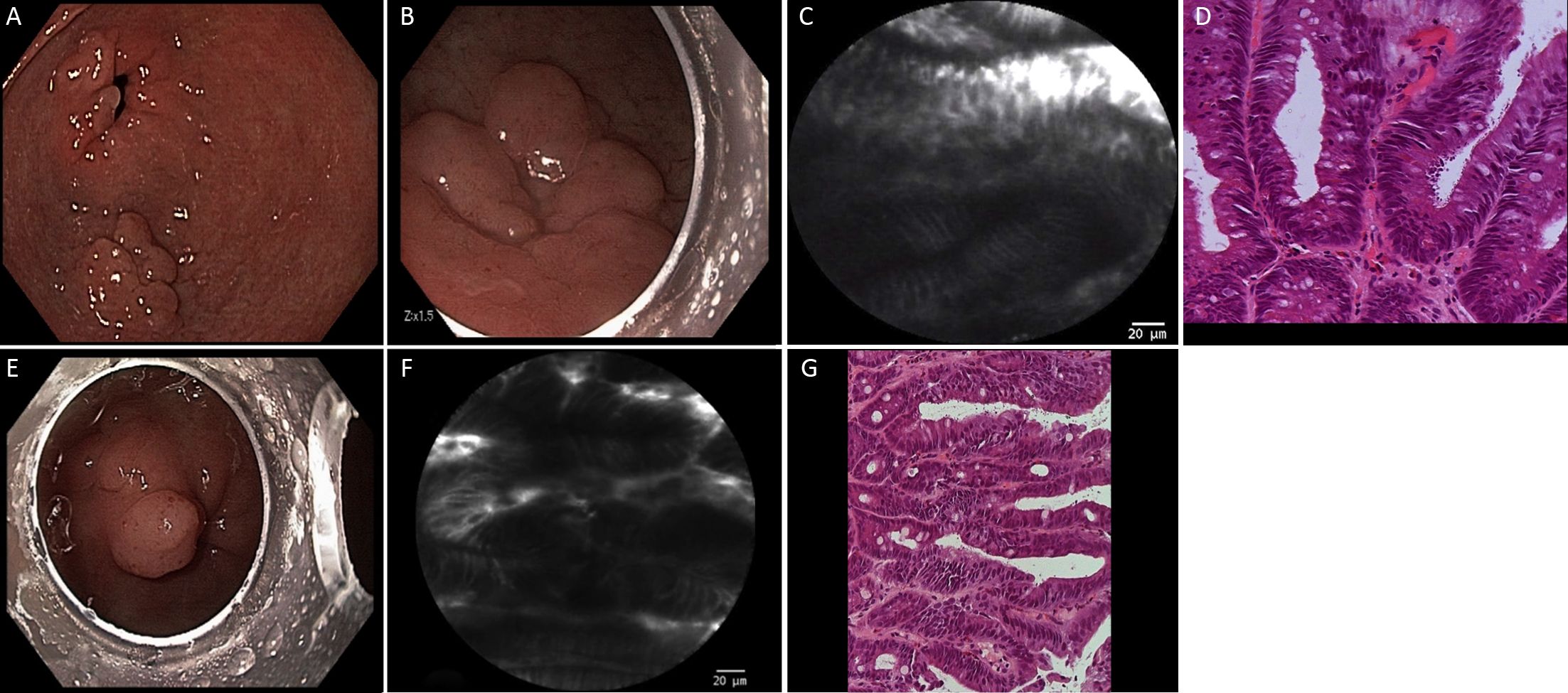
pCLE classification was based on Li et al. (8) previous report, taking into consideration details of pit and vascular patterns. In summary, according to this classification, regular pits with wide, round and slit-like openings are considered normal findings in cardia, corpus and antrum, respectively. Regular pits with elongated openings and increased fluorescence in stroma are characteristics of inflammatory gastric mucosa. On the other hand, reduced pits with dilated openings are features of atrophic mucosa and the appearance of goblet cells with dark mucin is features of intestinal metaplastic mucosa. Low-grade intraepithelial neoplasia is considered when mild to moderate irregular pits are found, and high-grade when prominent distorted pits with irregular epithelial lining are present. Appearance of atypical glands and dispersion of irregular dark cells are characteristics of differentiated and poorly differentiated adenocarcinoma. Based on vascular architecture, regular capillaries with normal calibers are found in normal mucosa, while increased capillaries with elevated leakage are encountered in inflammatory gastric mucosa. Irregular and dilated capillaries with heterogeneous leakage are found in neoplastic gastric mucosa.
Histopathology
Diagnostic pCLE was followed by biopsies for histology, although small suspicious lesions were endoscopically resected, avoiding biopsy-related fibrosis or distortion that could interfere with further endoscopic workup.
Biopsy specimens (n=10) or resected endoscopic specimens (n=10) were fixed in formalin. A senior pathologist blinded to the pCLE results examined all the histologic specimens.
Results
Demographics
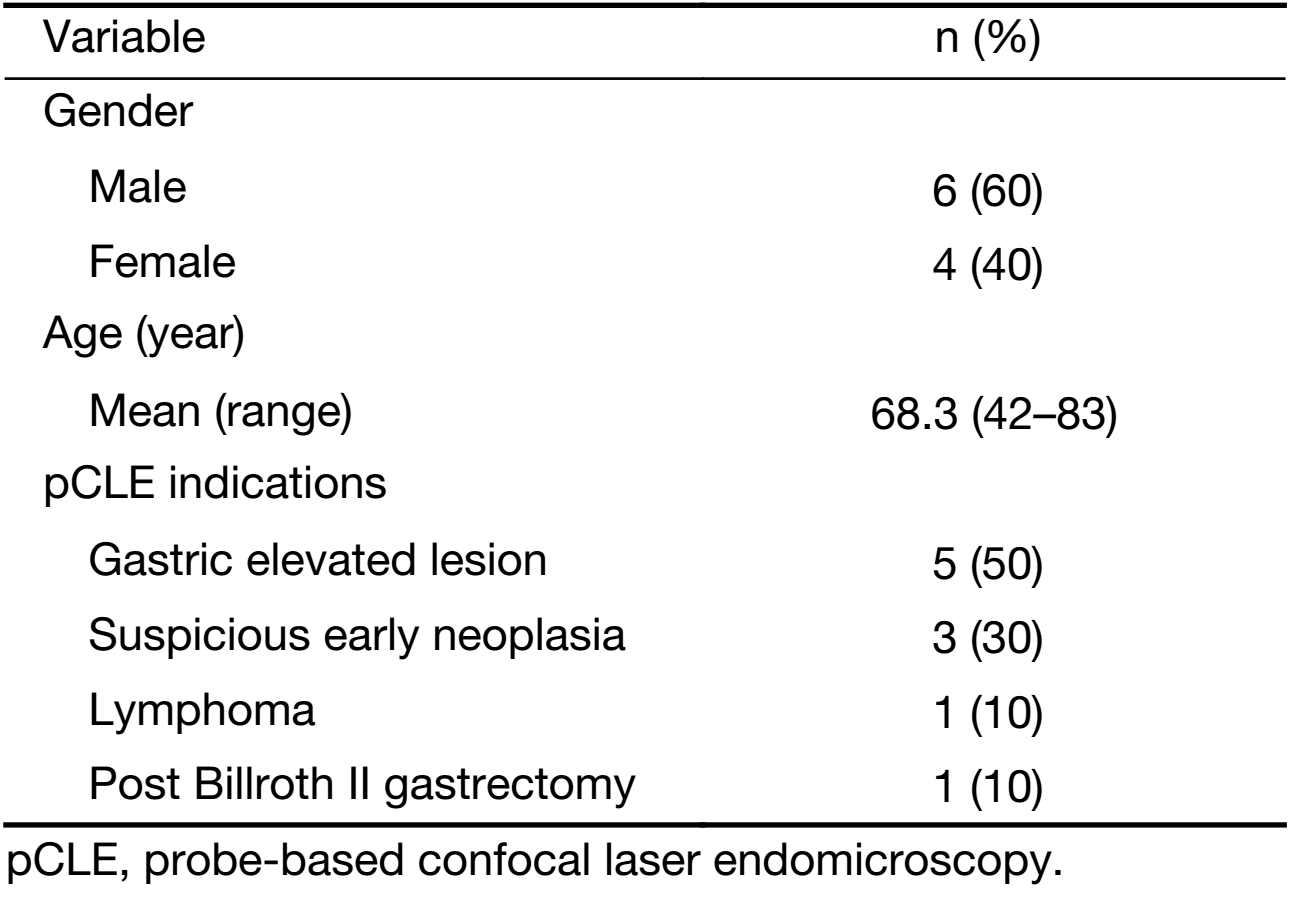
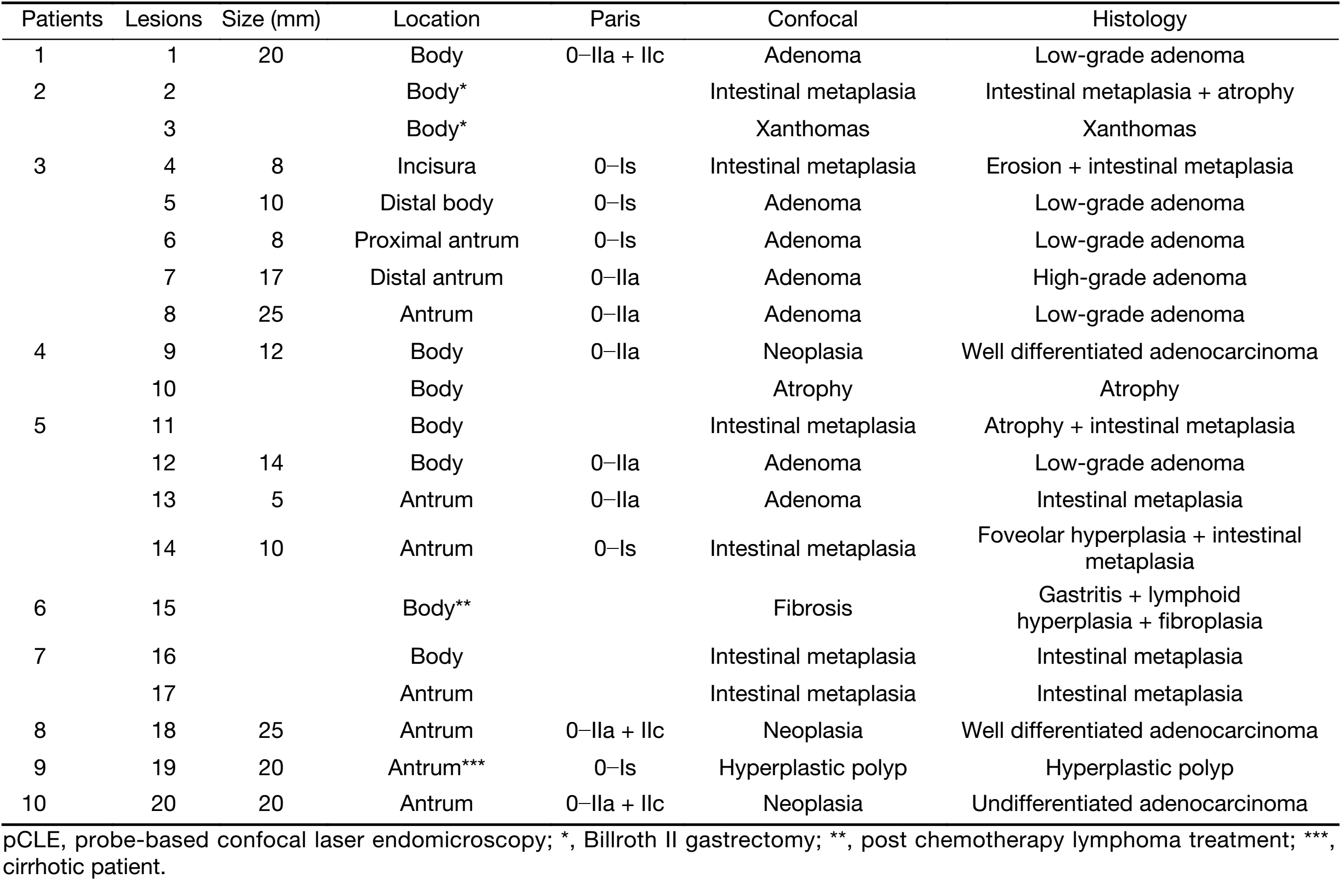
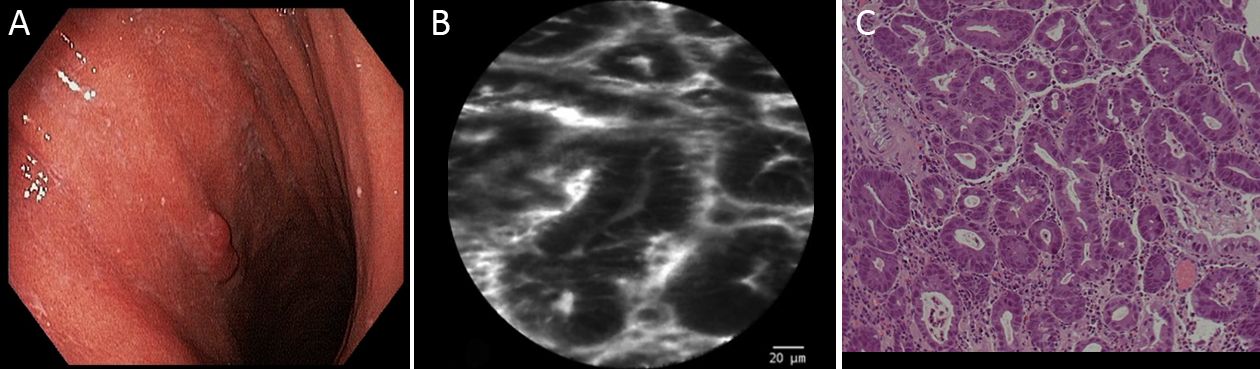
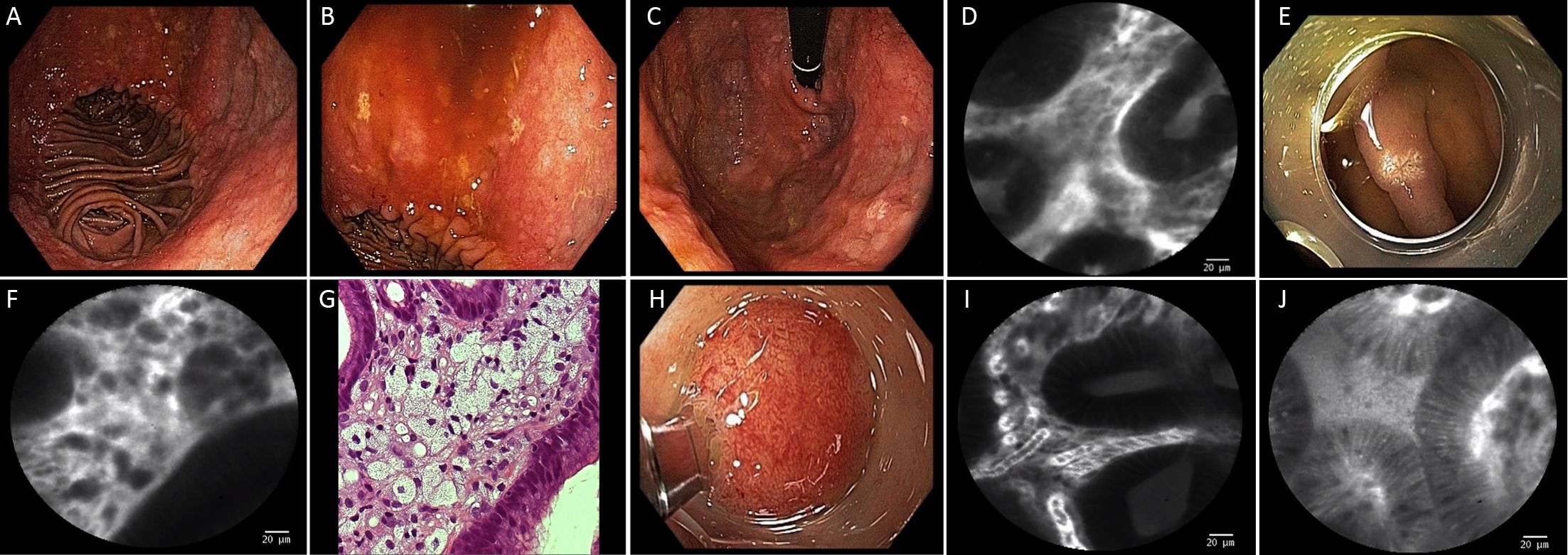
Twenty gastric mucosal lesions from ten patients were examined by pCLE. Patients’ mean age was 68.3 (range, 42–83) years and six were men (Table 1). Thirteen suspicious flat or elevated lesions (classified as 0−Is, 0−IIa or 0−IIa+IIc) and seven benign lesions (atrophy and intestinal metaplasia) were evaluated (Table 2; Figures 1−5). One of these patients was followed up after chemotherapy for gastric lymphoma and one was evaluated during follow-up after partial gastrectomy and presented severe atrophy and intestinal metaplasia, and xanthomas at the stump mucosa (Figure 6). The location of gastric lesions was in the body (n=10 lesions), the antrum (n=9) and the incisura angularis (n=1).
Full table
Full table
pCLE results and histopathological data
pCLE properly diagnosed all the neoplastic lesions (n=9), and all but one benign lesions (n=10). Three pCLE suspected malignant early lesions were confirmed histologically after endoscopic resection. Five resected elevated lesions were diagnosed as low-grade adenoma and one as high-grade adenoma. pCLE incorrectly diagnosed one small antrum lesion (5 mm in size) as low-grade adenoma, however the final diagnosis was intestinal metaplasia, in a patient with three other lesions. The final histological diagnosis was neoplastic in 9 and benign lesions in 11. Thus, pCLE diagnostic accuracy was 95% (19/20 lesions).
Adverse events caused by the intravenous injection of fluorescein were not observed, although changes in skin and urine discoloration were seen in all patients.
Discussion
The incidence of gastric cancer remains high in some countries of Eastern Asia and South America and still represents the third leading cause of cancer deaths worldwide (1,10).
In western countries, the majority of patients with gastric cancer are diagnosed in an advanced stage disease, resulting in high mortality rate (11). When early neoplastic lesions are diagnosed, they can be managed by endoscopic mucosal or submucosal resection due to the low risk of lymph node metastasis (12). Either standard or extended endoscopic criteria, recommended by the Japanese Gastric Cancer Association, and by Gotoda et al., allow curative resection of gastric lesions with good prognosis (13). Therefore, early diagnosis is imperative to achieve less invasive treatment, and consequently better quality of life.
Gastric cancer screening represents the optimal method for early detection of neoplastic lesions in countries with high incidence of this malignancy, although such strategy has not been performed worldwide, except in few Asian countries such as Japan or Korea (14). However, few patients who were followed up for other malignancies at the São Paulo Cancer Institute have benefited from upper gastrointestinal endoscopy for screening.
pCLE has been used with high accuracy for the diagnosis of gastric lesions (8). This strategy permits examination at cellular and subcellular levels, allowing a 1,000 times magnification (4,15). Patients with high risk of pre-neoplastic lesions, including patients with partial gastric resection, in which the pouch might increase the chance of development of intestinal metaplasia and atrophy, could benefit from the use of pCLE, since this procedure allows easy detailed visualization of such alterations. Moreover, atrophic stomach in patients with familial gastric cancer risk might represent another indication for pCLE, in order to avoid multiple tissue biopsies. In one case in our series, it was also possible to identify, at the cellular level, the presence of xanthomas in a gastric atrophic pouch. Patients with portal hypertension may also benefit since pCLE might differentiate benign from dysplastic lesions, as occurred in one patient in our series who presented an elevated lesion at the antrum diagnosed as hyperplastic polyp, which did not require resection.
Additionally, vascular patterns evaluated by pCLE might assist in the diagnosis of gastric lesions, since neoangiogenesis is essential for tumor growth (16). A classification of vascular alterations by Li et al. may help distinguish pre-neoplastic versus neoplastic lesions. Irregularity and dilated capillaries with heterogeneous leakage may indicate malignant alterations (8). Furthermore, pCLE has been utilized for studying the neoangiogenesis before and after neoadjuvant therapy for patients with advanced gastric disease, determining those patients who had better response (16). In our case series, pCLE was useful for detecting pre- and early neoplastic lesions with high accuracy. Using the combination of cellular and vascular patterns, pCLE could improve the diagnosis and delineation of the margins of gastric lesions that have not been clearly differentiated by high-definition white light endoscopy, NBI and chromoendoscopy.
In summary, our study corroborates that pCLE allows real time in vivo diagnosis of gastric lesions, regarded as an optical biopsy. This strategy could have a relevant impact on the treatment management avoiding further diagnostic procedures. Tissue biopsies could be safely eliminated and not interfere with the endoscopic resection.
This study has some limitations, including the small number of patients from a single institution and the large variety of included gastric lesions.
Conclusions
pCLE was accurate for real time histology of gastric lesions and could be useful for the surveillance of gastric neoplastic lesions in high-risk patients. pCLE may alter the management of patients with gastric mucosal alterations, guiding biopsies and endoscopic resection. Therefore, pCLE may contribute to early diagnosis of gastric lesions and might have an influence on the therapeutic strategy.
Acknowledgements
The authors are indebted to Richard Huneke for reading and correcting the manuscript. This study was partially supported by FINEP (Financiadora de Estudos e Projetos) from Brazilian Ministry of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interests to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [PubMed] DOI:10.1002/ijc.29210
- Tsai MC, Wang CC, Lee HL, et al. Health disparities are associated with gastric cancer mortality-to-incidence ratios in 57 countries. World J Gastroenterol 2017;23:7881–7. [PubMed] DOI:10.3748/wjg.v23.i44.7881
- Meining A, Saur D, Bajbouj M, et al. In vivo histopathology for detection of gastrointestinal neoplasia with a portable, confocal miniprobe: an examiner blinded analysis . Clin Gastroenterol Hepatol 2007;5:1261–7. [PubMed] DOI:10.1016/j.cgh.2007.05.019
- Wallace M, Lauwers GY, Chen Y, et al. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy 2011;43:882–91. [PubMed] DOI:10.1055/s-0030-1256632
- Safatle-Ribeiro AV, Baba ER, Faraj SF, et al. Diagnostic accuracy of probe-based confocal laser endomicroscopy in Lugol-unstained esophageal superficial lesions of patients with head and neck cancer. Gastrointest Endosc 2017;85:1195–1207. [PubMed] DOI:10.1016/j.gie.2016.09.031
- Zhang JN, Li YQ, Zhao YA, et al. Classification of gastric pit patterns by confocal endomicroscopy. Gastrointest Endosc 2008;67:843–53. [PubMed] DOI:10.1016/j.gie.2008.01.036
- Bok GH, Jeon SR, Cho JY, et al. The accuracy of probe-based confocal endomicroscopy versus conventional endoscopic biopsies for the diagnosis of superficial gastric neoplasia (with videos). Gastrointest Endosc 2013;77:899–908. [PubMed] DOI:10.1016/j.gie.2013.01.018
- Li Z, Zuo XL, Li CQ, et al. New classification of gastric pit patterns and vessel architecture using probe-based confocal laser endomicroscopy. J Clin Gastroenterol 2016;50:23–32. [PubMed] DOI:10.1097/MCG.0000000000000298
- . The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3–43. [PubMed] DOI:10.1016/S0016-5107(03)02159-X
- Luo G, Zhang Y, Guo P, et al. Global patterns and trends in stomach cancer incidence: Age, period and birth cohort analysis. Int J Cancer 2017;141:1333–44. [PubMed] DOI:10.1002/ijc.30835
- Guedes MT, de Jesus JP, de Souza Filho O, et al. Clinical and epidemiological profile of cases of deaths from stomach cancer in the National Cancer Institute, Brazil. Ecancermedicalscience 2014;8:445. [PubMed] DOI:10.3332/ecancer.2014.445
- Pereira MA, Ramos MFKP, Dias AR, et al. Risk factors for lymph node metastasis in western early gastric cancer after optimal surgical treatment. J Gastrointest Surg 2018;22:23–31. [PubMed] DOI:10.1007/s11605-017-3517-8
- Gotoda T, Iwasaki M, Kusano C, et al. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg 2010;97:868–71. [PubMed] DOI:10.1002/bjs.7033
- Hamashima C, Sano H. Association between age factors and strategies for promoting participation in gastric and colorectal cancer screenings. BMC Cancer 2018;18:345. [PubMed] DOI:10.1186/s12885-018-4244-6
- Baba ER, Safatle-Ribeiro AV, Paduani GF, et al. Probe-based confocal laser endomicroscopy for the differential diagnosis of gastric tubular adenoma and intestinal metaplasia in a patient with severe atrophic pangastritis. Gastrointest Endosc 2016;84:183. [PubMed] DOI:10.1016/j.gie.2016.02.033
- Spessotto P, Fornasarig M, Pivetta E, et al. Probe-based confocal laser endomicroscopy for in vivo evaluation of the tumor vasculature in gastric and rectal carcinomas. Sci Rep 2017;7:9819. [PubMed] DOI:10.1038/s41598-017-10963-1
